Phenprocoumon-induced acute liver failure with unfavourable prognosis regarding plasma disappearance rate of indo-cyanine green
Received: 26-Apr-2018 Accepted Date: May 30, 2018; Published: 05-Jun-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Acute liver failure (ALF) involves a rapidly emerging impairment of liver function carrying a high mortality. Phenprocoumoninduced hepatotoxicity is a rare but potentially serious complication of oral-anticoagulation. As liver transplantation should be considered only for patients with unfavourable prognosis, there is further need to improve prognostic scoring systems. CASE PRESENTATION: This report is about a 68 year-old woman with phenprocoumon-induced ALF. Patient’s King’s College criteria and the dynamic assessment of liver function by plasma disappearance rate of indocyanine green (ICG-PDR) indicated a poor prognosis for spontaneous recovery. Transplantation was performed 8 days after admission and patient made a complete recovery. CONCLUSION: Considering the high mortality of ALF and organ shortage, current available models like King’s College criteria fail to adequately predict patients outcome. The dynamic liver evaluation by non-invasive ICG-PDR measurement might improve the appreciation of spontaneous recovery or candidacy for transplantation. Key Words: Acute liver failure (ALF); Indo-cyanine green (ICG); Plasma disappearance rate (PDR); Phenprocoumon-induced hepatotoxicity
Keywords
Acute liver failure (ALF); Indo-cyanine green (ICG); Plasma disappearance rate (PDR); Phenprocoumon-induced hepatotoxicity
Abbreviations
IAH: Intraabdominal Hypertension; ALF: Cute Liver Failure; INR: International Normalized Ratio; DILI: Drug-Induced Liver Toxicity; OLT: Orthotopic Liver Transplantation; ICG: Indo-Cyanine Green; PDR: Plasma Disappearance Rate; ALAT: Alanine Aminotransferase; ASAT: Aspartate Aminotransferase; PT: Prothrombin Time; ICU: Intensive Care Unit; MELD: Model of End-Stage Liver Disease
Acute liver failure (ALF) is a rare but life-threatening clinical syndrome characterized by rapid impairment of previously regular liver function [1]. The established definition of ALF is primarily based on disturbed coagulation with an international normalized ratio (INR) >1.5 and any degree of encephalopathy in patients without pre-existing hepatic disease [2]. Most frequent causes of ALF are drug-induced liver injury (DILI), viral hepatitis, autoimmune liver disease and hypoperfusion during shock [3,4].
ALF still involves a high mortality despite advances in clinical management and a tendency to more benign etiologies such as acetaminophen [5]. Transplantation is a well-established treatment option for individuals lacking spontaneous recovery [2-6]. Orthotopic liver transplantation (OLT) has improved the overall short-term survival rate (1-year) to more than 60%. However, OLT can only be justified for patients with unfavourable prognosis for spontaneous hepatic regeneration [3]. Various scoring systems were proposed to appreciate the prognosis, but all currently available prognostic scores fail to sufficiently predict the course of ALF [1,2]. King’s College criteria still represents the most frequently applied model in clinical practice [7]. Improvement of prognostic validity may be achieved by additional methods for accurate assessment of liver function in addition to conventional laboratory tests. One of the most promising techniques seems to be the dynamic appraisal of functional liver status by plasma disappearance rate of indo-cyanine green (ICG-PDR) [8]. According to King’s College criteria “drug-induced etiology” belongs to indicators for unfavourable prognosis [7]. Despite the large number of agents potentially involved in DILI only a few cases of hepatotoxicity induced by oral-anticoagulants have been reported world-wide. However, a closer look on the available cases demonstrates that phenprocoumon-induced hepatic injury is a rare but potentially serious complication that may progress from mild hepatitis even to ALF [9]. The following report concerns a case of phenprocoumon-induced ALF in a 68 year-old woman. In consideration of progressive hepatic dysfunction indicating unfavourable prognosis our patient was selected for OLT.
Case Presentation
A 68 year-old Caucasian woman was admitted to our emergency department with acute onset of painless jaundice progressing within a time period of 7 days. Medical history revealed a permanent atrial fibrillation with catheter ablation 3 month ago, implying an indication for long-term oralanticoagulation using phenprocoumon. Our patient convincingly denied the intake of any other medication including herbal drugs, mushroom eating, alcohol consumption or former hepatic affections. Blood tests showed substantial disorders typical for ALF with elevated liver enzymes (ALAT 2355 U/L, ASAT 1897 U/L), increased bilirubin of 10.0 mg/dL and serious impairment of plasmatic coagulation (PT<10 %, INR>7.0). The activity of the vitamin K-independent coagulation factor V was reduced to 43 %. A mild raise in ammoniac level (92 μg/L) combined with increased fatigue and abnormal tiredness was compatible with encephalopathy grade I. Otherwise, the physical examination was without pathological findings. A timeline summarizing important data and relevant treatment steps is illustrated in Table 1.
| Timeline of important data and treatment steps | |
|---|---|
| 3 months before admission | Initiation of oral-anticoagulation with phenprocoumon |
| 7 days before admission | Progressing painless jaundice |
| Day 1 | Admission to emergency department: Medical history; physical examination; laboratory tests Diagnosis of Acute liver failure: bilirubin 10.0 mg/dL, INR > 7.0, hepatic encephalopathy grade I |
| Day 2 | Admission to ICU-unit: Analyses to exclude virological, autoimmune or hereditary etiology; Ultrasound; Magnetic resonance tomography; Initial ICG-PDR assessment: 2.2 %/min |
| Day 7 | Fulfilment of King´s College criteria: bilirubin 31.6 mg/dL, age, phenprocoumon-induced ALF |
| Day 8 | Orthotopic liver transplantation |
| Day 15 | Stable clinical conditions; INR 1.1 |
| 3 months | Full recovery and medical care via outpatient department |
Table 1: Timeline of important data and treatment steps
On admission to our intensive care unit (ICU) the patient presented with stable cardiopulmonary condition and regular renal function without evidence for severe affection of central nervous system such as flapping tremor or sopor. Phenprocoumon therapy was stopped immediately and no other medication was applied except lactulose and N-acetylcysteine. At an early stage we performed detailed analyses to exclude a virological, autoimmune or hereditary genesis of ALF. Furthermore, we excluded obstructive cholestasis or major circulatory disorders by ultrasound and magnetic resonance tomography. In consequence of markedly deranged coagulation combined with the presumable drug-induced etiology of ALF we refrained from aquiring a liver biopsy. To further extend the assessment of liver function we supplemented laboratory tests with daily measurements of ICG-PDR by using non-invasive LiMON technology (Pulsion® Medical Systems, München, Maquet Getinge Group). ICG-PDR on admission to ICU was significantly reduced to 2.2 %/min (normal range 18-25 %/min).
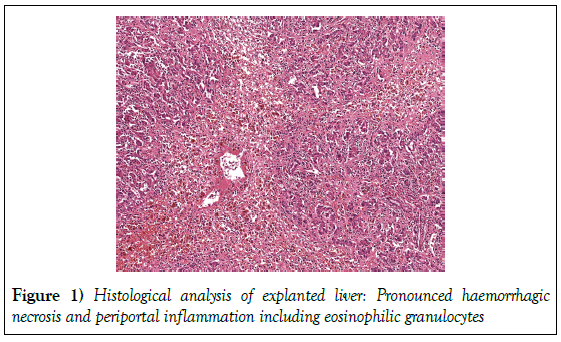
Continuous monitoring of vital parameters and blood tests twice a day were performed. We mentioned a continual decline in levels of elevated liver enzymes (ALAT 1177 U/l, ASAT 773 U/l). As opposed to this, coagulation did not improve and bilirubin ascended to a maximum of 31.6 mg/dL 7 days after admission. Because of persistent abnormalities of coagulation we initiated a treatment with vitamin K. Administration at a dose of 10 mg per day provoked an increase of prothrombin time to a maximum of 37 % (INR 2.1), but could neither achieve a complete normalization of coagulation parameters nor avoid a further escalation of bilirubin. In conformity with consensus recommendations we performed an individualized assessment of patient’s prognosis. Considering fulfilment of King’s College Criteria combined with pathological ICG-PDR as indicators for unfavourable prognosis, we came to the interdisciplinary decision for high-urgency liver transplantation; 8 days after admission patient received an allogenic OLT. The histological analysis of the explanted liver displayed extensive confluent, haemorrhagic necrosis and periportal inflammation including eosinophilic, reconcilable with a drug-induced ALF (Figure 1).
Figure 1: Histological analysis of explanted liver: Pronounced haemorrhagic necrosis and periportal inflammation including eosinophilic granulocytes
Following OLT our patient recovered completely with stable cardiopulmonary condition, good renal function and a normal vigilance without evidence for encephalopathy. Blood tests showed regular coagulation 7 days after OLT (INR 1.1) and declining levels of bilirubin. Non-invasive measurement of ICG-PDR 7 days post-transplant was 9.3 %/min. Within a time period of 3 months, bilirubin levels returned to normal. Patient made a complete recovery and is currently under regular medical care via our outpatient department.
Discussion
This case report illustrates some of the decisive questions related to ALF. The primary task is to draw a distinction between ALF and acute-on-chronic liver disease, undoubtedly of importance because of differences in clinical management and prognostic criteria [1-3]. In this case it was essential to exclude underlying chronic hepatic affection by anamnesis as well as to except pre-existing cirrhosis, cancer or Budd-Chiari-syndrom by imaging methods. Special laboratory tests were performed to disclose Wilson´s disease, autoimmune or chronic viral hepatitis. Another question in this case is whether coagulopathy is just a result of anticoagulant-overdosing or must be regarded as a feature of DILI. Patient’s prolonged prothrombin time (INR>7.0) theoretically may be a result of increased phenprocoumon effect. However, simultaneous impairment of liver function and depression of vitamin K-independent coagulation factor V indicated a more complicated problem than just an overdosing. Liver damage is an overall rare side effect of oral-anticoagulants but potentially of high relevance. The Swiss Drug Monitoring Centres SANZ and IKS reported on a total of 10 oralanticoagulant- induced liver injuries (1.5%) among 674 cases of drug-induced hepatitis from 1981 to 1995 (10). According to the largest retrospective analysis of 8 cases by Schimanski et al., [9] phenprocoumon-induced hepatotoxicity may range from mild hepatitis to even ALF in a mainly dosageindependent manner. In the present case histological analysis of explanted liver showed a predominantly hepatitic injury with massive necrosis and increased eosinophilic granulocytes as reported in most phenprocoumoninduced liver diseases [11]. Daily administration of vitamin K could not normalize prothrombin time (minimum INR 2.1) and seemed to be inconsiderable regarding the clinical course of ALF. The third question deals with the difficult but crucial acquisition of prognostic factors to predict outcome of ALF. King’s College Criteria and MELD-Score serve as most prevalent scoring systems, but neither with satisfying prognostic accurancy. King’s College Criteria is suffering from poor sensitivity [7-12]. Conventional assessment of liver function is mainly based on static laboratory tests [8]. Bilirubin and INR are substantials of scoring systems but significance may be restrained by concomitant diseases, concurrent medication or medical attendance like substitution of coagulation factors or albumin-dialysis. Dynamic tests related to short-term functional status seem to be more precise in assessing liver function and predicting outcome of critically ill patients [13]. They may offer a more objective evaluation of liver status less susceptible to iatrogenic confounders. Most promising experiences were acquired with the dye indo-cyanine green (ICG), as ICG-PDR can be measured noninvasively and reflects liver blood flow, hepatocellular function and biliary excretion, with a normal ICG-PDR ≥ 18 %/min [14]. The significance was already illustrated for early assessment of liver dysfunction, outcome of septic patients, morbidity after liver resection and for predicting survival of cirrhotic patients by incorporating ICG-PDR into MELD-Score [8-15]. A prospective analysis of 25 patients by Merle et al. evaluated the validity of ICG-PDR in predicting outcome of ALF. They demonstrated a sensitivity and specificity of 85.7 % and 88.9 %, respectively for an ICG-PDR ≤ 6.3 %/min on day 1 for predicting an absent recovery in ALF [16]. This study suggests that ICGPDR might be of high prognostic relevance in addition to King’s College criteria. Advancement of prognostic scores plays the decisive role in the challenge to accurately weigh up the necessity of OLT. Transplantation is the only available treatment option for individuals without spontaneous recovery and has improved overall 1 year-survival to over 60 % [1-3]. Longterm course for patients receiving OLT is satisfying with a survival rate of 70- 75 % after 3 years, but 1-year survival is less than that described in patients transplanted for cirrhosis [17]. Consequently, there is always a high medical responsibility for an individualized risk-benefit-analysis for transplantation in ALF. Outcome prediction should optimally allow a rapid identification of patients that will most likely benefit from OLT, whereas it must be avoided to transplant ALF-patients needlessly in view of organ-shortage and livelong immunosuppression [18]. In our case patient’s individual parameters fulfilled King’s College Criteria and ICG-PDR on admission was 2.2 %/ min, suggesting an unfavourable prognosis for spontaneous recovery. In the absence of contraindications such as severe underlying diseases, cardiopulmonary instability, sepsis or progressive neurological disorders, patient received an OLT 8 days after admission to hospital. The additional establishment of dynamic liver tests like ICG-PDR may be the decisive factor in optimizing prognostic scoring systems for ALF. All improvements should help to timely determine candidacy for transplantation.
Conclusion
Phenprocoumon-induced liver injury is a rare but potentially severe side effect of oral-anticoagulation. Regarding the high mortality of ALF and the general scarcity of organs, it is mandatory to improve prognostic scoring systems for more accurate prediction of patient’s outcome and necessity for transplantation. Non-invasive ICG-PDR measurement is a promising dynamic liver test to improve the validity of prognostic scores.
Declarations
Ethics approval and consent to participate
This case report was approved by the institutional review board (Ethikkommission Technische Universität München; Fakultät für Medizin).
Consent for publication
Informed consent was obtained by the patient presented in this case report.
Availability of data and materials
More detailed data are available upon request. To receive anonymized data readers are welcome to contact the corresponding author: Dr. Ulrich Mayr, Klinik und Poliklinik für Innere Medizin II, Klinikum rechts der Isar der Technischen Universität München, Ismaninger Strasse 22, D-81675 München in Germany.
Competing interests
Wolfgang Huber collaborates with Pulsion Medical Systems SE, Feldkirchen, Germany, Maquet Getinge Group, as member of the Medical Advisory Board. All other authors have no conflict of interest to disclose.
Authors’ contributions
UM and TL designed the case report and draftet the manuscript.
IH, GB, RS and WH participated in the analysis and helped to draft the manuscript.
REFERENCES
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013; (26): 2525-2534.
- Lee WM. Acute liver failure. Semin Respir Crit Care Med 2012; (1): 36-45.
- Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; (12): 947-954.
- Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis 2014; (2):134-144.
- Lee WM. Etiologies of acute liver failure. Semin Liver Dis 2008; (2):142-52.
- Bernal W, Lee WM, Wendon J, et al. Acute liver failure: A curable disease by 2024? J Hepatol 2015;(62): 112-120.
- O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;(2): 439-445.
- Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007;(2): 207-214..
- Schimanski CC, Burg J, Mohler M, et al. Phenprocoumon-induced liver disease ranges from mild acute hepatitis to (sub-) acute liver failure. J Hepatol 2004; (1): 67-74.
- Ciorciaro C, Hartmann K, Stoller R, et al. (Liver injury caused by coumarin anticoagulants: experience of the IKS (Intercanton Monitoring Station) and the SANZ (Swiss Center for Drug Monitoring)). Schweiz Med Wochenschr 1996;(49): 2109-2113.
- De Man RA, Wilson JH, Schalm SW, et al. Phenprocoumon-induced hepatitis mimicking non-A, non-B hepatitis. J Hepatol 1990;(3): 318-321.
- McPhail MJ, Farne H, Senvar N, et al. Ability of King's College criteria and model for end-stage liver disease scores to predict mortality of patients with acute liver failure: A meta-analysis. Clin Gastroenterol Hepatol 2016;(4): 516-525 e5; quiz e43-e45.
- Sakka SG, Klein M, Reinhart K, et al. Prognostic value of extravascular lung water in critically ill patients. Chest 2002;(6): 2080-2086.
- Faybik P, Hetz H. Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc 2006; (3): 801-802.
- Zipprich A, Kuss O, Rogowski S, et al. Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut 2010;(7): 963-968.
- Merle U, Sieg O, Stremmel W, et al. Sensitivity and specificity of plasma disappearance rate of indocyanine green as a prognostic indicator in acute liver failure. BMC Gastroenterol 2009;(9):91.
- Roberts MS, Angus DC, Bryce CL, et al. Survival after liver transplantation in the United States: A disease-specific analysis of the UNOS database. Liver Transpl 2004;(7) :886-8897.
- Lake JR, Sussman NL. Determining prognosis in patients with fulminant hepatic failure: when you absolutely, positively have to know the answer. Helpatology 1995;(3):879-882.