Histological study on epididymis of African striped ground squirrel (Xerus erythropus)
Received: 17-Jun-2018 Accepted Date: Oct 10, 2018; Published: 20-Oct-2018
Citation: Rachel Mngu-suur K, Hambolu Joseph O, Suleiman Mohammed H, et al. Histological study on epididymis of african striped ground squirrel (Xerus erythropus). J Histol Histopathol Res 2018;2(2): 32-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The epididymis has been regarded as a highly specialized ductal system that forms an integral part of the male reproductive system. In this study, we obtained and studied the epididymis of ten African striped ground squirrels (Xerus erythropus). The animals were obtained from the wild around Zaria and environs between January and May, 2017, and conveyed in constructed cages to the research laboratory of the Department of Veterinary Anatomy, Ahmadu Bello University, Zaria, where they were acclimatized for a period of three weeks, during the period of acclimatization, they were fed with sweet potatoes, cabbage, groundnuts, water-melon and water ad libitum. The animals were euthanized with 30 mg of thiopental sodium given intramuscularly on the quadriceps femoris muscle. An incision was made on the abdominal region extending to the pubic area to locate the testis, on which the epididymis is attached. The epididymis was observed to be highly convoluted at the cranial aspect of each testis as the catput epididymis, the medial aspects of the testis as the corpus epididymis and caudal aspects of the testes as the caudal epididymis. In the African striped ground squirrel, the caudal epididymis had two aspects as the fixed and free portions. Histologically, apart from Haematoxilin and eosin stain the Masson-Trichome and PAS were used to study collagen and glycogen reaction respectively. The epithelium lining the epididymal ducts stained positive for both Masson-Trichome and PAS. In conclusion, this study established that there is histo-morphological variation between the catput, corpus and cauda epididymis. The ducts of the cauda epididymis seemed to be larger than those of both the catput and corpus epididymis. The epithelium was pseudostratified, and changed to tall columnar and low columnar with cilia in the catput, corpus and cauda epididymis respectively.
Keywords
Histology; Epididymis; Squirrel
he mammalian epididymis is an exceptionally long, convoluted ductal system that serves to connect the ductuli efferentes, which drain the testes, to the ductus deferens. The anatomic divisions of the epididymis include the initial segment, the head also referred as the caput, the middle segment, the corpus and the distal segment, the caudal epididymis. Each region consists of a lumen and a polarized epithelium composed mostly of principal and basal cells [1]. Although these three anatomical regions of the epididymis are easily identified in most adult male mammals [2], histological and ultrastructural segmentation of this organ varies among the different species of mammals [2]. The epididymal duct is currently recognized as a channel that transports, concentrates and preserves the spermatozoa. It is also known that the spermatozoa leaving the testis are immovable, immature and unable to fertilize an oocyte, and that under androgen control, the epididymal epithelium secretes proteins within the intra-luminal compartment that create a very complex environment which surroundes the spermatozoa [3]. This luminal compartment of the epididymis stores the spermatozoa until ejaculation. Not only does the epididymis preserve the spermatozoa, it also prepares the spermatozoa for fertilization by providing the essential requirements in terms of temperature, oxygen tension, pH and an available energy substrate [1]. The catput epididymis is thought to be most active in terms of protein synthesis and secretory activity [4]. The catput epididymis is also the region where spermatozoa first begin to swim in a progressive manner, and continue to develop in the corpus epididymis before becoming fully developed in the caudal epididymis.
Materials and Methods
Experimental animals
Ten African striped ground squirrels (Xerus erythropus) were obtained from the wild with the aid of traps around Zaria and environs through the months of January to May and transported in constructed cages to the Department of Veterinary Anatomy, Ahmadu Bello University, Zaria where the research was conducted. After three weeks of acclimatization, the animals were euthanized with 30 mg/kg of thiopental sodium given intra-muscularly on the quadriceps femoris muscle. An incision was made into the pubic region extending towards to abdomen to locate the testes on which the epididymides attach. The epididymides were identified as catput, corpus and caudal portions and were removed, labeled and fixed for 48 hours in 10% neutral buffered formalin (5). Dehydration was done through a series of graded alcohol (70%, 80%, 90%, 95% and 100%). Then cleared with xylene and infiltrated with molten paraffin wax, and sections of 4 μ thick were made from the embedded tissues using a microtome.
Staining of sections
Haematoxilin and Eosin (H&E): The sections of the tissues were mounted on grease free glass slides and the Haematoxylin and Eosin (H&E) staining for routine histological studies was carried out according to the protocol of Romeis Mikroskopishe Technik [5,6]. The sections were immersed in Haematoxilin solution for 7 minutes, which was done immediately after removal from distilled water. This was washed in running tap water for 20 minutes and then passed into 50% ethanol for 2 minutes. The sections were then counterstained with eosin for 3 minutes and immersed in 2 changes of 96% ethanol, isopropanol and xylol in that order for 2 minutes each. Tissues were mounted using oil mountant and cover slips. The slides were then examined using a light microscope at varying magnifications. Photomicrographs of the tissues were obtained by using an Amscope MT series microscope camera connected to a computer.
Masson and trichome: Masson trichome stain was employed to study the distribution of collagen fibres [7]. The sections were taken to water and covered with Weigert’s Haematoxilin for 20 minutes at RT. Then covered with cytoplasmic stain for 2 minutes and covered with dodecamolybdophosphoric acid hydrate for 5 minutes. The sections were then covered with the light green stain for 2 minutes, and washed again in water. Sections were dehydrated, cleared and mounted in Distyrene plasticizer xylene (DPX), which is a permanent mountant that protects the stained sections from fading and dust, and also functions to increase the refractive index of the slides to make the sections appear clearer. The slides were then examined using a light microscope at varying magnifications. Photomicrographs of the tissues were obtained by using an Amscope MT series microscope camera connected to a computer.
Periodic acid Schiff: Periodic acid schiff (PAS) was the special stain used for studying glycogen distribution within the reproductive organs. The cut sections of 4 μm were placed in water and then covered with periodic acid for 5 minutes. The sections were then covered with Schiff reagent for 15 minutes at RT º C and covered with Haematoxilin for 2 minutes. Sections were dehydrated, cleared and mounted in DPX mountant. The slides were later examined using a light microscope at varying magnifications. Photomicrographs of the tissues were obtained by using an Amscope MT series microscope camera connected to a computer.
Results
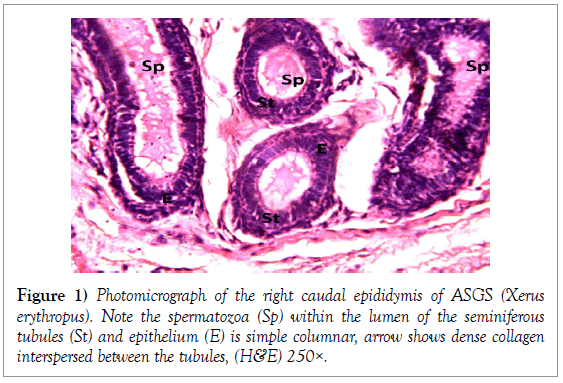
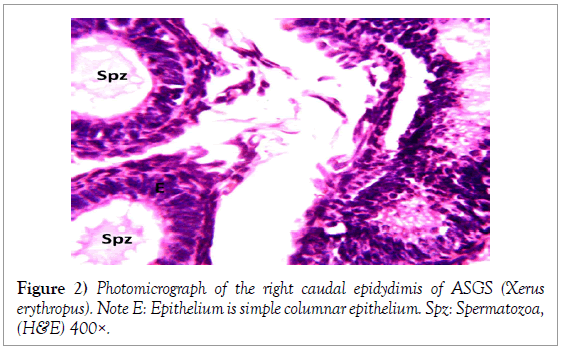
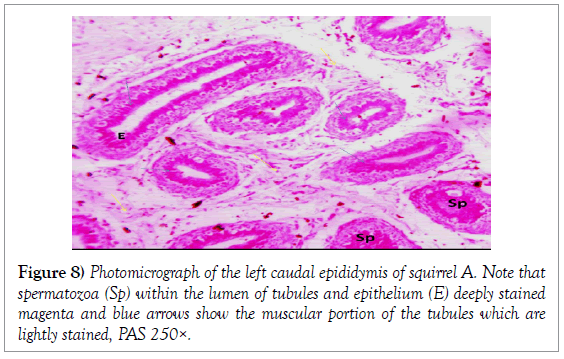
The epididymis was composed of tightly packed ducts with the head of the epididymis consisting of the most packed ducts as compared to the tail of the epididymis. The epithelium of the epididymal ducts was pseudostratified columnar epithelium in the cranial epididymis and simple columnar epithelium with stereo cilia in the caudal epithelium. The packed ducts were interwoven by bundles of collagenous and reticular fibres which are prominent and formed the meshwork of the epididymis. The right caudal epididymis consistently contained larger volumes of spermatozoa within the lumen of the tubules, and to a lesser extent the corpus epididymis and less prominent within the lumen of the catput epididymis (Figures 1 and 2).
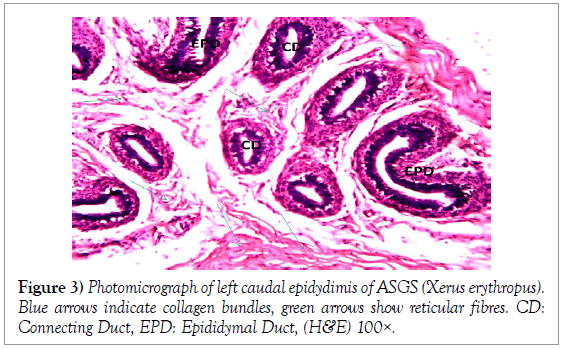
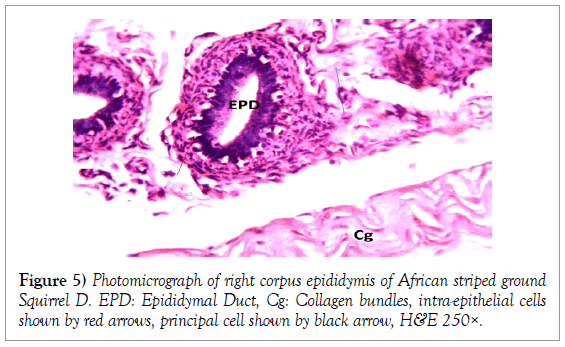
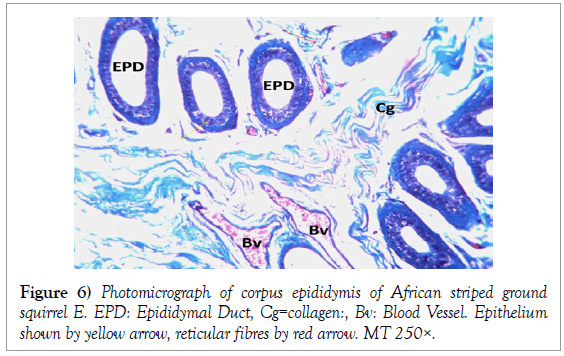
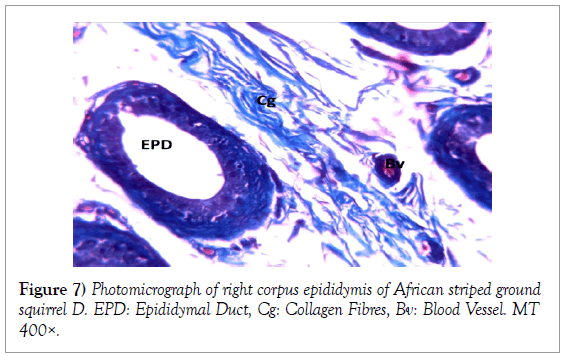
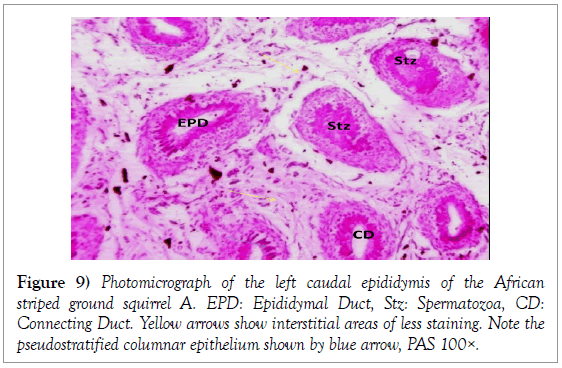
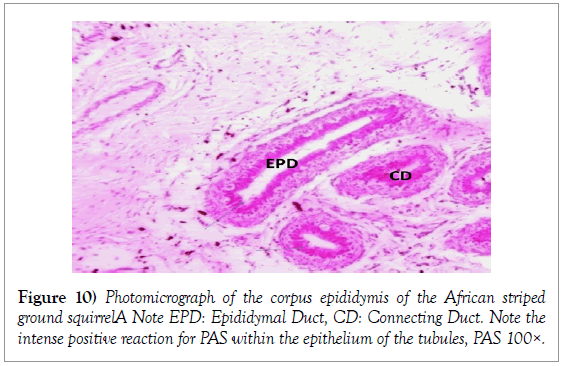
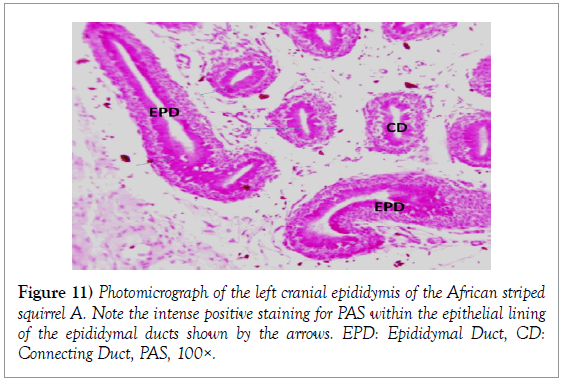
Histology of the epididymis revealed principal cells at the basement membrane (Figures 1 and 2) and some intra-epithelial glands occurring within the simple columnar epithelium of the epididymal ducts which were termed intra-epithelial glands (Figures 3 and 4). The epididymal ducts formed lobes, and each lobe was surrounded by heavy bundles of collagenous fibres (Figures 3-5). The epididymal ducts stained positive for Masson- Trichome stain which was most evident in the ducts and the inter ductular meshwork and the surrounding lobes of epididymal ducts (Figures 6 and 7). The epithelium of the epididymal ducts stained positive for periodic acid shiff (PAS). Spermatozoa within the lumen of the ducts were also positive for PAS (Figures 8-11).
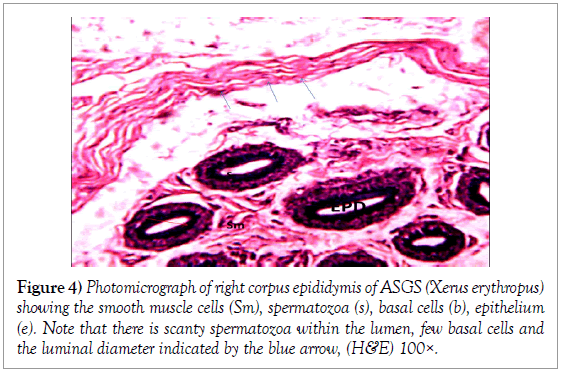
Figure 4: Photomicrograph of right corpus epididymis of ASGS (Xerus erythropus) showing the smooth muscle cells (Sm), spermatozoa (s), basal cells (b), epithelium (e). Note that there is scanty spermatozoa within the lumen, few basal cells and the luminal diameter indicated by the blue arrow, (H&E) 100×.
Figure 5: Photomicrograph of right corpus epididymis of African striped ground Squirrel D. EPD: Epididymal Duct, Cg: Collagen bundles, intra-epithelial cells shown by red arrows, principal cell shown by black arrow, H&E 250×.
Discussion
Histologically, the proximal segment of the epididymis of African striped ground squirrels showed the most convolutions as compared to the caudal epididymis. The luminal diameter of the epididymis of African striped ground squirrels seemed to be smaller in the catput and larger in the caudal epididymis. This finding corresponds with the report of Boukenaouli-Ferrouk in the morphometric study of the epididymis of sheep. In their study, they reported that the catput was notably more convoluted and elongated, and also narrower in diameter. The reason for this could be attributed to the natural phenomenon where the epididymis has been anatomically modified to meet the varying needs of the spermatozoa at the various segments of the epididymis. Spermatozoa are known to spend a longer time in the catput epididymis and a shorter period of time in the caudal epididymis as compared to the catput (Boukenaouli-Ferrouk et al., 2017). Noviana et al. [8] reported in Kacang goats and local sheep that the diameter in the corpus was smaller than the caput and cauda regions, due to the narrowed and elongated anatomical structure of the corpus epididymis. But tubular diameter increased significantly with the age in the cauda, because spermatozoa were stocked in this epididymis area. For the domesticated adult African great cane rat (Thryonomys swinderianus) [9], and in age-related study in the rat [10], the epididymal luminal diameters increased progressively from caput to cauda. The morphometric variations within the catput, corpus and caudal epididymis could be due to the fact that the cauda epididymis acts as a sperm reservoir, while both the caput and corpus are responsible for sperm maturation [3]. From the present study, we observed that the height of the epithelium of each region decreased from catput to caudal epididymis. The epithelial height of caput was more developed than in corpus and cauda epididymis. In some previous studies involving one-humped camel (Camelus dromedaries), and rat, the highest epithelium was seen in the caput and decreased gradually toward the caudal epididymis [10,11]. This is thought to be due to the fact that the proximal epididymal duct may play a mechanical role in facilitating the passage of spermatozoa toward the terminal segment, where they remain stored until ejaculated [11]. Histologically, spermatozoa were seen consistently in the right caudal epididymis. This finding further confirms that the African striped ground squirrel used for the present study had attained sexual maturity. Senger also stated that the generally accepted sign for onset of puberty in male mammals involves the presence of spermatozoa within the lumen of the seminiferous tubules [12]. This finding corresponds to that of Montero et al. who reported the presence of viable spermatozoa in the caudal epididymis of non-breeding mole rats (Fucomys anselli) [13]. The periodic acid shiff stain showed areas of positive for glycogen which showed a more intense staining around the epithelium of the epididymal tubules in the epididymis and the spermatogonia stained more intense within the testis, also the spermatozoa within the epididymal ducts had an intense positive reaction for glycogen. It is striking to note that the intensity of positive staining for PAS signifying presence of glycogen was not uniform within the testes, epididymis and ductus deferens. This could be attributed to the degree of maturity of seminiferous epithelium, since glycogen is thought to play an important role in the maturation of germ cells.
Conclusion
In conclusion, the present study established that the epididymis of African striped ground squirrels has three histologically distinct portions as catput, corpus and cauda epididymis. The epithelium of the catput epididymis is pseudostratified columnar, as it progresses to the corpus, it changes to tall columnar and in the cauda epididymis, the epithelium becomes simple low columnar epithelium. The ducts of the cauda epididymis appear significantly larger than those of both corpus and catput epididymis. Spermatozoa is consistently found in the caudal epididymis of the matured male African striped ground squirrel (Xerus erythropus). The epididymal ducts formed lobes that were surrounded by heavy bundles of collagen fibres, and interwoven by reticular fibres. The epithelium of the epididymal ducts were positive for PAS and the spermatozoa within the ducts, and this suggests that much energy is available in form of glycogen in the epithelium for spermatogenesis. Also, the positive reaction of spermatozoa to PAS sugeests that spermatozoa are filled with sufficient amount of energy in form of glycogen. The epididymal ducts were interwoven by reticular fibres and surrounded by bundles of collagen fibres and this suggests that the epididymis is modified for contractile activities, during movement of spermatozoa.
REFERENCES
- Dacheux JL, Castella S, Gatti LJ, et al. Epididymal cell secretory activities and the role of the proteins in boar sperm epididymis. Theriogenology. 2005;63(2):319-41.
- Smithwick EB, Young LG. Histological effects of androgen deprivation on the adult chimpanzee epididymis. Tissue and Cell. 2001;33(5):450-61.
- Sullivan R. Male fertility markers, myth or reality. Animal Reproduction Science. 2004;82-83:341-347.
- Aitken RJ, Nixon B, Lin M, et al. Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian J Androl. 2007;9:554–64.
- Onuoha EO. A Practical Guide for the Preparation of Histological, Histopathological and Cytological Slides. Fepam ventures, Nigeria. 2010.
- Mulisch M, Welsch U, Aescht E, et al. Romeis Mikroskopische Technik. (19thedn), Heidelberg Spektrum Publishers. 2010;330-40.
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques, (3rdedn), Churchill Livingstone, London, Torento. 1992.
- Noviana C, Boediono A, Wresdiyati T. Morphology and histo-morphometry of testis and epididymis of Kacang goat (Capra sp) and local sheep (Ovis sp.). Media Veteriner. 2000;7(2):12-6.
- Olukole SG, Obayemi TE. Histomorphology of the testes and epididymis in the domesticated adult African great cane rat. International Journal of Morphology. 2010;28(4):1251-4.
- Markey CM, Meyer GT. A quantitative description of the epididymis and its microvasculature: An age-related study in the rat. Journal of Anatomy. 1992;180(2):255-62.
- Zayed AE, Aly K, Ibrahim IA, et al. Morphological studies on the epididymal duct of the one-humped camel (Camelus dromedaries).Open Journal of Veterinary Medicine. 2012;2:245-54.
- Senger PL. Pathways to Pregnancy and Parturition. 2003.
- Montero AG, Vole C, Burda H, et al. Non-breeding Eusocial Mole-rats produce viable sperm-spermiogram and functional testicular morphology of Fukomys anselli. PLoS One. 2016;11(3):eo150112.