Current surgical approach for left ventricular restoration
Received: 28-Jul-2017 Accepted Date: Sep 07, 2017; Published: 11-Sep-2017
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Coronary artery disease is not a simple coronary lesion that causes angina, but also combined heart problem that leads to severe ischemic complications as dilated heart failure, valve insufficiencies, heart rhythm disturbances, thrombus formation to secondary ischemic/embolic complications. In order to manage it, several worldwide surgical treatment months, wound assessment, measurement of limb oxygenation, ankle brachial pressures as well as McGill pain questionnaire will be carried out. Blood will also be collected at baseline and at the end of every month of the treatment period (for a total of 5 collections) for the measurement of different biomarkers were divided in two groups: Those with ST elevation myocardial infarction (STEMI) (Group 1, 54 patients), and those with non-ST elevation. We present here our single center and initial experience on surgical approach for left ventricular restorations and, we found it in conclusion that the outcome of these procedures was quite sufficient.
Keywords
Coronary; Artery; Disease; Heart; Ventricular; Cardiomyopathy
Abbreviations
CABG: Coronary Artery Bypass Grafting; COPD: Chronic Obstructive Pulmonary Disease; LV: Left Ventricle; TEE: Transesophageal Echocardiography; ECMO: Extracoroporeal Membrane Oxygenation; VSD: Ventricular Septal Defet; MV: Mitral Valve; TV-Tricuspid Valve; SVR: Surgeical Ventricular Restoration; PVL: Pressure Volume Loop: NYHA: New York Heart Association; EDV: End Diastolic Volume; ESV: End Systolic Volume; EF: Ejection Fraction; LVEDTD: Left Ventricular End-Diastolic Transverse Diameter; LVEDLD: Left Ventricular End-Diastolic Longitudinal Diameter
Ischemic cardiomyopathy is one of the possible complications related to coronary artery disease, and presents ischemic heart dilatation with concomitant mitral valve regurgitation and left ventricular (LV) thrombus formation [1,2]. Surgical ventricular restoration (SVR) is a procedure designed to restore or remodel the left ventricle to its normal, spherical shape and size in patients with akinetic segments of the heart, secondary to either dilated cardiomyopathy or post infarction left ventricular aneurysm [2-4]. The SVR procedure is usually performed after coronary artery bypass grafting (CABG) and may proceed or be followed by mitral valve repair or replacement and other procedures such as endocardectomy and cryoablation for treatment of ventricular tachycardia. A key difference between surgical ventricular restoration and ventriculectomy is that in SVR the ventricle is reconstructed using patches of autologous or artificial material that are placed to close the defect while maintaining the desired ventricular volume and contour Additionally, SVR is distinct from partial left ventriculectomy (the Batista procedure) which does not attempt to specifically resect akinetic segments and restore ventricular conture [4,5].
The actuality is this problem in Uzbekistan is still significant related to the patients’ late diagnosis and hospitalization which leads to development of severe heart failure and left ventricular (LV) thrombus formation. The procedures for such complications are simply explained of performing coronary bypass surgery, LV remodeling with thrombus removal and additionally mitral valve repair, if necessary. The results of LV remodeling associated with coronary artery bypass grafting in patients with ischemic dilated cardiomyopathy are demonstrated by STITCH trial, but, still some literatures show that LV reformation continued LV remodeling, and recurrent mitral valve (MV) regurgitation may occur after ventricular restoration [6-8].
Over the last decades, from time to time, the surgical strategies towards left ventricular restoration operations have been changing and several techniques have been described in order to reduce LV volumes and diameters. We would like to demonstrate our current approach for SVR and present it in our small cohort of patients group [9].
15 consecutive symptomatic patients: male/female-12/3 patients, aged 61.7+6.7 years, with a history of a previous large anteroseptal myocardial infarction are primarily diagnosed in outpatient department of Republican Scientific Center of Surgery and hospitalized in cardiac surgery division and included into study. All patients have NYHA III-IV class and present mostly concomitant diseases as diabetes, obesity and COPD, correspondently. Moreover, in 12 (80%) of cases, we had LV thrombus existence and post infarction VSD which gave us obvious explorations inside left ventricular cavity. Table 1 describes all the patient characteristics.
| Male | 12 (80%) |
| Female | 3 (20%) |
| NYHA III-IV | 15 (100%) |
| Age | |
| 50-60 | 8 (53.3%) |
| 60-70 | 4 (26.6%) |
| >70 | 3 (20%) |
| Diabetes on insulin | 8 (53.3%) |
| Obesity BMI >30 | 11 (73.3%) |
| COPD | 7 (46.6%) |
| LV thrombus | 12 (80%) |
| 3 coronary disease | 7 (46.6%) |
| 2 coronary disease | 6 (40%) |
| 1 coronary disease | 2 (13.3%) |
| Post-infarction VSD | 2 (13.3%) |
| Mitral regurgitation | 8 (53.3%) |
| Mitral valve repair | 3 (20%) |
| Mitral valve replacement | 2 (13.3%) |
| Post-infarction VSD | 2 (13.3%) |
| Operative technique | |
| Semicircular stitch | 10 (66.6%) |
| Linear patch repair | 5 (33.3%) |
Table 1: Patient’s characteristics (n-15)
Operative Technique
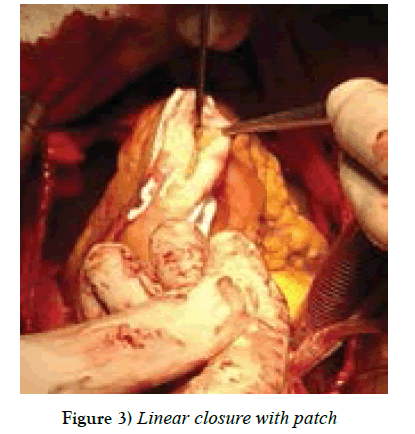
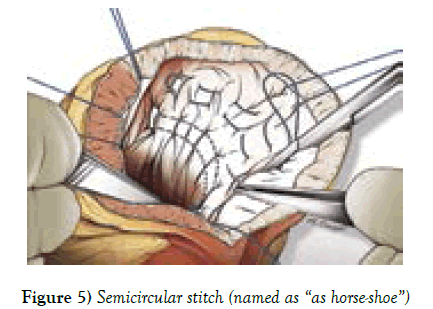
The surgical protocol consisted of the insertion of a prophylactic intra-aortic balloon pump (1 case), induction of cold crystalloid cardioplegia, complete myocardial revascularization, MV repair (for grade +2 regurgitation), and the placement of a permanent LV epicardial lead (in case of dyssynchrony). 2 patients (13.3%) underwent MV annuloplasty. After conventional start of every case, we instituted cardiopulmonary bypass on a standard fashion. We arrested the heart with cold crystalloid cardioplegia with topical ice packing if necessary. After conventional coronary distal anastomosis has been done, we usually targeted on our access in LV scarred area. In small LV aneurysms we used a linear patch technique as described by D. Cooley, with removal of LV thrombus. We faced mostly organic thrombus formations inside LV, firmly attached to myocardium those are detached, cleaned and flushed the LV cavity with isotonic solution. In quite large dilatations, instead of Dor [3] procedure, We used semicircular stitch technique similar to “horse-shoe technique” as described by Ferrazzi P., in 2009, so that, parallel purse-string sutures (polypropylene 2/0 or 3/0) are positioned in scarred interventricular septum, acting on the LV circumference: one at equatorial level (which is located at the middle of the insertion of the anterior papillary muscle) and one displaced 1 cm toward the apex. The equatorial purse string is tied first to obtain an elliptic shape, and then, the second purse string is tied to reshape the new apex [10]. In case of much enlarged LV cavity, up to 4 horseshoe purse-string sutures can be necessary. In order to reshape the LV cavity one has to string all the placed purse-string sutures and we never placed the mannequin balloon inside the LV chamber as described by Di Donato [11] for measuring end diastolic volume which should be around 50-60 ml/ m2. 1-2 index fingers were sufficient and helped us to obtain possible size of LV EDV. The ventriculotomy we closed with interposition of a pericardial patch or sometimes with reinforcing felt strips. This secures the bleeding complications from the stitch lines. We applied this technique in 10 patients with antero-septal myocardial infarction, regardless of LV dimensions and other 5 patients have got benefit with Cooley linear patch repair. An early postoperative device ECMO “Bioconsole” was implanted in 1 (6.66%) (Tables 1 and 2) (Figures 1-5).
| Pre-operative | Follow-up (30 day) | P value |
|---|---|---|
| NYHA class 2.8+0.7 | 1.3 + 0.5 | 0.002 |
| LVEF (%) 35 + 4.6 | 45.1 + 10.1 | 0.001 |
| LVEDV (mL) 242.4 + 45.3 | 140.9 + 32.2 | <0.001 |
| LVESV (mL) 164.8 + 46.7 | 140.9 + 32.2 | 0.008 |
| LVEDTD (mm) 164.8 + 46.7 | 57.3 + 6.5 | 0.008 |
| LVEDLD (mm) 99.5 + 13.4 | 57.3 + 6.5 | 0.008 |
NYHA - New York Heart Association; LVEF - Left Ventricular Ejection Fraction; LVEDV - Left Ventricular End-Diastolic Volume; LVESV - Left Ventricular End- Systolic Volume; LVEDTD - Left Ventricular End-dDastolic Transverse Diameter; LVEDLD - Left Ventricular End-Diastolic Longitudinal Diameter
Table 2: Pre-operative and follow-up data.
Results
No in-hospital mortality was occurred. After a mean follow-up of 30 days, 14 patients were still alive; one patient who weaned from ECMO died of multiorgan failure in 2 weeks after surgery. Patients who presented concomitant diseases as COPD and obesity ventilated in ICU longer than the usual ones. Fortunately, we do not encountered any postoperative bleeding or perioperative acute heart failure complications. We evaluated pressure- volume loops in 2 patients before and directly after cardiopulmonary bypass as previously described and found a decrease in the EDPVR slope.
Discussion
Post infarction aneurysm or akinetik LV apical zones make blood circulation in this area difficult. By Virchow’s triade, the lowering the flow speed could conjugate blood components and further formation of clot formation (9). Our operative protocol is consists of surgical re-exploration of LV cavity in case of presents of LV thrombus on TEE, which in our series, it presented in 12 (80%) patients, in those performed removal of LV thrombus. But, sometimes, severe hypertrophied muscles (2 in our series) positioned on LV apex might conceal the thrombus presence and the experienced TEE experts should resolve the masking problems, otherwise any masked doubts on Echo, would be checked by open ventriculotomy. LV thrombus floatation definitely needs to its removal by not manipulating the heart before aortic cross-clamping, which might bring poor ischemic stroke complications [9-12].
We divided our patients into 2 groups: in first 10 patients, in who severe dilated LV cavity presented are performed semicircular stitches those help to reduce LV volume and diameter. In 3 patients who has developed severe mitral regurgitation are performed valve repair by Carpentier ring, and in other 2 (13.3%), MV replacement has been performed. For patients, the severity of MV insufficiency estimated as functional <++2, any procedure on MV has been encountered. We completely agreed with Di Donato [3] and colleagues who have evaluated the effectiveness of SVR and un-repaired mild to moderate ischemic mitral regurgitation on left ventricular geometry, cardiac and functional status, and the author obtained satisfactory outcome. We also have left 1-2 degree functional tricuspid regurgitation without any surgical involvements. In group 2, who has developed LV thrombus but the LV aneurysm presents up to 30% of whole LV cavity, we repaired these patients with linear patch technique as described by Cooley. The technique is simple to perform, and we closed the ventriculotomy with Teflon felts or pericardial patch of both ventriculotomy sides. LV aneurysm consisting less than 30% of all LV cavity mostly left untouched.
Regarding the possible LV volume after correction has caused some doubts. In 2004, Menicanti L [2] and Di Donato published excellent work on LV volume reshaping procedure by using mannequin balloon corrected on 50 ml/m2 and leaving inside the LV chamber. These patients’ outcomes on more than 1000 patients show that SVR is safe and effective in improving pump function, clinical status and survival in patients with post infarction ischemic cardiomyopathy. We did not use a mannequin balloon, and while using semicircular stitches, maximum 2 fingers have to completely fill the LV cavity once the stitches are stringed. The technique has been evolved by Dr. Ferrazzie’s operative skills and helped us to evolve this procedure. It seems to be blindly described but we did not observe any low cardiac output syndrome after surgical correction. Also, we checked pressure volume loops (PVL) after correction and it showed significant decrease of PVL in these series.
Joyce et al. stated that as techniques for the management of patients with ischemic heart failure have evolved, controversy has arisen with respect to the roles of revascularization and ventricular restoration procedures. The STICH trial definitely ascertain if SVR adds any health benefits to CABG alone in patients with left ventricular dysfunction and dilated ventricle. Ribeiro et al. and Ratcliffe stated that there are subsets of patients with ischemic cardiomyopathy for whom the optimal treatment strategies are not clear. These investigators studied 137 consecutive patients with anterior myocardial infarction and they concluded that the short-term and mid-term outcomes of CABG alone in patients with a large left ventricle are inferior to CABG plus SVR. We also follow current surgical approaches as performing CABG and SVR combined, if patients present chronic ischemic cardiomyopathy. We bypassed sufficient revascularization in order to obtain satisfactory results and our bypass grafts ranged around 1-3 anastomosis which was performed after SVR procedure had been done. We might be limited only with CABG procedures ones the diameter of LV aneurysm not containing thrombus formation consists of less than 30% of whole LV cavity.
The ventricular remodeling operation (also known as the Batista procedure, partial left ventriculotomy, heart reduction surgery, and wedge resection of the heart) has been proposed as a surgical procedure to replace or postpone heart transplantation in patients with dilated non-ischemic cardiomyopathy. But this technique definitely did not get its supporters over time, and our idea is also not to involve any surgical procedure, if the myocardium is viable without scar zones even the LV presents its large volume. We think these patients have to gain more support by implanting Ventricular Assist Devices rather than removing partial viable myocardium, for further preparation to future heart transplantation.
Post infarction ventricular septal defects (VSD) are usually common. Yamomoto [10] published various techniques for repair of ventricular septal perforation complicating inferior myocardial infarction, but no standard method has been established. An effective technique for closing ventricular septal perforation using double patches via a right atrial approach is described. In their experience, no residual shunt was observed after repair using this procedure. In our series, 2 case have presented chronic VSD formation complicated after myocardial infarction, which are simply closed by running suture (4/0 prolene) without any residual shunt during in hospital period.
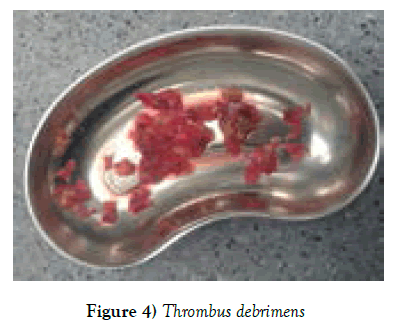
Yet, we have small group of patients, but with some complications. 1 patient has bled after surgery which are stopped in proper time. Ventilation related complications has been occured in most patients who present COPD in history, but no fatal complications. Even we had a single death in patient who weaned from ECMO and developed end organ failure later on, we consider the survival is optimal and greater than would be expected in patients with post-infarction dilated ventricles and depressed left ventricular function. We also have not faced embolic related stroke complications in whom the thrombus removal has been processed. Clean visual debridement of organic thrombus and flushing LV cavity carefully helped us to obtain such satisfactory outcomes.
Conclusion
We conclude that the patients with chronic ischemic cardiomyopathy require combined approach to treat. In order to get a perfect outcome, the combination of procedures as complete coronary bypass surgery, surgical ventricular restoration with left ventricular thrombus removal and mitral valve surgery have to be encountered. Even, we studied small cohort of patients, by the way, it gave us satisfactory and quite good in-hospital outcome. Of course, it needs to be studied large series of patients with long term results in what we will hope to get in the future.
REFERENCES
- Paolo F, Michele T, Attilio L, et al. Surgical ventricular restoration by means of a new technique to preserve left ventricular compliance: The horseshoe repair. J Thor Cardiol Surg 2001;136:5.
- Menicanti L, LV aneurysm reshaping technique.
- Di DM, Sabatier M, Dor V, et al. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J Thorac Cardiol Surg 2001;121:91-6.
- Tulner SAF, Steendijk P, Klautz RJM, et al. Surgical ventricular restoration in patients with ischemic dilated cardiomyopathy: evaluation of systolic and diastolic ventricular function, wall stress, dyssynchrony, and mechanical efficiency by pressure-volume loops. J Thorac Cardiovasc Surg 2006;132:610-20.
- Dor V. Left ventricular aneurysms: the endoventricular circular patch plasty. Semin Thorac Cardiovasc Surg. 1997;9:123-30.
- Ferrazzi P, Matteucci MLS, Merlo M, et al. Surgical ventricular reverse remodeling in severe ischemic dilated cardiomyopathy: the relevance of the left ventricular equator as a prognostic factor. J Thorac Cardiovasc Surg. 2006;131:357-63.
- Guilmet D, Popoff G, Dubois C, et al. A new surgical technique for the left ventricular aneurysm: The overcoat aneurysmoplasty. Preliminary results. 11 cases. Arch Mal Coeur Vaiss. 1984;77:953-8.
- Cirillo M, Amaducci A, Brunelli F, et al. Determinants of postinfarction remodeling affect outcome and left ventricular geometry after surgical treatment of ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2004;127:1648-56.
- Yamamoto N, Ohara K, Nie M, et al. Double patch closure using gelatin resorcin formal glue of a ventricular septal perforation following acute myocardial infarction. Jpn J Thorac Cardiovasc Surg 2002;50:294-7.
- Yamomoto N Repair of ventricular septal perforation after inferior myocardial infarction. Asian Cardiovasc Thorac Ann 2010;18:185-187.
- Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: Comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg 1998;116:50-9.
- Athanasuleas CL, Stanley AW Jr., Buckberg GD, et al. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. The RESTORE group. Reconstructive endoventricular surgery, returning torsion original radius elliptical shape to the LV. J Am Coll Cardiol 2001;37:1199-209.