Some speculations on the histogenesis of desmoplastic melanoma
Received: 10-Sep-2018 Accepted Date: Sep 30, 2018; Published: 10-Oct-2018
Citation: Filotico M. Some speculations on the histogenesis of desmoplastic melanoma. J Histol Histopathol Res 2018;2(2): 25-31.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Opportunity to have observed a case of Desmoplastic Melanoma showing a somewhat particular clinical presentation led to the review of the literature on the subject and offered an opportunity for some speculation about the histogenesis of this lesion. From the examination of our case and from the review of the literature, it emerged that the starting factor is a dedifferentiated tumorigenic cell clone originated in a Lentigo Maligna. This clone would be capable of triggering in the surrounding dermis as a proliferative phenomenon with the characters of a fasciitis-like PMP (Pseudosarcomatous Myofibroblastic Proliferation) that would constitute the desmoplastic component of the tumor. This phenomenon of the PMPs-related tumor has been widely demonstrated to date, only in association with squamous superficial carcinom of mucous membranes. In our case, the “fulminant” relapse finds an explanation in the onset of a Spindle cell Postoperative Nodule, lesion, even this one, belonging to PMPs group, characterized by rapid onset after a surgical trauma.
Keywords
Desmoplastic melanoma; Immunohistochemistry; Histogenesis
The peculiarity of the clinico-pathologic presentation of a case of Desmoplastic Melanoma, has led to a series of considerations regarding the histogenesis of this particular neoplasia. CASE-Male Physician 54-year old, have long-term[from birth?], In the right deltoid region, a brown-colored skin mole of round and dome-shaped, at net margins, with a diameter of 9 mm, unmodified over time. The Patient reports that the lesion, after minor trauma [scratching?], has been partially removed. Following this event, after a few days, it is subject to the removal of the residue of the neoformation. About ten days after surgery, at the dermal site, below the suture scar, nodularity appeared, initially attributed to a suture granuloma. The nodule has increased rapidly, so that within a few weeks it reaches the dimensions of 3 x 3 x 3 cm, and shows ulceration of the overlying skin. After about eight weeks from the first operation, the Patient is subjected to the nodule’s excision. On this occasion, it is also noticed as the presence of the right axillary lymphadenopathy. The patient is then subjected to an expansion of local excision and axillary lymphadenectomy.
Materials and Methods
The anatomical samples from the various surgical procedures were fixed in formalin and paraffin embedded, stained with hematoxylin-Eosin and Masson’s Trichrome.
A large panel of antibodies has been performed (Table 1).
Table 1: A large panel of antibodies.
| AB | DIL |
|---|---|
| S100 | 1.400 |
| HMB45 | 1:50 |
| MEL A | 1:30 |
| SOX10 | 1.250 |
| P75 | 1:300 |
| CD34 | 1:20 |
| CD68 | 1:50 |
| DESM | 1:50 |
| SMACT | 1:50 |
| MSA | 1:50 |
| CALP | 1:50 |
| VIM | 1:50 |
| LCA | 1:200 |
| CD99 | 1:500 |
| CD117 | 1:400b |
| EMA | 1:50 |
| ALK | 1;25 |
| CK AE1-AE3 | 1:50 |
| FATTXIIIa | 1:500 |
| KI67 | 1:100 |
Pathology
Villalba suggested 1) The sample of the first surgical intervention consists of a skin lozenge of the size of 2 x 1 x 0.5 cm, interesting the skin and a very thin strip of the subcutaneous tissue.
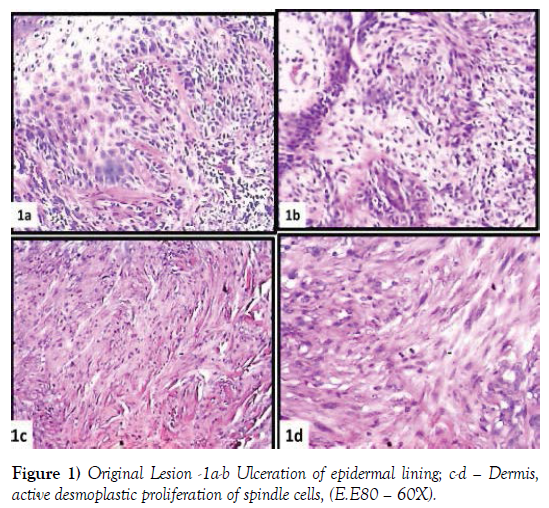
The epidermis appears to be extensively ulcerated and replaced by a band of amorphous necrotic material (Figure 1a and 1b).
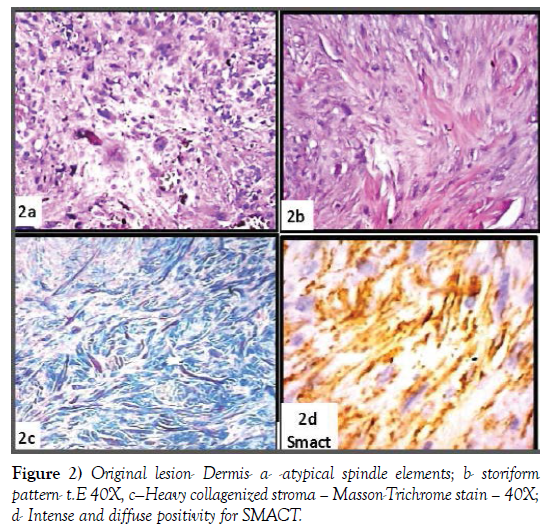
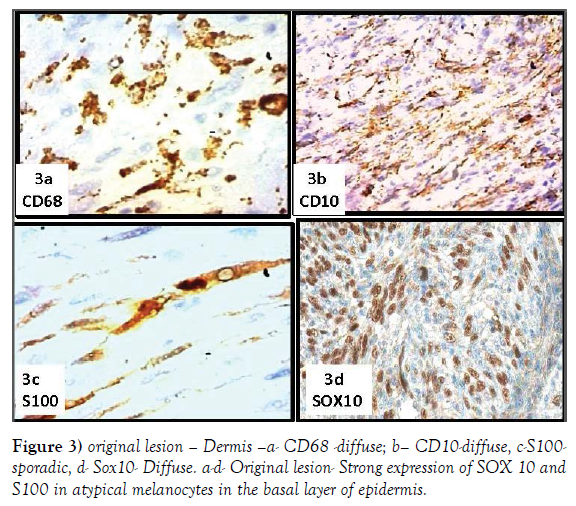
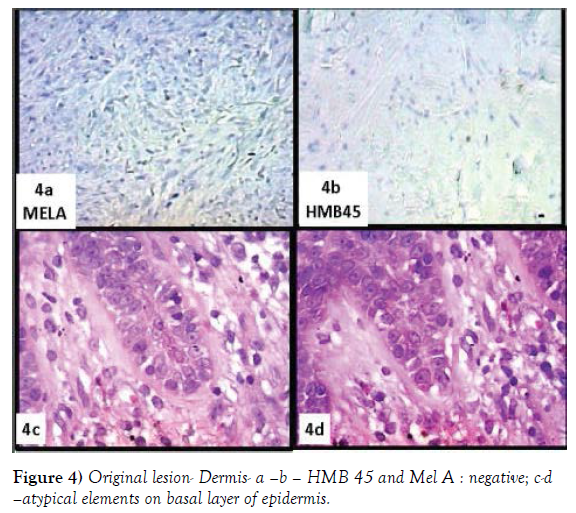
The dermis is occupied by a pleomorphic proliferation of spindled elements, sometimes arranged in a storiform fashion (Figures 1c, 1d, 2a and 2b) and heavy collagenized, as evidenced by a trichrome stain (Figure 2c). These elements diffusely express vimentin and very frequently SMACT, CD68, CD10 (Figures 2d, 3a and 3b) and focally S100 (Figure 3c and 3d). Totally not expressed HMB45 and MEL A (Figure 4a-4d).
At first glance, a dermatofibroma diagnosis is advanced, based on the morphological pattern that the total negativity of the specific markers for the melanoma (MEL A, HMB45). The very rare S100-positive elements were, initially, interpreted as dendritic cells.
After the histological evaluation of the recurrent lesion, it proceeded to a revision of the original lesion, and to the immunohistochemistry panel, which was added to the antibodies SOX10, P75, FATT XIIIa.
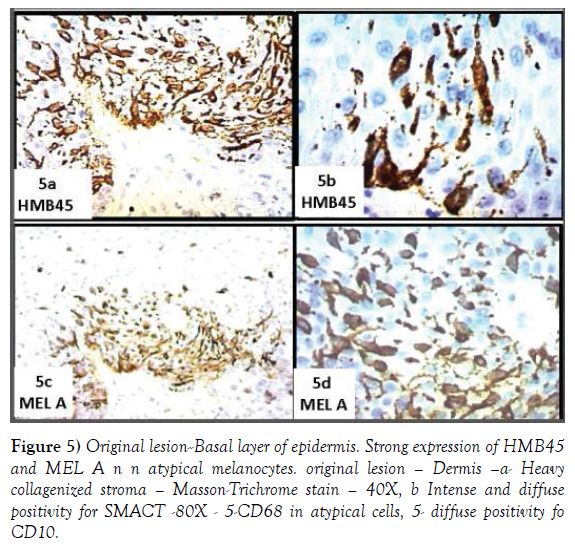
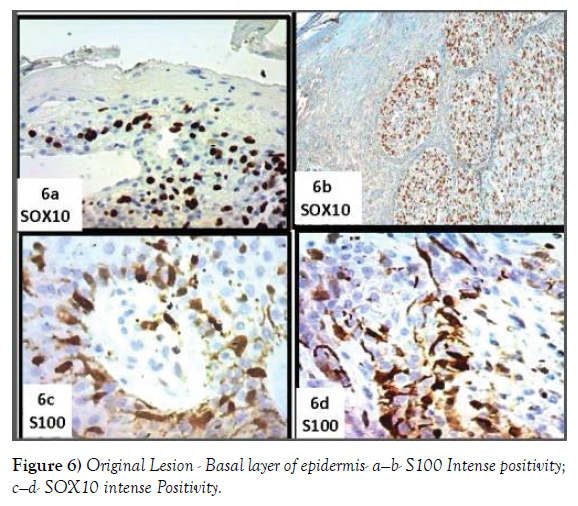
A re-evaluation of the very small residual part of the epidermal lining, of the original lesion, showed, at basal layer, the presence of bulky, roundish elements, irregularly arranged, with hyperchromatic and dysmetric nuclei (Figure 4c and 4d). Immunohistochemistry shows that these typical cells express intensely, Hmb45, MEL A (Figure 5a-5d), SOX10, S100 (Figure 6a-6d). These elements frequently exhibit extensions between the epithelial epidermal cells. The latter aspect is particularly evident at the level of the annexes.
Figure 5: Original lesion--Basal layer of epidermis. Strong expression of HMB45 and MEL A n n atypical melanocytes. original lesion – Dermis –a- Heavy collagenized stroma – Masson-Trichrome stain – 40X, b Intense and diffuse positivity for SMACT -80X - 5-CD68 in atypical cells, 5- diffuse positivity fo CD10.
At dermal level, this further evaluation showed, mixed to cells expressing mesenchymal antigens, atypical cells of oval shape with hyperchromatic and dysmetric nucleus expressing intensely and diffusely SOX10 (Figure 3d) and P75. Negative Fact XIIIa
Following the revaluation the lesion was renamed as Desmoplastic Melanoma in Lentigo Maligna
2) The sample of the “recurrent” lesion of roundish shape and in size of 3 x 3 x 3 cm, fleshy, translucent to the pinkish section. It is covered with highly ulcerated skin.
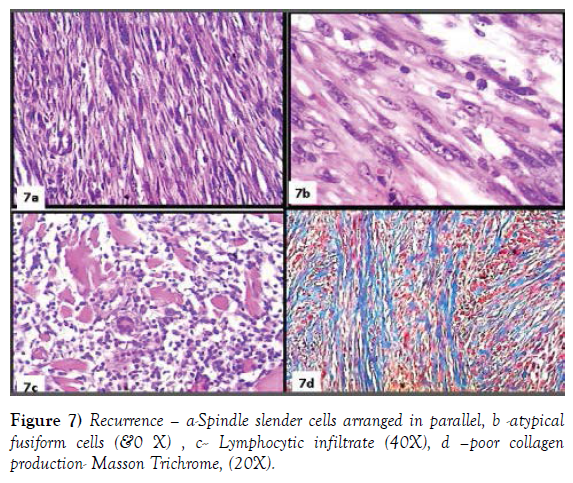
The tissue is made up of a slender proliferation of spindle elements, with the elongated nucleus and weakly eosinophylic cytoplasm. The elements tend to arrange in parallel bundles (Figure 7a). These elements are mixed with a second cellular population, consisting of more voluminous cells, with irregular oval hyperchromatic, and dysmetric nucleus (Figure 7b). A nonnegligible lymphoid component permeates the tissue forming on peripheral surface follicular aggregates (Figure 7c). Trichrome staining shows only a modest stromal collagenization (Figure 7d).
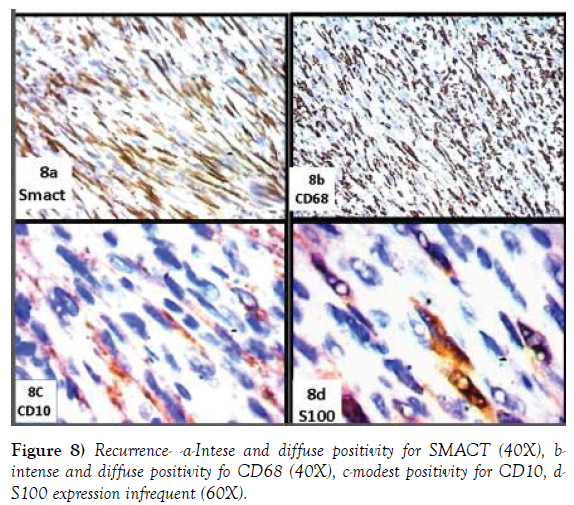
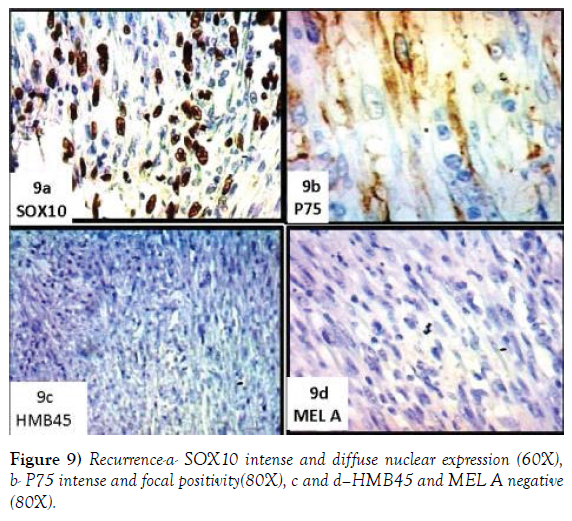
The two cellular populations show a different immunophenotypic profile. The former diffusely expresses SMACT (Figure 8a), CD68 (Figure 8b), CD10 (Figure 8c and 8d) and focally Calponin; the second express focally S100 (Figure 8d), diffusely SOX10 (Figure 9a) and focally P75 (Figure 9b). LCA decores the ymphoids aggregation, absolutely absent HMB 45 and MEL A (Figure 9c and 9d), Ki67 is valued >30%.
3) The surgical sample of axillary lymphadenectomy consists of a multi-lobed tissue fragment about the size of 3.5 cm x 5 x 2. The section surface is of homogeneous appearance, of pink color, translucent.
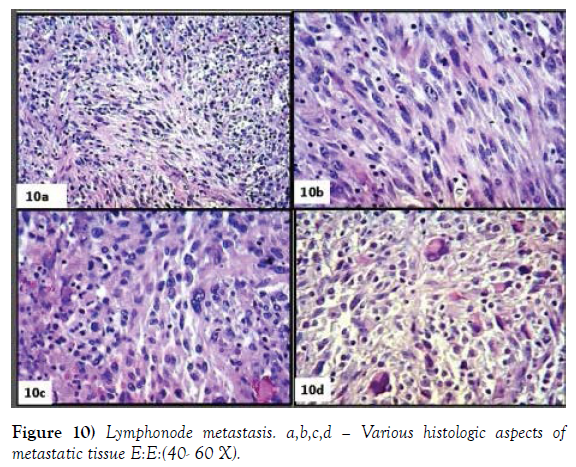
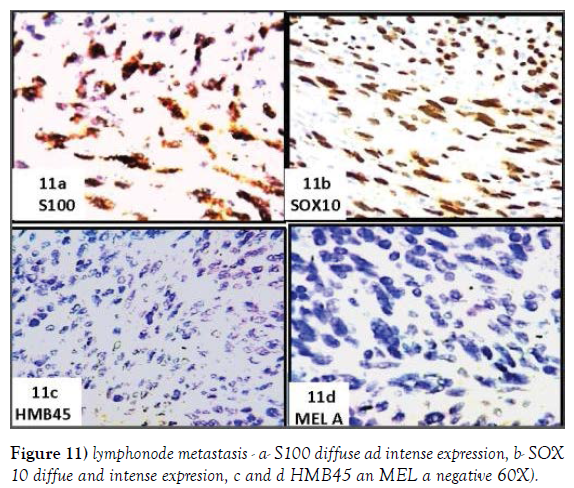
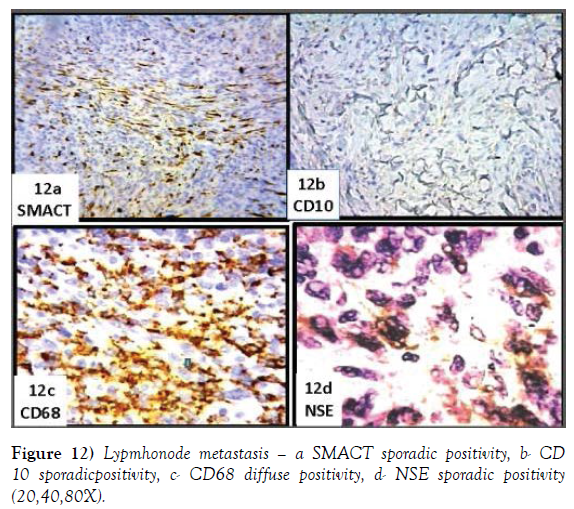
The nodal structure that virtually disappeared is preserved only in a narrow marginal area. The remaining tissue consists of a disordered and an atypical proliferation of spindle cells, with a hyperchromatic nucleus, sometimes monstrous and multiple, often showing a brisk mitotic activity. No desmoplasia is evident. The tissue is permeated by a widespread dispersion of lymphoid elements (Figure 10a-10d). Immunohistochemistry shows intense and widespread positivity for S100 (Figure 11a), SOX10 (Figure 11b), negative HMB45 and MELA (Figure 11c and 11d), very sporadic for SMACT (Figure 12a) and CD10 (Figure 12b) diffuse CD68 (Figure 12c), focale NSE (Figure 12d).
The histopathologic diagnosis was a nodal metastases of spindle cell melanoma -S100+, SOX10+, HMB45 and MELA negative.
Table 2a and 2b summarizes the immunophenotypic profiles of the various lesions (Table 2a).
P=Primitive, e=Epidermis, d=Dermis, R=Relapse, M=Metastasis (Table 2b).
Table 2A and 2B: Summarizes the immunophenotypic profiles of the various lesions.
| S100 | HMB45 | MELA | SOX10 | P75 | CD34 | CD68 | DESM | Smact | MSA | CALP | VIM | LCA | CD10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pe | + | + | + | + | ND | - | - | - | - | - | - | - | ND | - |
| d | + | - | - | + | + | + | - | + | +- | + | + | + | ||
| R | + | - | - | + | + | + | + | +- | + | +- | + | + | + | + |
| M | + | - | - | + | + | - | + | - | -/+ | - | - | + | ND | -/+ |
| A | CD3 | CD5 | CD20 | GD21 | CD23 | CD38 | CD99 | CD117 | EMA | ALK | FATTXIIIa | KI67 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pe | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | - | ND |
| d | - | - | ||||||||||
| R | + | + | . | - | - | - | - | - | - | - | ND | >20% |
| M | ND | ND | ND | ND | ND | ND | ND | ND | - | ND | ND | >20% |
Discussion
The Desmoplastic Melanoma (DM) was described for the first time in 1971 by Conely and Coll, as a rare variant of malignant melanoma [1]. The description of the lesion and the clinical pathology considerations drawn by the Authors are still the most complete and exhaustive, to deserve their full citation: “That Malignant Melanoma many assume varied histologically and the clinical patterns are widely recognized. The lesion described here as Desmoplastic malignant melanoma is a rare and hitherto not described variant of spindle cell melanoma; it arises from a superficial melanocytic lesion and evolves into an aggressive, infiltrating, and potentially metastasizing tumor characterized locally by large subcutaneous tumefactions of fibrous appearance. The clinical and microscopic appearances of this apparently new pathologic entity may suggest benign fibrosis, invasive fibromatosis, or fibrosarcoma. The outcome is usually fatal, resulting from widespread dissemination”…….“We have to conclude that this peculiar clinicopathologic sequence of events which, starting from an inconspicuous superficial malignant melanoma leads to an invasive and metastasizing desmoplastic spindle cell tumor with fibromatosis-like features, is a spindle cell melanoma. The mechanism of the fibroplasia remains unexplained. There is at least a theoretical possibility that collagen can be produced directly by neoplastic melanocytes, as suggested by the experimental work of Green et al. We believe that it is most important for the practicing pathologist and surgeon to be aware of the fact that if in the history of a stubbornly recurrent “fibroma” or “fibromatosis,” there is an associated or antecedent inconspicuous pigmented lesion of the overlying skin, they may well be dealing with this rare variant of malignant melanoma”.
In addition to the original description, there are data in the presence of an infiltration of lymphoid elements in the context of desmoplastic proliferation, until the formation of nodular aggregates in peripheral areas [2-4]. Clinically, it remains a fact that the lesion prefers subjects in old age, It is usually situated in head and neck, is very often devoid of pigment and is presented as an infiltrated hard lesion with the appearance of fibroids. Local recurrence is very frequent. These clinical features correlating with morphological patterns have been in the past, and today, still the cause of frequent erroneous diagnosis of neoplasias and proliferation, usually benign of a fibrohistiocytic type.
Since the first reports have been reported, a very frequent association with an atypical melanocytic intraepidermal component, type lentigo maligna, considered by some precursor, and any other as a particular variety of melanoma in situ, susceptible to evolve in the tumorigenic infiltrative phase called lentigo maligna melanoma [5,6]. The tumorigenic component is usually represented by a spindle cell melanoma. From the literature, the tendency to consider DM a Spindle Cell Melanoma variant is evident (SCM) [7,8]. Recent research, to which we will refer most widely later, would demonstrate the clear difference between these two lesions-
Of DM if they describe two variants: one called “Pure” and ‘the other “Combined or Mixed”, when the desmoplastic component is associated with other conventional type [9-12].
The discussion on the origin and significance of desmoplasia intersects with that of the histogenesis of this peculiar neoplasia. From the first description, the Authors are faced with this issue. In fact, even if in hypothetical terms, they favored the possibility that the production of collagen would be performed by the same neoplastic melanocytes [1]. In 1979 Valensi in an ultrastructural research points out.” On the basis of evidence presented, it is concluded that the cells are dedifferentiated cells of the malignant melanoma with fibroblastic features and probably fibroblastic functions rather than host-engendered fibrobasts in response to invasive melanoma” [13]. Subsequent research, again on the ultrastructural basis would confer schwannian characteristics to desmoplastic cells [14]. Other authors, always on the basis of ultrastructural research, believe that “the desmoplastic component of these malignant melanomas derives from melanocytes have undergone adaptive fibroplasia” [15]. Analog hypothesis, however, had already been advanced in Conley’s report, [1]. It is now believed by most workers that the proliferating malignant spindle cells undergo a progressive metaplasia, with differentiation toward fibroblastic, myofibroblastic or even schwannian cells [16].
The introduction of the immunohistochemical investigation has allowed further progress in the knowledge of this neoplasia. Numerous publications were dedicated to this topic [17-20].
From the literature, it is apparent that lentigo maligna may generate two different tumorigenic cell clones: a conventional one from which originate the lentigo maligna melanoma> Spindle cell melanoma (SCM), expressing the specific melanocytic antigens (HMB45, MELA) characteristics of conventional melanoma, and another, dedifferentiated, expressing only the neuroectodermal antigens such as S100, SOX10, P75 and not those specifically melanocytic as HMB4 and MELA. From this second clone would originate the DM whose immunophenotypic profile is characterized by the expression of neuroectodermal antigens and by the absence of melanocytic markers [21-25]. This immunophenotypic anomaly, of course, contributes to further increasing the already high risk of diagnostic error.
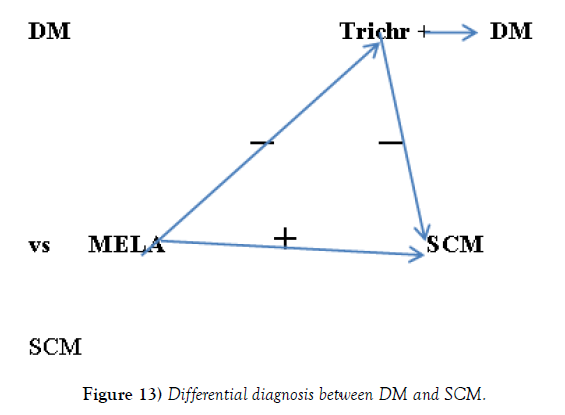
For the differential diagnosis between DM and SCM algorithm (Figure 13).
In the same research was highlighted Braf mutations in 31% of spindle cell and 5% of desmoplastic melanoma, with an overall mutation frequency of 16% (n ¼ 6/38) [26].
Also reported in the desmoplastic proliferation is the presence of heterologous elements SMACT’+, Desmin+ and CD 68+ [27-29].
The case of our observation has all the clinical, morphological, and immunophenotypic characteristic of a pure DM, including that of not being diagnosed at the first assessment.
In the original lesion, the intraepidermal melanocytic proliferation, of freckled appearance, shows the immunophenotypic expressiveness of a conventional lentigo maligna: HMB45+, MELA+, S100+, SOX10+, P75+.
The desmoplastic dermal proliferation, shows a somewhat different immunophenotypic profile, lacking the expression of the melanocytic markers (HMB45 and MELANA) with a widespread and intense expressiveness of SOX10 and of modest S100. Moreover, there is presently no negligible number of elements positive for SMACT, CD68, and CD10. The proliferation is associated with an intense production of collagen, well documented with Trichrome stain.
From these observations it has emerged that: the neoplastic cell clone of the intradermal tumorigenic proliferation is immunophenotypically different from that of the intraepidermal fraction. This cell clone is considered to be dedifferentiated because it lacks the specific melanocytic antigens and expresses only S100 SOX10 and P75 which are present in most of the neurocristic derivatives. The non-neuroectodermal component has a pleomorphic composition of mesenchymal elements who express Vimentin, SMACT, CD10, CD68.
The morphological pattern and the immunophenotypic profile of the latter component is overall fasciitis-like and may be compared to that of a group of lesions that goes under the umbrella definition of “Pseudosarcomatous Myofibroblastic Proliferations” (PMPs) [30,31], whose immunophenotypic profile is characterized by positivity for vimentin and usually for smooth muscle actin, muscle actin, CD10, Smooth muscle myosin, CD68 [32]. PMPs Includes the Nodular Fasciitis [33] in its various expressions (Proliferative Fasciitis, Proliferative Myositis, and Ossificans), lesions from time to time labelled under various designations such as Visceral Fasciitis, Pseudosarcomatous Fibromyxoid Tumor, Spindle Cell Pseudomalignant Proliferation, Postoperative Spindle Cell Nodule (PSCN) [34-37], and Inflammatory Pseudotumor. The extensive nomenclature attributed to these lesions is due to the great variability of clinical presentation, although there are subtended morphological pattern and their immunophenotypic profile is quite constant. These lesions most often occur in the genitourinary system (bladder, prostate, ureter, vagina, and vulva) [38,39] but can occasionally arise in the gastrointestinal tract or in the organs of the upper aerodigestive tract (pharynx, larynx, nasal cavities, and mouth). Proliferations of this type have been known for a long time, in the form of polyploid vegetations, associated with epithelial neoplasms, squamous type, usually in situ or superficial and ulcerated, with preferred locations in the high aero-digestive tract, but also in other districts. We ourselves have been for some time and on several occasions interested in the study of this type of lesions [40-43].
In our patient, the recurrence, in the form of a small nodule at the suture scar, was noted, about 10 days after surgery. The mass was growing rapidly and, when it was removed about 8 weeks later, had a diameter of 3 cm, Initially, the lesion was interpreted as a suture granuloma, which resulted in a delay in its removal.
The morphological pattern of the recurring lesion appears somewhat different from the primitive. The proliferated tissue is more cellularized and much less collagenized, consisting of spindle thinner elements, with an elongated nucleus and tend to be arranged in parallel bundles. These cells express extensively and intensively SMACT and CD10, CD68, Desmin and focally Calponin. Mixed with these elements are present, spindle cells, plumper with a voluminous and hyperchromatic clearly atypic nucleus. Such elements express S100, SOX10, P75, and CD68. This lesion, therefore is composed of two different cell populations: a first, formed by spindle thinner cells, not atypical, that express VIM, SMACT, CD10 and CD68, a second substantially neoplastic constituted by atypic elements expressing S100, SOX10, P75, CD68. Expressivity for Cd68, as well as in PMSs, is also reported in 90% of cases of conventional melanoma and DM [44]. So the recognition of its expressiveness in both cellular populations is entirely compatible. Absolutely not expressed are HAMB45 and MELA.
The immunophenotypic profile of the first cell population, even in recurrence, is characteristic of that lesion group called PMPs. For its morphology and, primarily, for its clinical presentation should be labeled as Spindle Cell Postoperative Nodule (PSCN)” [34]. This lesion, in fact, occurs rapidly, on the order of a few weeks, from surgery or trauma and presents a morphological pattern very similar to that found in our case.
Lymph node metastasis consists of a massive proliferation of spindled elements expressing intense and diffuse S100, SOX10, and CD68. HMB45 and MELA are completely unexpressed. As well as are completely absent elements expressing mesenchymal antigens. This confirms the non-neoplastic nature of the desmoplastic component.
Although in our observation there are no alterations of this type, it is appropriate at this point of the discussion to examine, albeit briefly the phenomenon of the neurotropism that we have deliberately previously left out.
Not long after the reporting of this morbid entity was added the finding of its tendency to neurotropism [45]. Consisting of the tumor cell infiltration of the nerve structures of the region. This phenomenon in literature has been mainly used for prognostic evaluations, while it is very significant for the purposes of an istogenetic interpretation. Beyond all argument, we just observe the way to penetrate and be disposed of neoplastic cells around the nerve fibers to be convinced of their neurilemma attitude. Numerous studies have been devoted to this phenomenon with almost a unanimous conclusion on the schwannian vocation of this cell clone [14,28,46].
In addition to these data, the literature reports a series of reports of variously composed hybrid lesions difficult to classify: DM micking PNST, Sarcomatoid melanoma, neural transforming melanoma, They are incomplete or poorly defined derived from neurocrest [47-50].
Conclusion
In order to draw some conclusions on the histogenesis of this Tumor, it seems quite clear that the key process is the appearance, generated by a lentigo maligna precursor, of a dedifferentiation, in the schwannian sense, tumorigenic clone. This cell clone triggers, in the cutaneous dermis, a myofobroblastic differentiation and a proliferation, PMPs type, that goes to constitute the desmoplastic component of the tumor. PMPs- tumorrelated, has been reported multiple times in superficial ulcerated epithelial mucosal tumors, whereas, so far, it had not been considered in relation to melanocytic neoplasms. That this may occur, it is necessarily a factor relating to the characteristics of the neoplastic cells. The proof of the reliability of this statement lies in the fact that not all ulcerated superficial squamous carcinomas give rise, in the surrounding stroma, to a pseudosarcomatous proliferation, as not all Lentigo Maligna results in a DM, but only when it generates a dedifferentiated tumorigenic clone.
This interpretation, based on a paraphysiologic phenomenon such as that of the myofibroblastic paraneoplastic proliferation, is much more acceptable than the very suggestive metaplasias of melanocytes that become fibroblasts and vice versa. The absence of PMP in the nodal metastases confirms the non-neoplastic nature of the desmoplastic proliferation.
In our case, while in the primitive lesion PMP is fasciitis –like, in the recurrence the PMP presents the clinical and morphological characteristics of a PSCN, probably triggered by surgical trauma, if we take account of its extremely rapid clinical presentation, as it occurs in this type of lesions.
Where to place the DM on the nosography, it is not easy. Considering it as a simple Melanoma variant, on the basis of the data at our disposal, this seems to be reductive. It is a complicated histogenesis tumor because it is based on not well-defined cell clone, certainly not fully melanocytic nor completely schwannian, capable of triggering a paraneoplastic proliferation in the surrounding mesenchyme. It is one of those unfinished creatures of the archipelago of neurocrest derivatives.
In our opinion, the DM is to be considered a Tumor-related Pseudo sarcomatous (PMP), myofibroblastic proliferation, in which the tumoral component is represented by a dedifferentiated malignant melanocytic proliferation. Together with Lane’sTumors could constitute, within PMP, a sub-group called PMP-TR (Tumor-Related)
These speculations have not only attempted to give a more rational interpretation of DM histogenesis and its nosographic placement. It has also shown that PMPs are a paraphysiological phenomenon, which if searched and recognized, can be identified in the most varied pathological situations. The protagonist of this phenomenon is the Myofibroblast, a strange cell not present in healthy tissue, from the obscure origin claimed by several cell lineages (fibroblasts, smooth muscle cells, pericytes and endothelial cell) more recently, bone marrow-derived circulating fibrocytes (BMDCF) and Epithelial-to-mesenchymal transition (EMT), present in various pathological situations: reparative, reactive, paraneoplastic, neoplastic benign, and malignant [51]. Our observation would demonstrate that PMPs–tumorrelated can also occur in other types of tumors, beyond those of the squamous, superficial ulcerated carcinoma type, so far reported.
The study of this case, both in primary lesion and in local recurrence, demonstrates how the clinical presentation is the result of several associated and overlapping pathologic processes, which should be acknowledged and evaluated for a proper management of the morbid event.
The best conclusion seems to be saying with Patterson “This entity is at high risk for future reclassification [52].
Acknowledgements
In memory of my never forgotten mentor Raffaele Lattes, who first described this peculiar neoplasm.
Conflict of Interest
There is no conflict of interest
REFERENCES
- Conley J, Lattes R, Orr W. Desmoplastic Malignant Melanoma (a rare variant of spindle cell melanoma). Cancer 1971;28:914-36.
- Bruijn JA, Mihm MC, Barnhill RL. Desmoplastic melanoma.Histopathology 1992;20:197-205.
- Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol 2006;19: S41-S70 .
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic Melanoma: A Review. J Am Acad Dermatol 2013;68:825-33.
- Flotte TJ, Mihm MC. Lentigo maligna and malignant melanoma in situ, lentigo maligna type. Hum Pathol 1990;21(12):1199-201.
- Tannous ZS, Lerner L H, Duncan LM,et al. Progression to Invasive Melanoma From Malignant Melanoma In Situ, Lentigo Maligna Hum Pathol 2000;31:705-8.
- Thelmo MC, Sagebiel RW, Treseler PA, et al. Evaluation of sentinel lymph node status in spindle cell melanomas. J Am Acad Dermatol 2001;44:451-5.
- Hawkins WG1, Busam KJ, Ben-Porat L, et al. Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol 2005;12(3):207-13.
- de Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: A clinicopathologic analysis of 113 cases. Am J Dermatopathol 2008;30:207-15.
- Busam KJ, Mujumdar U, Hummer AJ, et al. Cutaneous desmoplastic melanoma: reappraisal of morphologic heterogeneity and prognostic factors. Am J Surg Pathol 2004;28:1518-25.
- Busam KJ. Cutaneous desmoplastic melanoma. Adv Anat Pathol 2005;12:92-102.
- George E, McClain SE, Slingluff CL, et al. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol 2009;36:425-32.
- Valensi QJ. Desmoplastic malignant melanoma: study of a case by light and electron microscopy. J Dermatol Surg Oncol 1979;5:31-5.
- Warner TFCS, Hafez CR, Finch RE, et al. Schwann cell features in neurotropic melanoma. J Cutan Pathol 1981;8:177-87.
- From L, Hanna W, Kahn HJ, et al. Origin of the desmoplasia in desmoplastic malignant melanoma. Hum Pathol 1983;14:1072-80.
- HM, Goellner JR, Woods JE, et al. Desmoplastic melanoma of the head and neck. Cancer 1987;60:2269-74.
- Argenyi Z, Cain C, Bromley C, et al. S-100 protein negative malignant melanoma: fact or fiction? A light microscopic and immunohistochemical study. A J Dermatopathol 1994;16:233-40.
- Longacre TA, Egbert BM, Rouse RV. Desmoplastic and spindle-cell malignant melanoma. An immunohistochemical study. Am J Surg Pathol 1996;2:1489-500.
- Shah IA , Gani OS, Wheler L. Comparative immunoreactivity of CD-68 and HMB-45 in malignant melanoma, neural tumors and nevi Pathol Res Pract 1997;193:497-502.
- Ferringer T. Immunohistochemistry in Dermatopathology Arch Pathol Lab Med 2015;139:83-105.
- Tacha D, Yu C. An Immunohistochemical Comparison Study of SOX10, Pan Melanoma Cocktail and S100 in Malignant Melanoma. Biocare Medical, Concord.
- Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 2008;32:1291-8.
- Lazova R, Tantcheva-Poor I, Sigal AC. P75 nerve growth factor receptor staining is superior to S100 in identifying spindle cell and desmoplastic melanoma. J Am Acad Dermatol 2010;63:852-8.
- Kanik AB, Yaar M, Bhawan J. p75 nerve growth factor receptor staining helps identify desmoplastic and neurotropic melanoma. J Cutan Pathol 1996;23(3):205-10.
- Karamchandani JR, Nielsen TO, Van de Rijn, et al. Sox10 and S100 in the Diagnosis of Soft-tissue Neoplasms. Appl Immunohistochem Mol Morphol 2012;20(5):445-50.
- Weissinger SE , Keil P , Silvers D N, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol 2014;27:524-34.
- Banerjee S, Bishop P, Nicholson C, et al. Malignant melanoma showing smooth muscle differentiation. J Clin Pathol 1996;49:950-1.
- Carlson JA, Dickersin GR, Sober AJ, et al. Desmoplastic neurotropic melanoma. A clinicopathologic analysis of 28 cases. Cancer 1995;75:478-94.
- Riccioni, Luca, Di Tommaso, et al. Actin-Rich Desmoplastic Malignant Melanoma: Report of Three Cases. Am J Dermatopathol 1999;21:537-41.
- Albores-Saavedra J, Manivel JC, Essenfeld H, et al. Pseudosarcomatous myofibroblastic proliferations in the urinary bladder of children. Cancer 1990;66:1234-41.
- Ro JY, el-Naggar AK, Amin MB, et al. Pseudosarcomatous fibromyxoid tumor of the urinary bladder and prostate: immunohistochemical, ultrastructural, and DNA flow cytometric analyses of nine cases. Hum Pathol 1993;24:1203-10.
- Miettinen M. Modern Soft Tissue Pathology. Cambridge University Press 2010;185).
- Filotico M, Bernardini E. Pseudo-Sarcomatous Subcutaneous Fibromatosis (Nodular Fasciitis). Anatomoclinical Study Based On 4 Personal Cases). Riv Anat Patol Oncol 1963;14:21-36.
- Proppe KH, Scully RE, Rosai J. Postoperative spindle cell nodules of genitourinary tract resembling sarcomas. A report of eight cases. Am J Surg Pathol 1984;8:101-8.
- Carla TG, Filotico R, Filotico MV. Postoperative spindle cell nodules (PSCN) resembling sarcomas: an atypical granulation tissue or atypical pyogenic granuloma with exuberant myofibroblastic component?”. Pathologica 1990;82:279-286.
- Wick MR, Mills SE, Ritter JH, et al. Postoperative /Posttraumatic Spindle Cell Nodule of the Skin. Am J Dermatopathol 1999;21:220-4.
- Garijo MF, Val-Bernal JF, Vega A, et al. Case Report .Postoperative spindle cell nodule of the breast: Pseudosarcomatous myofibroblastic proliferation following endo-surgery. Pathology International 2008; 58:787-91.
- Stark GL, Feddersen R, Lowe BA, et al. “Inflammatory pseudotumor (pseudosarcoma) of the bladder”. Send to J Urol 1989;141:610-2.
- Harik LR, Merino C, Coindre JM, et al. “Pseudosarcomatous myofibroblastic proliferations of the bladder: A Clinicopathologic Study of 42 Cases”. Am J Surg Pathol. 2006:30;787-94.
- Lane N. “Pseudosarcoma (polypoid sarcoma-like masses) associated with squamous-cell carcinoma of the mouth, fauces, and larynx; report of ten cases.” Cancer 1957;10:19-41.
- Filotico M, Filotico R. “Lane’s type pseudosarcoma of glans penis. A case report,” Pathologica. 2017;109:114-9.
- Filotico M, Trabucco M. “Contribution to the knowledge of so-called ‘pseudosarcoma’ associated with laryngeal carcinoma”. Tumori 1966; 52:295-301.
- Filotico M, D’Amuri A. Polypoid Carcinoma of the Oropharynx with Stromal Ossifying Myofibroblastic Proliferation: A Case Report and Literature Review. Case Report in Pathology 2016;ID 2540407:1-7.
- Shah IA , Gani OS, Wheler L. Comparative immunoreactivity of CD-68 and HMB-45 in malignant melanoma, neural tumors and nevi. Pathol Res Pract 1997;193:497-502.
- Reed RJ, Leonard DD. Neurotropic melanoma (a variant of desmoplastic melanoma). Am JSurg Pathol 1979;3:301-11.
- Quinn MJ, Crotty KA, Thompson JF. et al. Desmoplastic and desmoplastic neurotropic melanoma: experience with 280 patients. Cancer 1998;83:1128-35.
- Kiuru-M, McDermott G, BergerM, et al. Desmoplastic Melanoma with Sarcomatoid De-differentiation. Am J Surg Pathol 2014;38: 864-70.
- Machado I, Llombart B, Cruz J, et al. Desmoplastic melanoma may mimic a cutaneous peripheral nerve sheath tumor: Report of 3 challenging cases. J Cutan Pathol 2017;0:1-7.
- Smithers BM, McLeod GR, Little JH. Desmoplastic, neural transforming and neurotropic melanoma: A review of 45 cases. Aust NZ J Surg 1990;60:967-72.
- Kenji Yorita, Satoka Yoshii, Naoto Kuroda, et al. Jun Iwata A case of desmoplastic melanoma that was difficult to distinguish from malignant peripheral nerve sheath tumor. Human Pathology: Case Reports 2018;11:6-10.
- Eyden B, Banerjee SS, Shenjere P, et al. The myofibroblast and its tumours:a review. J Clin Pathol 2009;62:236-49.
- Patterson JW. Weedon's Skin Pathology-, fourth ed. Pp:1245.