Effects of chicken manure and chicken manure -derived biochar on the bioavailability and concentration of Lead (Pb) in two Brassica vegetables
Received: 12-Jan-2023, Manuscript No. PULJMBR-23-6044; Editor assigned: 16-Jan-2023, Pre QC No. PULJMBR-23-6044 (PQ); Accepted Date: Jan 25, 2023; Reviewed: 20-Jan-2023 QC No. PULJMBR-23-6044 (Q); Revised: 22-Jan-2023, Manuscript No. PULJMBR-23-6044 (R); Published: 27-Jan-2023, DOI: 10.37532/puljmbr.2023.6(1).14-18
Citation: Mulenga M, Mukumbuta I, Chishala B, et al. Effects of chicken manure and chicken manure -derived biochar on the bioavailability and concentration of Lead (Pb) in two Brassica vegetables. J Mic Bio Rep. 2023;6(1):14-18.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Lead (Pb) contamination has been widely reported in Kabwe town soils due to mining activities. The bioavailability and excessive amounts of Pb in soils are toxic to both plants and microorganisms. Manure and biochar have been reported to immobilize heavy metals in soil. This study, thus evaluated the effects of chicken manure and chicken manure-derived biochar on the bioavailability of Pb in the soil and its uptake by plants in polluted garden soils of Kabwe. Pots containing Pb contaminated soil with five treatments of manure and biochar in the following proportions: CT (un-amended polluted soil, control); 2% and 4% of CM (chicken manure); 2% of and 4% of CMB (Chicken Manure-Derived Biochar) were used in this study. The manure and biochar were applied as percentage of total mass of soil in the pot. Brassica napus (rape) was planted and grown in the soils under greenhouse conditions for nine weeks. After harvesting the rape, Brassica rapa (Chinese cabbage) was planted in the same pots and grown for eight weeks. The planting of Chinese cabbage was done to ascertain the residual effects of the amendments. Soil pH and bioavailable Pb was measured six times during the experiment and after the harvest, Pb concentration in plant tissues were determined using Atomic Absorption Spectroscopy (AAS-Z series 2010).
Concentrations of bioavailable Pb were higher in the amended soils of 2% and 4% CM (18.90 mg Pb/kg ± 1.5 mg Pb/kg; 21.18 mg Pb/kg ± 2.3 mg Pb/kg), 2% and 4% CMB (29.16 ± 2.4; 41.15 mg Pb/kg ± 2.6 mg Pb/ kg) compared to CT (18.36 mg Pb/kg ± 1.6 mg Pb/kg). Despite higher bioavailable Pb in the manure and biochar amended soils; the concentration of Pb in both rape and Chinese cabbage tissues was lower than that in unamended polluted soil (CT). Concentration of Pb in rape from 2% and 4% CM were less than detection limit, 2% and 4% CMB were 12.79 mg Pb/kg ± 2.49 mg Pb/kg and 8.4 mg Pb/kg ± 0.20 mg Pb/kg while concentration of Pb in rape from CT was 35.13 mg Pb/kg ± 13.72 mg Pb/kg. In Chinese cabbage, all amendments had Pb concentrations in plant tissues less than detection limit while the value in CT was 8.13 mg Pb/kg ± 0.97 mg Pb/kg. The experiment showed that, amendment application with increase in dose application results to reduced Pb uptake by the plants and concentration, despite increased Pb mobilization in soil. It is recommended that these amendments can be used for remediating Pb contaminated soils, as they seem to reduce Pb concentrations in plant tissues
Keywords
aminated Lead (Pb) Soil; Bioavailability; Remediation; Amendments
Introduction
Globally, many studies have been conducted on the health risks of Pb once consumed. Pb exposure to the environment can influence the neurologic system, kidneys, and blood circulation in humans, especially in children, infants, and fetuses [1]. Other studies have revealed that elevated Lead (Pb) in the environment can result in decreased growth and reproduction in plants and animals. In view of the aforesaid, growing of vegetables on contaminated soils with Pb has negative effects on human health. The sources of Lead (Pb) to ecosystems include the direct discharge of waste streams to water bodies and mining. This problem of Pb pollution to the environment has affected even most African countries and Zambia inclusively. Lead (Pb) pollution from the Mine in Kabwe town has resulted in the contamination of soils leading to most backyard gardeners with no option but to grow their vegetable crops on the same soil. Lead (Pb) polluted soils severely affect plant growth parameters by influencing physical-biochemical processes, oxidative defense systems, and nutritional quality. Several studies on Kabwe have revealed that the area has been adversely affected by previous mining activities. As a result, areas that are adjacent to the old Zinc and Lead Mine show high levels of Pb contamination in the soil samples [2]. Pb contamination poses health risks through food consumption [3].
Chicken manure is used as an organic fertilizer, especially for soils low in nitrogen [4]. Chicken manure is sometimes pelletized before being used as a fertilizer. Biochar is a low-cost porous pyrogenous substance produced by the pyrolysis of organic materials in oxygen-deficit conditions. It has been reported to have a high Cation Exchange Capacity (CEC), a large surface area, and higher porosity compared to other organic amendments [5]
Biochar and manure amendments can render heavy metals immobile and non-bioavailable by various Physico-chemical means, which disrupt their pathway of exposure and reduce their health risk [6,7]. The application of these amendments to soils, from a remedial point of view, is justified by their relatively low cost, compared to ‘hard’ engineering solutions, and by their prevalence as wastes, which require other forms of disposal such as burial in landfill, incineration among others. Therefore, the justification for the addition of biochar and manure to the soil is that they can save as sorbents for metals in the solution, where they establish new equilibrium levels between the concentrations of adsorbents in the solution and that in the solution. The other justification for the addition of biochar and manure is that they are readily available and provide nutrients and habitat to organisms living in the soil. Though other studies on the remediation options of Pb in the soil have been done such as liming, phytoextraction, and phytoaccumulation among others, these remedial options are expensive compared to biochar and manure use. Therefore, it was in this regard this research was conducted to investigate the effects of chicken manure and chicken manure-derived biochar to minimize the uptake of Pb by Brassica vegetables (rape and Chinese cabbage). Brassica vegetables are crops and vegetables for human consumption. They have a greater ability to accumulate heavy metals in their edible parts from contaminated soils and are mostly grown in the backyard gardens in Kabwe hence chosen for this study [8].
Goals and Objectives
To evaluate the effects of chicken manure and chicken manure–derived biochar on the bioavailability and concentration of Pb in two Brassica vegetables (B.napus and B. rapa).
To achieve the main objective, the study had the following specific objectives:
• To evaluate the effects of chicken manure and chicken manurederived biochar on changing soil pH.
• To evaluate the effect of chicken manure and chicken manurederived biochar on the bioavailable Pb in polluted soils.
• To evaluate the effect of chicken manure and chicken manurederived biochar on the concentration of Pb in rape and Chinese cabbage grown in polluted soils.
Materials and Methods
Study area
Kabwe town is located in the Central Province of Zambia about 136 km north of Lusaka, the capital of Zambia. The town is characterized by Acrisols although Podsols and Vertisols are also found. Vegetation is mainly characterized by Miombo woodlands [9]. Kasanda residential area was chosen for soil sample collection because it is near and adjacent to Kabwe Zinc and Lead Mine (Figure 1). Most studies also reveal that Kasanda residential area is highly contaminated with Pb from mining activities.
Soil collection and characterization
Soil samples were collected at 0 cm-20 cm depth using a Trowel at 5 points and thoroughly mixed using plastic buckets. At each point, soil samples from the 5 gardens were collected then soil samples were mixed to form one composite sample representative of the whole sampling area.
The collected soil sample was air-dried, and visible roots and other debris were removed. The larger soil aggregates were gently broken using fingers sieved through a 2.0 mm sieve and mixed using a Trowel in plastic buckets thoroughly to prepare a composite soil sample. The sieving of the composite soil sample was done to make a homogeneous soil sample. The sieved soil was characterized before setting up the pot experiment for soil pH, total Pb concentration, electrical conductivity, ammonia, nitrates, and phosphorous
Chicken manure
Chicken manure was collected from the University of Zambia, School of Agricultural Sciences Field Station poultry house. It was air-dried and sieved using a 2 mm sieve. The chicken manure collected was from broiler chickens and the bedding material was sawdust. The chicken manure was characterized for pH, total Pb concentration, electrical conductivity, organic carbon, cation exchange capacity, and some metalloids
Chicken manure-derived biochar
The chicken manure-derived biochar was made using the pit method under limited oxygen conditions (Figure 2), and then, the charred materials were ground using a mortar and pestle and sieved through a 2 mm sieve. The chicken manure–derived biochar was equally characterized for pH, total Pb concentration, electrical conductivity, organic carbon, cation exchange capacity, and some metalloids.
Experimental design and treatments
The Complete Randomized Design (CRD) was employed in this study. A pot experiment was conducted in a greenhouse at the University of Zambia. It was set up with 4 replications. The amendments application rates were calculated as a percentage of the mass of the soil in the pot. Chicken manure and Chicken manure-derived biochar were applied at rates of 2% and 4% of the soil mass.
For the pot experiment, 2500 g of soil was used and the manure and biochar amendments were set up with a control, meaning, CTno amendment was applied, 2% of 2500 g of the soil of which calculated 50 g of Chicken manure and Chicken manure-derived biochar was applied (CM2% and CMB2%), 4% of 2500 gram of soil of which calculated 100 g of chicken manure and chicken manure-derived biochar was applied (CM4% and CMB4%) as shown in Table 1 below. The treatments below were repeated with 200 grams soil for destructive sampling with similar application rates for analysis of soil pH and bioavailable Pb. All treatments were replicated 4 times
TABLE 1. Treatment set up showing application rates calculated by the percentage of the mass of the soil in the pots
| Treatment | Mass of soil (g) | Mass of amendment applied in grams |
|---|---|---|
| Control (CT) | 2500 | 0 |
| CM2% | 2500 | 50 |
| CM4% | 2500 | 100 |
| CMB2% | 2500 | 50 |
| CMB4% | 2500 | 100 |
CT is un-amended polluted soil; CM2% and CM4% are chicken manure treatments at 2% and 4% respectively; CMB2% and CMB4% are chicken manure-derived biochar treatments at 2% and 4% respectively.
Planting, irrigation, and crop management
Five seeds of each variety of vegetable, that is, rape (Brassica napus) and Chinese cabbage (Brassica rapa) were planted. After germination, two healthy seedlings per pot were maintained after thinning. Tap water was used to irrigate the treatments as needed.
The rape (Brassica napus) was sown on 12th March 2020 a day after soilamendments mixture preparations. It was then harvested on 13th May 2020 (harvested after 9 weeks). Thereafter, the pots were left for five weeks without any plants planted in them. This soil incubation was done to determine the residue effects of the amendments on bioavailable Pb in the soil. The incubation of soil samples is used to measure mineralization [10]. The second crop plant, Chinese cabbage (Brassica rapa), was planted on 21st June 2020 and was harvested on 11th August 2020 (8 weeks after). Chinese cabbage was chosen as a second crop because it is also a Brassica vegetable. It can accumulate Pb in its edible parts. It is an equally grown vegetable in Kabwe town. Management practices were the same for both the first and second crops.
Soil and amendment analysis
Soil analysis
During the experiment, soil samples were collected six times for analysis of bioavailable Pb and soil pH. Bioavailable Pb was extracted in DTPA–TEA (Diethylene Triamine Penta Acetic Acid, Triethanolamine) buffered at pH 7.3 [11]. Ten grams of soil was weighed and 20 ml of DTPA-TEA solution was added. The mixture was shaken for two hours using the mechanical shaker. The suspensions were filtered by gravity through the filter paper. Then filtrates were analyzed for Pb concentration using Flame Atomic absorption spectroscopy (FAAS-Z series 2010). To determine soil pH, 25 ml of CaCl2 was added to every ten grams of soil and shaken for thirty minutes using the mechanical shaker. pH was determined using a soil pH meter. The electrical conductivity of soil was determined by adding 25 ml of deionized water to ten grams of each sample. The mixture is shaken for one hour using a mechanical shaker. The filtrates were analyzed for electrical conductivity using an electrical conductivity meter.
Organic carbon was determined by Walkley and Black method. One gram of air dry soil was into a 250 cm3 conical flask to which ten centimeter cubic of 1N K2Cr2O7 (with a pipette) was added. Then rapidly 20 cm3 of concentrated H2 SO4 was added and directed into the stream suspension (an automatic pipette was used).
Cation exchange capacity was determined using the distillation method. 10 ml of leached solution (25 ml 1N NaCl solution in a 50 ml volumetric flask) was pipetted. Magnesium oxide (MgO) was added as a catalyst. Distillation was done for five minutes while collecting NH4+ in a 20 ml boric acid indicator. Thereafter, the indicator was titrated with 0.1 N HCl solution from green to purple, and then, meq CEC/100 grams soil was calculated.
Ammonium and nitrate were extracted using 2M KCl. Ten grams of soil sample was weighed into a conical flask and added to 100 ml 2 M KCl solution. Then the mixture had shaken for 30 minutes. Thereafter, filtered the solution and the clear filtrate taken for analysis within 24 hours.
The available phosphorous in the soil was determined using 0.5 M NaHCO3 (pH 5.8) as extracting solution. The soil texture of the soil sample was analyzed using the Hydrometer method.
Amendments analysis
The electrical conductivity for amendments (chicken manure and chicken manure-derived biochar) was determined by adding 25 ml of deionized water to ten grams of each sample and shaking for one hour. The filtrates were analyzed for electrical conductivity using an electrical conductivity meter. To determine the pH for amendments, 25 ml of deionized water was added to every ten grams of amendments and shaken for thirty minutes using the mechanical shaker. The pH reading was taken directly from the suspensions of chicken manure and chicken manure-derived biochar. At least 4 replicates were used for each analysis.
For the amendments, the organic carbon, cation exchange capacity, and available phosphorous were determined using the same methods outlined in soil analysis. While for the metallic elements in amendments, the samples were weighed 10 grams each and put into a 250 cm3 Erlenmeyer flask. Fifty centimeters cubic of NH4O ac, pH 7.0 was added. Shaken for 30 minutes on a shaker. The suspension was filtered using number forty-two filter paper, and then potassium, calcium, manganese, and iron among others were measured in the filtrate.
Lead (Pb) determination in soil and plants
To determine total Pb concentrations in soils, 0.05 grams of soil was weighed and placed in test tubes to which 5 ml 30% nitric acid (HNO3 ) and 1 ml hydrogen peroxide (H2 O2 ) was added. The mixture was digested for 45 minutes using the Speedway digester (Berghof, Germany). Thereafter filled up to 15 ml with deionized water in a 15 ml transparent bottle.
To determine Pb concentrations in the plants, the plants were oven dried for 72 hours at 70oC then ground with a pestle and mortar. Pb concentrations in plant samples were determined by weighing 0.05g ground plant sample to which 5 ml 30% nitric acid (HNO3 ) and 1 ml hydrogen peroxide (H2O2 ) was added. The samples were then digested using the Speedway digester (Berghof, Germany) digester for 45 minutes. Thereafter filled up to 10 ml with deionized water in a 15 ml transparent bottle.
Post-harvest soil was collected and bioavailable Pb was determined in 20 ml of DTPA–TEA as described in soil analysis. In both plant and soil, Pb concentrations were analyzed using Flame Atomic absorption spectroscopy (FAAS-Z series 2010).
Statistical analysis
All experiments were conducted with four replicates. The data were statistically analyzed using the one-way Analysis Of Variance (ANOVA) test using Graphpad Prism software 9.0.0. Tukey’s multiple comparison tests were conducted at a significance level of p<0.05 to separate the statistically significant treatment means. Pearson correlation was used to determine the relationship between soil pH and bioavailable Pb in soil. Other calculations and graphs were prepared using Microsoft Excel 2010.
Results and Discussion
Soil and amendment properties
The texture of the soil used in the experiment was sand clay loam and the soil was alkaline. The pH values of chicken manure and chicken manurederived biochar were 7.37 ±0.07 and 10.26±0.01, respectively, which showed an increase in pH by approximately 42.9% when chicken manure was pyrolyzed. These results are in agreement with the results of Chan and Cely who reported an increase in pH after manure pyrolysis [5,12]. Total lead Pb in the soil was 1849 mg Pb/kg ± 246 mg Pb/kg which is above the WHO recommended threshold of 100 mg/kg and the FAO threshold of 150 mg/kg respectively [13,14]. Generally, the soil was contaminated with Pb as evidenced by the total Pb of 1849 mg Pb/kg ± 246 mg Pb/kg detected compared to recommended soil concentration of 100 mg Pb/kg and 150 mg Pb/kg by WHO and FAO, respectively.
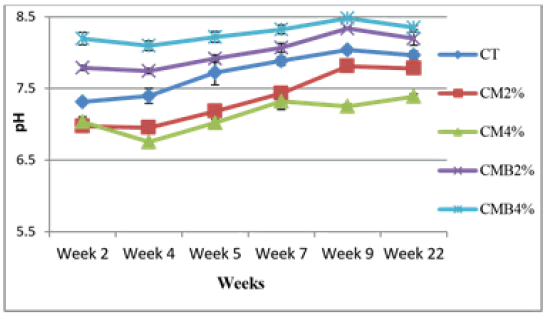
Effects of chicken manure and chicken manure-derived biochar on soil pH
The chicken manure reduced the soil pH compared to the control. The soil pH was observed to be lower in CM4% than in CM2% (Figure 3). This could be attributed to fewer base-forming ions released in the soil in the pot amended with CM4 [15]. In most cases, the application of chicken manure to the soil results in a soil pH increase because of the presence of basic cations in the chicken manure released upon microbial decarboxylation [16]. In this study, the pH for the soil-chicken manure mixture was found to be slightly neutral to alkaline which is in agreement with the results by Dikinya and Mufwanzala [4].
However, the addition of chicken manure-derived biochar application rates increased the pH of the soil (Figure 3). The increase in soil pH in pots amended with chicken manure-derived biochar was attributed to free basic cations present in biochar such as Calcium (Ca), Magnesium (Mg), and Potassium (K) which are readily released into the soil solution resulting in a net increase of soil Ph. The substantial pH increase or response with an increasing application rate of chicken manure derived biochar was also observed in this study which is in agreement with other studies when biochar is added to soil [17,18]. The pH for the soilamendments mixture was found to be alkaline.
The average pH values were 7.35 ± 0.36, 7.13 ± 0.23, 8.01 ± 0.23, 8.27 ± 0.14 and 7.72 ± 0.29 for CM2%, CM4%, CMB2%, CMB4% and CT treatments respectively. There was a significant difference among mean soil pH values (P<0.05)
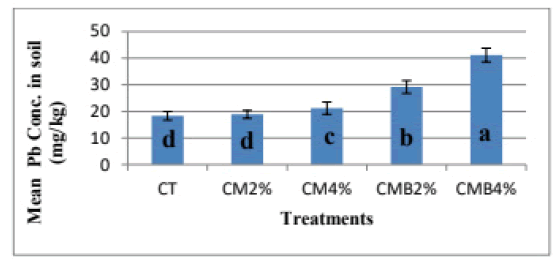
Effects of chicken manure and chicken manure-derived biochar on bioavailable Pb in the soil
The mean bioavailable Pb concentration was highest in CMB4% with a concentration of 41.15 mg Pb/kg ± 2.6 mg Pb/kg and followed by CMB2% with 29.16 mg Pb/kg ± 2.4 mg Pb/kg. The Pb concentration in CM4% was 21.18 mg Pb/kg ± 2.3 mg Pb/kg, 18.90 mg Pb/kg ± 1.5 mg Pb/kg in CM2% treatment compared to the un-amended control (CT) with 18.36 mg Pb/ kg ± 1.6 mg Pb/kg (Figure 4). Krol observed that Pb concentration in the soil was higher in alkaline conditions [19]. This agrees with the results of the chicken-manure-derived biochar treatments in this study. The increase in bioavailable Pb in amended soils could attribute to cation competition on adsorption sites of chicken manure and chicken manure-derived biochar which resulted in Pb ions being released into the soil solution [20]. Bloomfield further stated that microbial action due to the decomposition process results in the mobilization of Pb from carbonate and oxide forms by aerobic decomposition as may be the case in this study. This led to more bioavailable Pb in the soil of the amended pots. Many studies have revealed that low pH (acidic conditions) results in high mobility and subsequently high concentrations of Pb in the soil while high pH (alkaline conditions) results in low mobility and low concentrations of Pb in the soil [21,22].
However, the application of amendments to the soil influences the formation of soluble complexes and can result in solubilization and mobilization of Pb or soluble organo-metal complexes which accounts for the majority of metal content in the solution. Rieuwerts explain that the bioavailability of metals in soils depends to a large extent on their distribution between the solid and solution phases which results in the mobilization of metals [23-25].
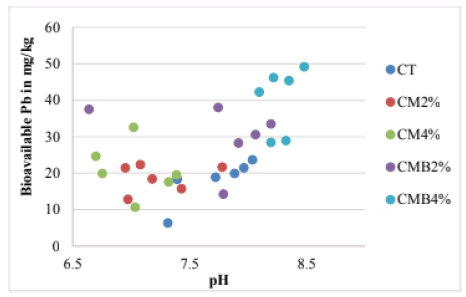
Soil pH influences the bioavailability of heavy metals in the soil, therefore, the current study evaluated the effect of pH change after the application of chicken manure and chicken manure-derived biochar. Figure 5 shows that bioavailable Pb in the soil decreased with increasing pH between soil pH 6.5 and 7.5 but after pH 7.5 the bioavailable Pb increased as the pH increased, representing a “V-shape” relationship between soil pH and bioavailable Pb concentrations. Similar results were observed [19, 26].
The behavior of Pb in alkaline conditions in this study is related to the amphoteric character of heavy metals i.e. representing a “V-shape” and such behavior is the result of the formation of hydroxyl complexes explains that Pb mobilization can occur in either acidic or alkaline conditions but at pH 7 (neutral) very little Pb is likely to be found in solution due to precipitation and sorption as observed in this study [19, 27].
Effects of chicken manure and chicken manure-derived biochar on the concentration of Pb in rape and Chinese cabbage tissues
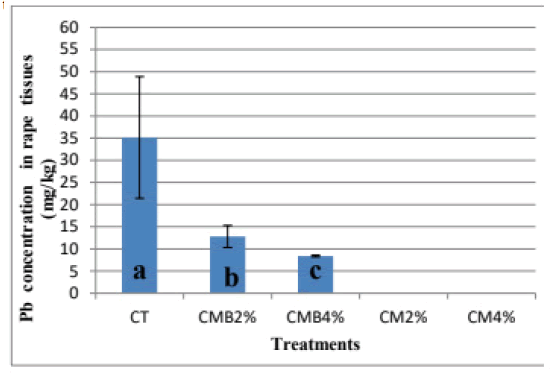
The mean Pb concentration in rape tissues was 35.13 ± 13.72 in the CT, CMB2%-12.79 ± 2.49, and CMB4%-8.4 ± 0.20 mg/kg, respectively (Figure 6). The Pb concentration in rape tissues was below detection in the manureamended treatments (CM2% and CM4%). It was also observed that an increase in dose application of chicken manure-derived biochar from 2% to 4% resulted in the reduction of Pb concentration in rape tissues from 12.79 ± 2.49 to 8.4 mg/kg ± 0.20 mg/kg. According to Rinklebe the reduction in Pb concentration in plant tissues in pots amended with CMB2% and CMB4% could be related to soil activities in the soil in which increased complexing agents (amendments) resulted in the mobilization of adsorbed metals leading to more complexation, cation exchange competition and Pb ion speciation in soil solution. These activities in the soil resulted in reduced plant uptake by disrupting the pathway [24]. It was observed that mobility and plant uptake of metals depend not only on their activity in the solution but also on the relation existing between solution ions and the solid phase, which may be the case in this study [28].
The reduction in plant-available Pb uptake in manure-amended pots compared to control was a result of complexation, presence, and concentration of dissolved chicken manure, cation exchange competition, Pb ion speciation, and distribution too in the soil. A large number of studies found that biochar and manure can decrease the mobility, leachability, and availability of the metals in contaminated soils, and reduce plant uptake [29,30].
Generally, a reduction in plant uptake was observed with increased dose application which is an indicator that with increased dose application of chicken manure-derived biochar amendments to the soil, Pb concentration in the plants can be reduced equally.
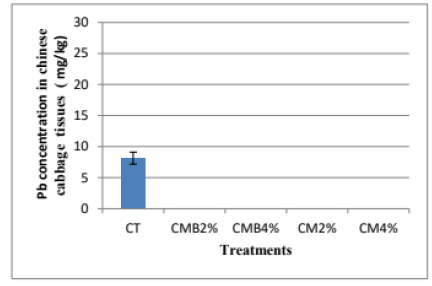
In Chinese cabbage tissues, the concentration of Pb was below the detection limit in pots with amended soils while in the CT it was 8.13 mg Pb/kg ± 0.97 mg Pb/kg (Figure 7). There was a reduction in Pb concentration in Chinese cabbage tissues in the CT (8.13 mg Pb/kg ± 0.97 mg Pb/kg) compared to rape tissues (35.13 mg Pb/kg ± 13.72 mg Pb/kg). This could be due to most of the bioavailable Pb being taken up by rape as observed in the results presented and shown in (Figures 6 and 7). The other factor could be due to the leaching of Pb in the pots. The maximum permissible concentration of Pb in vegetables is 0.3 mg/kg (WHO/FAO), therefore the concentration of Pb in the biochar amended treatments was higher than the permissible not suitable for human consumption.
The low Pb concentration in Chinese cabbage tissues in pots amended with manure and manure-derived biochar was the result of increased complexation and Pb speciation or distribution processes in time (Figure 7). This led to a reduction in the uptake and concentration of Pb in Chinese cabbage tissues. Further, the results observed in Chinese cabbage validate or ascertain that both manure and biochar have a significant role in reducing Pb concentration in plants and this could depend on the number of amendments applied to the soil.
Generally, amendments increased bioavailable Pb in the soil but reduced Pb concentration in the plant tissues [28]. According to Krol and Joris under alkaline conditions, Pb reacts with organic matter to form hydroxyl complexes which disrupt the movement of Pb from soil to the plant. This could be the reason for the observed contradicting results between the bioavailable Pb in the soil and the concentration of Pb in the plant tissues in this current study. The results in this study are contradictory to other studies that show the application of chicken manure and bio char immobilizing Pb in the soil [29,30].
Recommendations
Chicken manure and chicken manure-derived biochar application might be recommended as a cheap management method for remediating contaminated Pb soils because they reduced plant uptake and concentration of Pb in plant tissues. Though the WHO/FAO permissible vegetable Pb concentration is lower than what was investigated in this study, still it has given the indicator of the effects of amendments when applied to Pb-polluted soils.
This research also observed the increase in Pb concentrations in the soil when amendments were applied. More research must be conducted on the scientific cause of this because this research was limited to evaluating the effects of amendments to minimize the uptake and concentration of Pb in plant tissues and avoid associated health risks.
Given the aforesaid, the findings of this research will help gardeners or farmers in surrounding areas of Zinc and Lead Mine in Kabwe to use these amendments and grow their crops on these polluted soils and reduce the associated health risks.
Conclusion
Chicken manure and chicken manure-derived biochar application to Pbcontaminated soils led to increased bioavailable Pb in the soil but reduced the plant Pb concentration in plants. The experiment showed that amendment application with an increase in dose application results in reduced Pb concentration in plants, despite increased Pb mobilization in soil.
References
- Guo G, Lei M, Wang Y, et al. Accumulation of As, Cd, and Pb in sixteen wheat cultivars grown in contaminated soils and associated health risk assessment. Int j environ res public health. 2018;15(11):2601.
- Kribek B, Nyambe I, Majer V, et al. Soil contamination near the Kabwe Pb-Zn smelter in Zambia: Environmental impacts and remediation measures proposal. J Geochem Explor. 2019; 197:159-73.
- Pocknee S, Sumner ME. Cation and nitrogen contents of organic matter determine its soil liming potential. Soil Sci Soc Am J. 1997;61(1):86-92.
- Oagile D, Namasiku M. Chicken manure-enhanced soil fertility and productivity: Effects of application rates. J soil sci environ manag. 2010;1(3):46-54.
- Cely P, Gasco G, Paz-Ferreiro J, et al. Agronomic properties of biochars from different manure wastes. J Anal Appl Pyrolysis. 2015; 111:173-82.
- Bolan NS, Duraisamy VP. Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: a review involving specific case studies. Soil Research. 2003;41(3):533-55.
- Bernal MP, Clemente R, Walker DJ. The role of organic amendments in the bioremediation of heavy metal-polluted soils. Environ res lead edge. 2007:1-57.
- Mourato MP, Moreira IN, Leitao I, et al. Effect of heavy metals in plants of the genus Brassica. Int j mol sci. 2015;16(8):17975-98.
- Kribek B, De Vivo B, Davies T. Impacts of mining and mineral processing on the environment and human health in Africa. J Geochem Explor. 2014:387-90.
- Birge HE. What happens during soil incubations? Exploring microbial biomass, carbon availability and temperature constraints on soil respiration. Doctoral dissertation, Colorado State University. 2013.
- Lindsay WL, Norvell W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil science society of America journal. 1978;42(3):421-8.
- Chan KY, Van Zwieten L, Meszaros I, et al. Using poultry litter biochars as soil amendments. Soil Research. 2008;46(5):437-44.
- Mamtaz R, Chowdhury MH. Leaching characteristics of solid waste at an urban solid waste dumping site. Journal of Civil Engineering (IEB). 2006;34(2):71-9.
- Diouf J, Lowrey MP. Food and agriculture organization (FAO).
- McCauley A, Jones C, Jacobsen J. Soil pH and organic matter. Nutrient management module. 2009;8(2):1-2.
- Natscher L, Schwertmann U. Proton buffering in organic horizons of acid forest soils. Geoderma. 1991;48(1-2):93-106.
- Tang J, Zhu W, Kookana R, et al. Characteristics of biochar and its application in remediation of contaminated soil. Journal of bioscience and bioengineering. 2013;116(6):653-9.
- Thies JE, Rillig MC. Characteristics of biochar: biological properties. InBiochar for environmental management .2012: 117-138.
- Krol A, Mizerna K, Bozym M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. Journal of hazardous materials. 2020; 384:121502.
- Malarkodi M, Krishnasamy R, Chitdeshwari T. Phytoextraction of nickel contaminated soil using castor phytoextractor. Journal of plant nutrition. 2008 ;31(2):219-29.
- Tills AR, Alloway BJ. An appraisal of currently used soil tests for available copper with reference to deficiencies in English soils. Journal of the Science of Food and Agriculture. 1983;34(11):1190-6.
- Chuan MC, Shu GY, Liu JC. Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water, Air, and Soil Pollution. 1996;90(3):543-56.
- Rieuwerts JS, Thornton I, Farago ME, et al. Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals. Chemical Speciation & Bioavailability. 1998;10(2):61-75.
- Rinklebe J, Shaheen SM, Frohne T. Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere. 2016; 142:41-7.
- Dermatas D, Shen G, Chrysochoou M, et al. Pb speciation versus TCLP release in army firing range soils. Journal of Hazardous Materials. 2006;136(1):34-46.
- Dijkstra JJ, Meeussen JC, Comans RN. Leaching of heavy metals from contaminated soils: an experimental and modeling study. Environmental science & technology. 2004;38(16):4390-5.
- Cui Y, Chen J, Zhang Y, et al. pH-dependent leaching characteristics of major and toxic elements from red mud. Int j environ res public health. 2019;16(11):2046.
- Violante A, Cozzolino V, Perelomov L, et al. Mobility and bioavailability of heavy metals and metalloids in soil environments. Journal of soil science and plant nutrition. 2010;10(3):268-92.
- Beckers F, Awad YM, Beiyuan J, et al. Impact of biochar on mobilization, methylation, and ethylation of mercury under dynamic redox conditions in a contaminated floodplain soil. Environment international. 2019; 127:276-90.
- Mwilola PN, Mukumbuta I, Shitumbanuma V, et al. Lead, zinc and cadmium accumulation, and associated health risks, in maize grown near the Kabwe Mine in Zambia in response to organic and inorganic soil amendments. Int j environ res public health. 2020;17(23):9038.