Methimazole induced cholestatic jaundice in a 30-year-old hyperthyroid female patient
2 Faculty of Medicine, Department of Tropical Medicine, Alexandria, Egypt, Email: marwaibrahim90@yahoo.com
Received: 11-Dec-2017 Accepted Date: Jan 19, 2018; Published: 23-Jan-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Hyperthyroidism is one of the most common endocrinal disorders especially in females. Here, we described a 30-year-old female complaining of yellow discoloration of sclera, dark urine and pale stool. She had a history of controlled thyrotoxicosis under Methimazole one month before. tapering the Prednisolone dosage. She started treatment with Methimazole 20 mg daily, within one month she presented with acute cholestatic liver insult not otherwise attributed. Symptoms and laboratory findings were decreased on withdrawal, but not normalized. Her condition was controlled by beta blockers and subsequently Prednisolone.
Keywords
Hyperthyroidism; Methimazole; Cholestatic liver injury; Prednisolone
Hyperthyroidism is one of the most common endocrinal disorders especially in females. Treatment is either pharmacological, surgical or by radioactive iodine uptake [1]. Antithyroid drugs (ATD) are considered as the first line treatment unless they are contraindicated, with a very few cases who reported side effects [2]. We report a 30-year-old female with hyperthyroidism, she started treatment with Methimazole 20 mg daily, within one month she presented with acute cholestatic liver insult not otherwise attributed. Symptoms and laboratory findings were decreased on withdrawal, but not normalized. Her condition was controlled by beta blockers and subsequently Prednisolone.
Case Report
A 30-year-old female, recently married, visited our outpatient clinic, complaining of yellow discoloration of sclera, dark urine and pale stool. She had a history of controlled thyrotoxicosis under Methimazole one month before. Her laboratory investigation showed Free Thyroxine level (1.76 ng/ dl) reference range (0.2-8.00 ng/dl), Thyroid stimulating hormone (0.01 IU/ ml) reference range (0.25-4.00 IU/ml).
Elevated total bilirubin (8.9 mg/dl) (152.1 μmol/L), direct bilirubin (7.1mg/dl) (121.4 μmol/L) serum AST (123 IU/l), serum ALT (68 IU/L), serum Albumin (3.4 gm/dl) and normal coagulation profile were found. Serum Alkaline phosphatase of 200 U/L and GGT of 102 U/L Hepatitis serology and autoimmune markers are negative.
Her body weight of 69 kg and height of 175cm were noted. Heart rate was 120 beat/min, Blood pressure was 140/90 mmHg Physical examination did not show anything except slight icteric tinged sclera, even the Thyroid was not palpable. Abdominal ultrasound showed normal liver span, even intra hepatic biliary ducts were of normal caliber, with no obstruction.
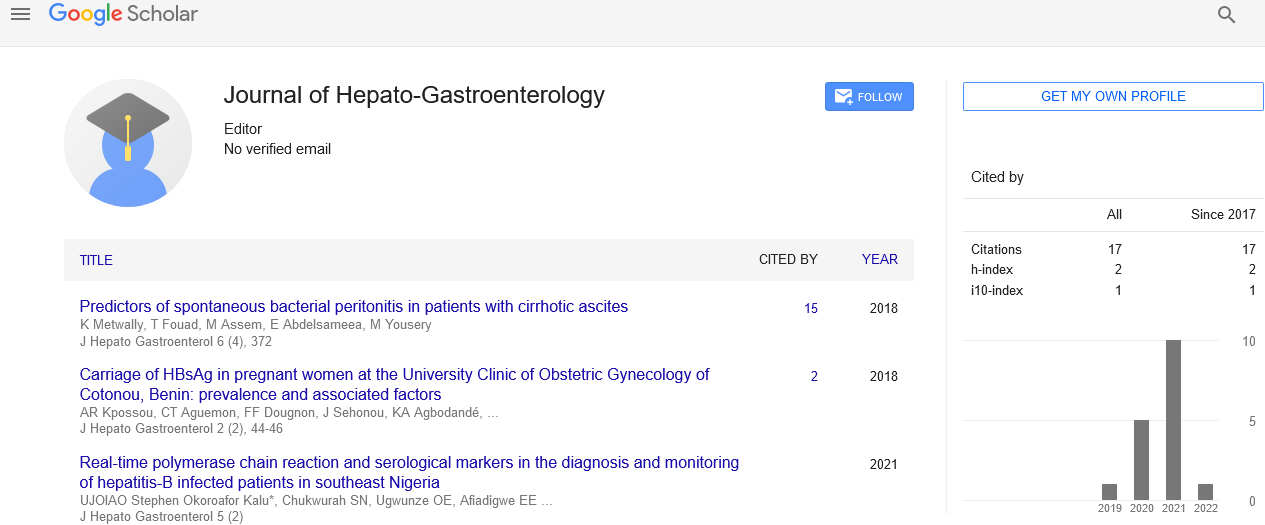
Cholestatic liver injury caused by Methimazole was suspected. A liver biopsy was performed that showed cholangio-dilatation with ductular reaction.
So, Methimazole was held and B-blockage (Propranolol 10 mg three times daily) was administrated. Twenty days later, liver profile was markedly decreased. She received Prednisolone (1 mg/kg/day), her liver function normalized through one month, as well as, free thyroxine level on contrary to thyroid stimulating hormone still suppressed.
Upon revaluation of two months, liver functions are still normal and we started down tapering the prednisolone dosage.
Discussion
Antithyroid medications have been available since 1940 for treatment of Grave’s disease. Antithyroid drugs (ATD), are the second line treatment option after RAI, Methimazole, is a prodrug; Carbimazole, and Propylthiouracil are the ATD available. ATD are preferred in young adult patients with a mild disease, as well as, in pregnant and lactating females. ATD act by inhibiting the action of the peroxidase enzyme; thus preventing organification, iodination, and coupling. PTU, in addition to blocking peroxidase effect, counteracts 5’ deiodinase and thus preventing the peripheral conversion of T4 to T3 [3].
Grave’s disease, the most common cause of hyperthyroidism, is treated with ATD for 12-24 months with 50-60% remission during this time period [4].
Observations over several decades have shown that Methimazole and its prodrug Carbimazole are better than Propylthiouracil in controlling more severe hyperthyroidism, having higher compliance rates, and causing less toxicity, especially when prescribed in lower doses. This has led to the recommendation that Carbimazole should be the first-line drug when antithyroid drug therapy is initiated, either for primary treatment or to prepare a patient for radioiodine or surgery [5].
Up to 15 percent of people who take an antithyroid drug have minor side effects which include itching, rash, wheals, joint pain and swelling. Fortunately, major side effects of antithyroid drugs are very rare, and include agranulocytosis, vasculitis, aplastic anemia [4].
Hepatic damage occurring from thyrotoxicosis per se has been ascribed to ischemic injury resulting from a relative decrease in blood flow despite increased metabolic activity of the liver [6].
A study conducted by Gurlek et al. [7] showed that 60.5% of 43 patients with hyperthyroidism had at least one liver abnormality at diagnosis.
Hence, a baseline liver profile is essential upon diagnosis of thyrotoxicosis. Routine testing of liver function during therapy with antithyroid drugs is not advocated due to alterations happening from the underlying disease itself [8].
Cholestasis may occur in patients with hyperthyroidism. Bile transport is interfered with due to the increase of hepatic oxygen consumption but without an increase of hepatic blood flow thus lowering the oxygen tension in the centri-lobular zone [9].
Thyroxine also can cause cholestasis directly. Of note, alkaline phosphatase (ALP) is not of a significant value in assessing hyperthyroidism/ATD induced liver insult as its rise can be simply correlated to the Thyroxin induced increase in the osteoblastic activity [10]. Autopsies in patients with hyperthyroidism demonstrate hepatic inflammation, fibrosis, and centrilobular necrosis. Organ oxygen consumption but not blood flow augments with the increase of the metabolic rate. The arterio-venous oxygen difference across the splanchnic bed increases, and hypoxia causes hepatic injury [11].
The estimated incidence of antithyroid agent’s associated hepatotoxicity is about 0.5%. PTU was reported to cause 23 liver transplants from 1990 to 2007 in the US and was ranked as the third most common cause of druginduced liver failure requiring transplants [12]. A transient increase in AST and ALT levels is observed in 30% of patients taking Propylthiouracil which usually normalizes 6 weeks plus from treatment initiation [13].
In one report, 389 patients receiving either PTU or MMI were studied. The adverse effects included five patients developing hepatotoxicity. Four out these five were treated with PTU and one with low-dose MMI [7].
ATD induced liver injury is thought to be based on an allergic host response. PTU triggers a cell mediated immune reaction. Lymphocytic sensitization occurs with subsequent release of cholestatic factors. Both carbimazole and propylthiouracil differ in their mechanisms of causation of liver damage CMI, being a sulphonamide, triggers an allergic reaction, which can lead to cholestatic jaundice as well as pancreatitis, erythema nodosum and type 2 DM.
The clinical presentation of patients with MMI-induced hepatotoxicity is similar to that of PTU, with patients presenting with symptoms of jaundice, fatigue, pruritus and malaise. However, it is known that MMI causes less severe liver toxicity, so the patients may not be as critically ill on presentation and may not progress to the severity of illness induced by PTU. CMI causes elevation of ALT by 2-3 folds; however, this 2-3-fold rise is not a recommendation to stop the treatment. Rather close monitoring should be done. If the rise is more than 3 fold up the upper normal limit then the drug should be stopped. Of note, liver enzymes keep rising for 1 week after stopping CMI then start to drop gradually returning back to normal by 6-8 weeks. Liver enzymes take about 5 month to normalize in case of PTU induced liver injury.
Conclusion
Confirmation of carbimazol induced liver injury (CMI) is made by the routine workup of drug induced liver injury. Drug induced liver injury workup entails proper history taking regarding the offending drug administration as well as use of any concomitant drugs. Exclusion of other causes of liver injury/cholestasis, dechallenge and rechallenge. Liver biopsy, although is not a must in all cases, remains the most confirmatory tool for CMI induced hyperbilirubinemia. Because cholestatic pattern is the most common clinical finding; biopsy of the liver is most likely to show expanded portal tracts with inflammatory cells. Proliferating cholangioles and bile plugs can also be seen. Diffuse swelling of hepatocytes is another feature that can be seen [14,15].
Ethical Approval
A written informed consent was obtained from the patient for publication of this case report.
Conflict of Interest
The authors declare no conflicts of interest
Funding
No funding received.
REFERENCES
- Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am 2007;(36):617-56.
- Wang J, Qin L. Radioiodine therapy versus antithyroid drugs in Graves' disease: A meta-analysis of randomized controlled trials. Br J Radiol. 2016.
- Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplantation. 2004;10:1018-23.
- http://www.uptodate.com/contents/pharmacology-and-toxicity-of-thionamides
- http://emedicine.medscape.com/article/169814-overview#a3
- Khan AH, Peter P, Tarigopula G, et al. Carbimazole induced cholestatic hepatitis. J Postgrad Med Inst. 2014;28:107-8.
- Gürlek A, ÇobankaraV, Bayraktar M. Liver tests in hyperthyroidism: Effect of antithyroid therapy. J Clin Gastroenterol. 1997;24:180-3.
- Kubota S, Amino N, Matsumoto Y, et al. Serial changes in liver function tests in patients with thyrotoxicosis induced by Graves' disease and painless thyroiditis. Thyroid. 2008;18:283-7.
- Chawla M, Bal CS. Four cases of coexistent thyrotoxicosis and jaundice: Results of radioiodine treatment and a brief review. Thyroid. 2008;18:289-92.
- Mosekilde L, Christensen MS. Decreased parathyroid function in hyperthyroidism: Inter relationship between serum parathyroid hormone, calcium phosphorus metabolism and thyroid function. Acta Endocrinol (Copenh). 1977;84:566-75.
- Ohshima T, Maeda H, Takayasu T, et al. An autopsy case of sudden death due to hyperthyroidism. Nihon Hoigaku Zasshi 1990;44:365-70.
- Vitti P, Rago T, Chiovato L, et al. Clinical features of patients with Graves' disease undergoing remission after antithyroid drug treatment. Thyroid. 1997;7:369.
- Davies TF, Larsen PR. Thyrotoxicosis. Williams Textbook of Endocrinology (11th ed). Saunders Elsevier, Philadelphia, USA. 2008;336.
- Martinez-Lopez J, Greenberg SE, Kling RR. Drug-induced hepatic injury during methimazole therapy. Gastroenterol. 1962;43:84-7.
- Majeed M, Babu A. Cholestasis secondary to hyperthyroidism made worse by methimazole Bymethimazole. 2006;332:51-3.