An unusual case of small bowel diarrhoea
Received: 01-Feb-2018 Accepted Date: Mar 03, 2018; Published: 07-Mar-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Since publication of 22 cases of olmesartan induced sprue like enteropathy by Rubio-Tapia et al. there have been publications of many case reports. It is now documented that olmesartan cause sprue like enteropathy with partial villous atrophy, leading to malabsorption and small bowel diarrhea. Sometimes it resembles celiac, in some it may resemble like collagenous or lymphocytic microscopic colitis. It may involve any parts of gastrointestinal tract. There are few case reports from India. We encountered a middle-aged female with severe diarrhoea leading to dehydration and acute kidney injury. After failed trial of gluten free diet, she responded only when olmesartan was stopped. AIM of reporting this case is- We as a clinician must be aware of this adverse effect of angiotensin receptor II blocker and should have a high index of suspicion so that we can avoid delay in the diagnosis as well as costly, frustrating unnecessary tests.
Keywords
Abdominal pain; Acute kidney; Colono-ileoscopy; Hypokalemia; Endoscopy
Sprue-like enteropathy associated with olmesartan, an antihypertensive;an angiotensin receptor blocker had been described by Rubia-Tapia et al [1] in 2012. There are approximately 100 reported cases [2,3]. Although coeliac disease is the most common cause of sprue like enteropathy, drug induced enteropathy should also be kept in mind. Here we report a patient with severe diarrhea, acute kidney injury, metabolic acidosis, dyselectrolemia after use of olmesartan for some duration. Histologically duodenal biopsy resembled that of coeliac disease. Clinical signs did not improve on gluten free diet but ceased upon drug discontinuation. Whether this adverse event is specific for olmesartan or is a class effect of angiotensin II receptor blockers is currently unknown.
Case Report
A 59-year-old housewife, diabetic for 5 years (on glimeperide) and hypertensive (on olmesartan for 1 year), presented with severe chronic non-bloody large volume diarrhea, 10-15 times a day, for 6 weeks duration. She had mild abdominal pain. There was no history of nocturnal diarrhoea, vomiting, fever, skin rash, oral ulcers, joint pain, palpitation, flushing or dizziness. Initially she received antibiotics, probiotics, cholestyramine and mesalamine as prescribed by her physician. Gradually she developed anorexia and fatigue without significant weight loss and was brought to our hospital. There was no history of travel abroad, similar history in the past or any family history of IBD.
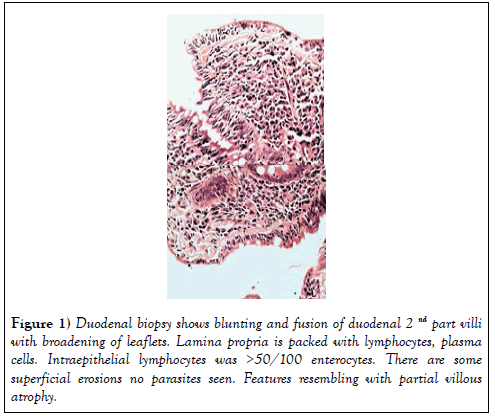
On clinical examination she was pale and dehydrated with blood pressure of 100/60 mmHg and pulse rate was 100/min. She was afebrile, without any with evidence of arthropathy, clubbing, lymphadenopathy, skin rash, tremor. The body mass index was 29 kg/m2. Investigation revealed normocytic normochromic anaemia- (Hb-9.2 gm%, PCV-27.9, MCV-80.2; TLC- 8200/ cumm, platelets-3.34 lakh/cumm, ESR-82 mm/hr), normal liver function with mild hypoalbuminaemia (total protein 6.2 gm/dl and albumin 3.0 gm/ dl), mild hypokalemia (K-3.2 meq/l), normal TSH, CRP, urea and creatinine (0.94 mg/dl). Vitamine B12, folate and iron profile were normal. HBsAg, AntiHCV and HIV were negative. Stool RE was normal with culture was negative for enteropathogens. Stool clostridium difficile PCR was negative. Chest X-ray and ultrasound abdomen were normal. Upper GI endoscopy was normal with no significant atrophy/scalloping of duodenal folds. D2 biopsyshowed blunting and fusion of villi with broadening of leaflets. Lamina propria was packed with lymphocytes, plasma cells and small superficial erosions, intraepithelial lymphocytes- >50/100 enterocytes at places, no parasites seen- partial villous atrophy, resembling coeliac disease (Figure 1) Duodenal biopsy-Serum AntitTgIGA for coeliac disease was negative (<1.4 u/ml) with normal Total IGA level.
Figure 1: Duodenal biopsy shows blunting and fusion of duodenal 2nd part villi with broadening of leaflets. Lamina propria is packed with lymphocytes, plasma cells. Intraepithelial lymphocytes was >50/100 enterocytes. There are some superficial erosions no parasites seen. Features resembling with partial villous atrophy.
Colono-ileoscopy–normal and biopsy ruled out microscopic colitis. Faecal calprotectin was high-1589.00 mg/kg. CT enterography- was normal.
On day 4, she had oliguria, tachycardia, tachypnoia and acidotic breathing but remained afebrile. ABG showed severe metabolic acidosis (Ph-7.0, HCO3-2.0, pCO2-7). BP-100/70 mmHg. She was shifted to ITU, sepsis screen was negative. Creatinine was raised to 3.0 mg/dl. FBS was 102 mg/ dl, urine showed no ketone bodies or pus cells. There was hypokalemia, hypomagnesaemia (K-2.7, Mg- 1.40) and normal Na level-139 meq/l. Differential diagnoses we thought at that time were contrast induced AKI, sepsis with AKI, severe dehydration with AKIor any other causes of severe diarrhoea. She was resuscitated with IV fluid and was given antibiotic. She improved in 48 hours. She was off olmesartan since admission. In view of severe hypokalemia and diarrhoea-serum chromogranin A was checked- 60.3 ng/ml, normal.
She was discharged after 12 days without any proton pump inhibitor and on gluten free diet.
She was readmitted after 7 days with severe diarrhoea, drowsiness, hypokalemia (2.7 meq/l), oliguria for 2 days and severe metabolic acidosis. (ABG -PH- 7.1, HCO3- 2.3, PAO2- 132, PCO2- 8 normal anion gap,) BP- 100/60 mmHg, PR-120/min, she was dehydrated, afebrile, CBS- 280 mg/dl. She was admitted to ITU and was given bicarbonate, hydrated with NS, RL. CVP was 3-4 mmHg. Investigation revealed creatinine 2.4 mg/dl, urea-48 mg/dl, Na- 151 meq/l, K-2.7 meq/l, HB-8.7, PCV- 28, MCV- 81, TLC-9010. Lactate 0.7, CRP- 23.2 mg/l. Blood C/S, urine C/S, stool RE and C/S were normal. Meanwhile recent history revealed that she had started olmesartan on her own after discharge.
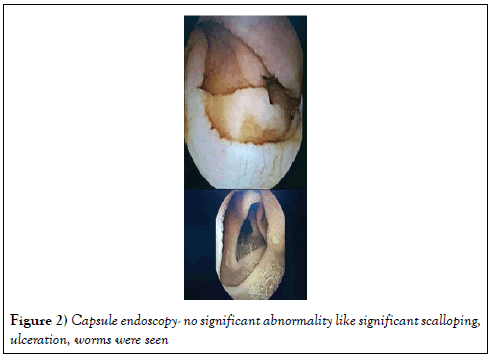
Screening for other causes like inflammatory bowel disease, Neuroendocrine tumor were also excluded. Capsule endoscopy did not show significant scalloping, inflammation, ulcer or mass. Serum chromogranin, fasting gastrin and urine 24 hrs HIAA were normal (Figure 2) Capsule endoscopy-She recovered with normal electrolytes and creatinine within 48 hours and was discharged and was categorically instructed not to restart olmesartan. She was followed up for 1 year and she remained asymptomatic, tolerating normal diet. Repeat UGI scopy and duodenal biopsy were nonspecific.
Discussion
The constellation of diarrhoea, malabsorption and villous atrophy raises the possibility of coeliac disease. Negative coeliac serology or nonresponse to a gluten-free diet implies a broad and challenging differential diagnosis which includes Crohn’s disease, enteric infections (e.g giardiasis), collagenous sprue, tropical sprue, Small intestinal bacterial overgrowth, common variable immunodeficiency, autoimmune enteropathy, hematological malignancies, HIV enteropathy and medication-associated enteropathy [1-3].
Drug causing enteropathy, include azathioprine, mycophenolate mofetil, neomycin, methotrexate, colchicine and angiotensin receptor blocker, like olmesartan. Telmisartan causing enteropathy like symptoms has also being reported [4,5].
Olmesartan induced enteropathy which is often indistinguishable from coeliac disease poses a diagnostic challange. It usually happens several months (6 m-1 yr) after initiation [2].
The adverse effects can be seen with any dose from 10 mg to 40 mg/day 1.
Most common symptoms are chronic diarrhoea, weight loss, anorexia, fatigue normocytic normochromic anaemia, hypoalbuminaemia and multiple electrolyte abnormalities. Dehydration and acute renal failure have been reported as the main causes of hospitalization [1] there are case report of olmesartan causing dermatological changes. A case of colonic perforation has also been documented [6].
Our patient had severe diarrhoea with acute kidney injury and metabolic acidodsis, anaemia and hypokalemia but without significant weight loss
Prevalence of HLA-DQ2 has been documented in 68% of patients with olmesartan-associated enteropathy, higher than for the general population (25%-30%) [1,7,8].
Histologically intestinal atrophy, mucosal inflammation, lymphocytic infiltration has been described. In one series, it has been found that 92/100 patients (92%) had total or partial villous atrophy, 5% had normal villi, 2% had increased IEL (25 or more per 100 enterocytes). Involvement of stomach and colon have also been described [8-12].
Clinical and histological improvement occurs earliest 48 hours and 6 months after withdrawal of olmesartan respectively [10].
Immune mediated damage attributed to increased TGF beta leading to apoptosis of endothelial cells [4,6,7,9,10,13].
Conclusion
This case report highlights a rare but devastating form of diarrhoeal disease resulting from a commonly prescribed antihypertensive olmesartan which needs wide appraisal.
REFERENCES
- Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe sprue like enteropathy associated with olmesartan. Mayo Clin Proc 2012;(87):732-8.
- Dreifuss SE, Tomizawa Y, Farber NJ, et al. Spruelike enteropathy associated with olmesartan: an unusual case of severe diarrhea. Case Rep Gastrointest Med 2013;2013:618071.
- Bhat N, Anupama NK, Yelsangikar A, et al. Olmesartan-related spruelike enteropathy. Indian J Gastroenterol 2014;(33):564-56.
- Tran H, Henlin L. Olmesartan and drug induced enteropathy. P &T 2014;(39):146-50.
- Mandhavdhare SH, Sharma V, Prasad KK, et al. Telmisartan induced sprue like enteropathy: a case report and a review of patients using non olmesratan angiotensin receptor blocker. Intest Res 2017;(15):419-21.
- Liliana C, Albino M, Adelina P, et al. Olmesartan induced sprue like enteropathy. Port J Gastroenterol 2016;(23):101-5.
- Scialom S, Malamut G, Meresse B, et al. Gastrointestinal disorder associated with olmesartan mimics autoimmune enteropathy. Plos ONE 2015;(10):e0125024.
- Jain M, Olmesartan induced chronic diarrhea. The antiseptic 2017;(114).
- Isabel HA, Alberto RT. Sprue like enteropathy associated with Olmesartan, a new kid on the enteropathy block. Portuguese J Gastroenterol 2016;(23):61-5.
- Choi EY, McKenna BJ. Olmesartan associated enteropathy. A review of clinical and histological findings. Arch Path Lab Med 2015;(139);1242-7.
- Hammoudi N, Dior M, Giraud V, et al. Olmesartan induced enteropathy ass with cutaneous lesion. Clinical Case Reports 2016;(4):379-82.
- Naik KD, Martelli Mathew. An atypicalmcase ofchronic diarrhea. Olmesartan induced sprue like enteropathy. BMJ Case Reports. 2015
- Herman ML, Rubio-Tapia A, Wu TT, et al. A case of severe sprue-like enteropathy associated with valsartan. ACG Case Rep J 2015;(2):92-4.