Partial intestinal obstruction due to small intestine gastrointestinal stromal tumor: A rare case
Received: 04-Nov-2022, Manuscript No. PULHG-22-5539; Editor assigned: 07-Nov-2022, Pre QC No. PULHG-22-5539 (PQ); Reviewed: 21-Nov-2022 QC No. PULHG-22-5539; Revised: 04-Jan-2023, Manuscript No. PULHG-22-5539 (R); Published: 13-Jan-2023
Citation: Peker E. Partial intestinal obstruction due to small intestine gastrointestinal stromal tumor: A rare case. J Hepato Gastroenterol 2023;7(1):1-3.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Gastro Intestinal Stromal Tumors (GIST) are submucosal tumors originating from the interstitial cells of canal located within the muscle layer from the digestive tract especially the stomach, small intestine, mesentery, colon and esophagus. GISTS are most common in the stomach (50%-70%), small intestine (20%-30%) and large intestine (about 10%). More rarely, it can be localized in other areas, such as the omentum and mesentery. Typically, more than 90% have a specific KIT or PDGFRA gene mutation. In this case report, we aimed to investigate the diagnosis and treatment of gastrointestinal stromal tumor of the small intestine detected in a patient who presented with partial intestinal obstruction.
Keywords
Pseudo capsule; Nausea; Extraluminal; Anthropyloric; Polyclonal
Abbreviations
GIST: Gastrointestinal Stromal Tumor; IHC: Immunohistochemistry; CT: Computed Tomography; FNAB: Fine Needle Aspiration Biopsy; PDGFRA: Platelet Derived Growth Factor Receptor A; EUS: Endoscopic Ultrasonic Examination; MRI: Magnetic Resonance Imaging; VEGFR: Vascular Endothelial Growth Factor
Introduction
Our patient was a 51 years old male. He was 165 cm tall and weighed 70 kg. In may 2022, she applied to the emergency department with complaints of abdominal pain, nausea and vomiting, which had been going on for about 2 days. Slight distension was present on abdominal examination, defense and rebound were not detected [1].
According to the vital signs, the pulse rate was 102 beats per minute, and the fever was normal. He had applied with complaints of severe pain in the left lower quadrant radiating to the left groin, nausea and vomiting, which started at night. He had defecated 1 day before, there was a gas outlet. On physical examination, defense in lower left quadrant was detected. The patient stated that nausea and vomiting had passed during clinical followup, but the pain continued [2].
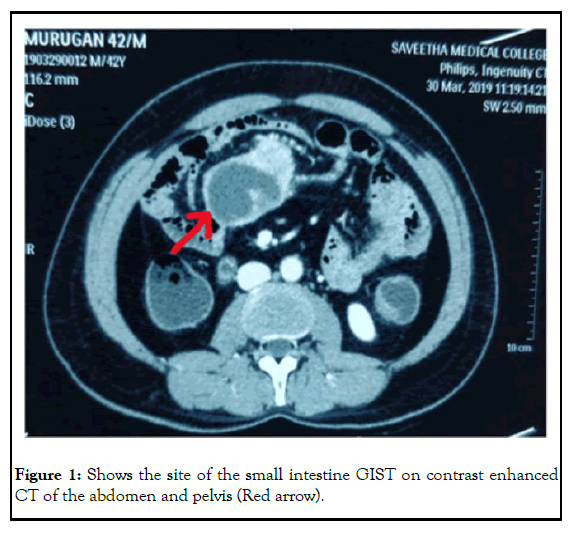
Abdominal tomographic examination revealed a lesion with a diameter of about 3 cm with intra and extraluminal components in the small intestine. Mild dilatation was detected in the small intestine sections proximal to this mass. The radiology department also reported that there was a 35 mm × 55 mm central hypo dense nodular lesion between the small intestine segments at the left pelvic entrance level. Liver, spleen, both adrenal glands and pancreas were normal. No intraabdominal free fluid was detected. Slight wall thickening was present in the anthropyloric region of the stomach and endoscopic examination was recommended [3].
There was no history of any chronic diseases, allergies and drug use. There were no features in his family history [4].
Case Presentation
The patient was taken to an emergency operation with a preliminary diagnosis of partial intestinal obstruction and small bowel mass. A superior inferior midline incision was made under general anesthesia, followed by skin antisepsis and sterile dressing, and the abdomen was entered. During the examination, a mobile mass with an increased vascularity of about 3 cm in diameter was detected in the abdomen, originating from the wall of the small intestine. No other pathologies were observed in the small intestine and abdomen. The small intestine of 5 cm, 150 cm distal to the ligament of Trietz, was held with a clamp and cut with a scalpel. It was seen that bloodletting was good at both ends. It was decided to perform an anastomosis manually. A jejuno-jejunal end to end anastomosis was performed with both ends facing each other axis and suspending sutures were placed. The posterior wall was sutured with separate 3-0 silk sutures, and the anterior wall was sutured with lambert sutures. The anterior wall was sutured in one layer with lambert sutures. Lumen opening was found to be sufficient on palpation. The meso aperture was closed with 3/0 vicryl sutures. The fascia was closed with the continuous loop PDS number 0 and finally the skin was closed with the help of a skin stapler [5].
His vitals were stable on the 1st postoperative days and oral regimen 1 was initiated. He received an oral regimen 2 on the 2nd postoperative day. On the 3rd postoperative day, his vitals were stable, he had gas discharge and defecation, and he was discharged with recommendations (Figure 1) [6].
Results
As a result of the pathology, the small intestine with a length of 4 cm, a diameter of 2.5 cm, was macroscopically monitored. A tumor lesion with a size of 6 cm x 3 cm x 3 cm, a properly limited part of 2.5 cm × 2.5 cm × 1.5 cm was observed in the lumen of the meso opposite wall. The lesion was at a distance of 1.2 cm and 1 cm from the surgical border. The lesion site was necrotic in appearance [7].
Immunohistochemical examination revealed CD117 (Thermofisher/ Polyclonal) diffuse strongly positive; DOG1 (Leica/K9) diffuse positive; SMA: (Scytek/1A4) diffuse and strongly positive in spindle cell component, negative in epithelioid component; desmin (Genemed/GM007) negative; CD34 (Leica/QBEnd/10) spindle cell component dispersed in positive, negative component epiteloid; S100 (Leica/Polyclonal) negative; SDBH (Biosb/BSB-131). Although the dye did not work optimally, it was interpreted that there was no loss of immunoreactivity due to local cytoplasmic immunoreactivity in neoplastic cells. There was no PDGFR-a (cell signaling/D1E1E) immunexpression. There was no BRAF (Spring/ VE1) immunosuppression. Ki-67 (Dako/MIB1) proliferation index was evaluated as approximately 7-8%. Mitotic rate was 7/22 BBA (5 mm²) and necrosis was present (prevalence 5%). No lymph node was observed in the specimen. The diagnosis was reported as mixed type, high grade (grade 2), unifocal, and stage pT3Nx, and histological type epithelioid (70%), and spindled (30%), gastrointestinal stromal tumor [8].
Our patient was referred to the oncology department after the postoperative pathology report was released, and imatinib treatment was started. No recurrence or residual tumor was detected in the abdominal tomographic examination taken 2 months after the operation. He is still being followed up under imatinib treatment [9].
Discussion
GISTs are tumors arising from mesenchymal/stromal cells located in the gastrointestinal tract. Approximately 30% of GISTS are malignant, and the prediction of malignant potential based on histopathological criteria is very important in identifying patients with a high probability of local recurrence or distant metastases [10].
The mean age of incidence is 55-60 years and equal in both men and women. They are the most common sarcomatous tumors of the gastrointestinal tract that have unpredictable biological behavior. They occur anywhere in the gastrointestinal tract, but most often in the stomach (40-60%), small intestine (30%), colon (15%) and duodenum (5%).
Common symptoms are abdominal pain, gastrointestinal bleeding, and abdominal mass. Approximately 50% of them have metastasized at the time of diagnosis and metastasize most frequently to the liver or peritoneum. Tumor size can be variable (1-2 cm to 20 cm).
When we looked at our case, we saw that he applied to the emergency department with abdominal pain and partial obstruction findings. We usually see this disease in the population over the age of 50. Our case was 51 years old. Radiotherapy and chemotherapy are usually ineffective in these tumors. In addition, close follow-up is required after resection and recurrences are seen in the first 2 years in these cases. GISTs almost never cause lymphatic metastases.
Large tumors can show cystic degeneration, necrosis and focal bleeding. GISTS are usually well confined and not encapsulated (pseudo capsules are rarely visible). Microscopically we see well differentiated smooth muscle cells. On the basis of cellular appearance, three histological types are distinguished: fusiform (77%), epithelioid (8%) and mixed (15%). Most GISTS are histologically seen as spindle shaped tumor cells, some as epithelioid tumor cells, or a mixture of both. Differential diagnosis from leiomyoma or leiomyosarcoma, consisting of spindle shaped tumor cells, can be difficult, since they are histologically similar.
The prognosis of this disease is associated with especially the tumor size and mitotic index. Gastric origin has a better prognosis than the small intestine. It is important to distinguish the epitheloid type from poorly differentiated adenocarcinoma, undifferentiated carcinoma and carcinoid tumors. Kit protein positivity is an important parameter in the differential diagnosis.
KIT is not only highly expressed in interstitial cells of Canal and GIST but also in hematopoietic stem cells, melanocytes, mast cells, and germ cells. While more than 95% of GISTs are Kit (+), other intra-abdominal tumors (malignant melanoma, seminoma, breast cancer, Ewing tumor/Peripheral Neuroectodermal Tumor (PNET), small cell carcinoma, adenoid cystic carcinoma, terato carcinoma, and thyme carcinoma) are rarely found as Kit (+). Most GISTs (∼85%) have KIT or PDGFRA oncogenic mutations that cause cell proliferation and survival. Weak Kit positivity can be detected when GISTs are associated with PDGFRA gene mutation. CD34 (+) is detected in 70-80% of GISTs in immunohistochemical studies.
GISTs usually present as subserosal or submucosal lesions in the gastrointestinal tract. Most subepithelial lesions are followed without a histological diagnosis. However, the prognosis of GISTS is associated with early histological diagnosis and requires R0 resection. Early treatment is important in this type of lesions. We need further studies for optimal follow-up. Very low risk GISTs may not require routine follow-up. When a smooth surfaced, hemispheric, non-ulcerous, and less than 2 cm mass is detected endoscopic follow-up is recommended every 1-2 years. Abdominal tomography, Endoscopic Ultrasonic examination (EUS) and Fine Needle Aspiration Biopsy (FNAB) are recommended if enlargement, ulceration or irregular border is detected in the follow-up. In the presence of a symptomatic submucosal tumor larger than 5 cm or pathologically diagnosed with GIST, imaging methods must be performed to detect preoperative invasion or for staging.
Surgery is the main method for histological confirmation of the diagnosis of GIST. The main purpose of surgery in GISTs is to resect with a macroscopically clean surgical margin. En-bloc resection should be performed in a way that does not damage the pseudo capsule.
For low risk tumors, follow-up with an abdominal CT scan or Magnetic Resonance Imaging (MRI) can be performed every 6 to 12 months for 5 years. For high risk patients during adjuvant treatment abdominal CT scan or an MRI follow-up should be 3 to 6 months for 3 years. After discontinuation of treatment, imaging should be performed every 3 months for 2 years and every 6 months until the end of the 5th year. For the next 5 years, once a year is sufficient.
Imatinib has been the first line therapy for both palliative and adjuvant treatments of GIST patient. Imatinib is relatively well tolerated. Orally administered imatinib should be the primary recommended treatment for recurrent GISTs. If surgical treatment is required in case of metastatic recurrence, close follow-up and imatinib are recommended after surgery. Sunitinib therapy may be one of the most important treatment options for unrespectable imatinib resistant GIST. Sunitinib is a multitargeted TKI with activity against KIT, PDGFR, VEGFR, and FLT-1/KDR. Imatinib and sunitinib resistance is usually caused by secondary mutations in the KIT and/or PDGFRA kinase domains. Sunitinib and regorafenib have been approved as second and third line therapies, respectively, for the treatment of advanced GIST after imatinib failure. Multikinase inhibitors have significantly advanced the field in the treatment of imatinib resistant GISTs because they increased the survival in patients. Most GISTS initially respond to front line imatinib treatment; however, complete eradication is rare. Eventually, almost all GISTs acquire resistance to imatinib, and patients treated with imatinib progress and die due to the appearance of clones with imatinib resistant mutations.
Our patient started taking imatinib treatment after the pathology report came out in the first month after surgery and is still continuing his treatment. No recurrence was observed in the abdominal tomographic examination performed at the 2nd month of the operation.
Conclusion
In conclusion, metastases in GISTS are usually intra-abdominal and most commonly in the liver. Although local recurrence is rare, surgery should be performed if there is recurrence due to a residue from previous surgery. Guidelines recommend abdominal imaging at 3-6 months intervals for the first 2 years in high risk patients. Patients with a significant risk of relapse should be treated with imatinib for at least 3 years. The optimal duration is unknown.
References
- Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018; 24(26):2806-17.
[Crossref] [Google Scholar] [PubMed]
- Schaefer IM, Marino-Enriquez A, Fletcher JA, et al. What is new in gastrointestinal stromal tumor? Adv Anat Pathol. 2017;24(5):259-67.
[Crossref] [Google Scholar] [PubMed]
- Von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol. 2018;36(2):136-43.
[Crossref] [Google Scholar] [PubMed]
- Huda T, Singh MP. Gastrointestinal Stromal Tumors of small intestine. Surg J. 2019;05(03):92-5.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Zhao Z, Wang W, et al. Current characteristics on small intestinal stromal tumor-a case control study. Ann Palliat Med. 2020; 9(1):98-107.
[Crossref] [Google Scholar] [PubMed]
- Peng F, Liu Y. Gastrointestinal Stromal Tumors of the small intestine: Progress in diagnosis and treatment research. Cancer Manag Res. 2020;12:3877-89.
[Crossref] [Google Scholar] [PubMed]
- Manxhuka-Kerliu S, Sahatciu-Meka V, Kerliu I, et al. Small intestinal gastrointestinal stromal tumor in a young adult woman: A case report and review of the literature. J Med Case Rep. 2014;8:321.
[Crossref] [Google Scholar] [PubMed]
- Zhou L, Liao Y, Wu J, et al. Small bowel gastrointestinal stromal tumor: A retrospective study of 32 cases at a single center and review of the literature. Ther Clin Risk Manag. 2018;14:1467-81.
[Crossref] [Google Scholar] [PubMed]
- Serrano C, George S, Valverde C, et al. Novel insights into the treatment of imatinib-resistant gastrointestinal stromal tumors. Target Oncol. 2017;(3):277-88.
[Crossref] [Google Scholar] [PubMed]
- Hayashi Y, Nguyen VTT. A narrative review of imatinib resistant gastrointestinal stromal tumors. Gastrointest Stromal Tumor. 2021; 4:6.
[Crossref] [Google Scholar] [PubMed]