The "Phenomenon of Life" in the behavior of the molecules of living matter
Received: 05-Dec-2023, Manuscript No. puljpam-23-6675; Editor assigned: 08-Dec-2023, Pre QC No. puljpam-23-6675 (PQ); Accepted Date: Jan 29, 2024; Reviewed: 02-Jan-2024 QC No. puljpam-23-6675 (Q); Revised: 07-Jan-2024, Manuscript No. puljpam-23-6675 (R); Published: 31-Jan-2024, DOI: 10.37532/2752-8081.24.8(1).01-05
Citation: Zhinzhiv G. The "Phenomenon of Life" in the behavior of the molecules of living matter. J Pure Appl Math. 2024; 8(1):01-05.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The properties of chains of biomolecules (amino acids, sugars, nucleic acids) are considered, geometrically investigating their structure in a space of higher dimension. It is shown that their movements and form are subject only to the conditions of their possible existence in given circumstances and are not subject to the imperatives set in advance from general considerations ("chiral purity").
We can say that the molecules of the chains of living matter prove by their existence the principle of the phenomenon of life.
Key Words
Chiral purity; Spatial image; Polarization; Nucleic acid; Amino -- acid molecules; Protein structures
Introduction
At the end of the 20th century, under the influence mainly of theworks of L. Pasteur, an idea was formed about the specialproperties of living matter. For example, in the work of N.F. Reimers we read: "L. Pasteur's law of chiral purity: living matter consists of chiral pure structures (chiral purity is the presence of only objects that are incompatible with their mirror image). Living proteins are built only from "left" amino acids (polarizing light to the left), nucleic acids are composed exclusively of "right" sugars, etc. Substances of non-biogenic origin are chiral symmetrical "left" and "right" molecules in them are equal in number. Chiral purity determines the specificity of the living, its irreducibility to the inanimate."
Similar views are repeated in the works of other authors. These ideas are based on a deep (unconscious) belief that the dimension of molecules cannot be more than three. However, in numerous works of the author, it has recently been proven that the dimension of biomolecules is more than three. In this regard, it is necessary to clarify what the phrase about the polarization of light to the right or to the left means, and how this is related to the structure of the corresponding molecules. To do this, one should refer to the initial experiments of L. Pasteur, taking into account the highest dimensionality of molecules. In addition, since the movement along the chain of biomolecules is usually accompanied by a rotation of the molecules at a certain angle, the possibility of rotation of the molecules in these movements should be considered, choosing to study the chain of amino acid molecules, the chain of sugar molecules and the chain of nucleic acid molecules, taking with account they dimension. This study is the subject of this work [1-6].
Spatial images of the molecules wine acid
The molecules of the wine acid are in two forms (L and D). The images obtained make it possible to explain the main property of tartaric acid-rotation of the plane of polarization of the incident light in different directions: in the case of the D form to the right, in the case of the L form to the left. It is a known device for rotating the plane of polarization of light, having the appearance of two folded triangular prisms, the boundary between which serves to reflect light. Can be said that the molecule of tartaric acid is a natural device for rotating the plane of light polarization. Two carbon atoms into structure molecules play the role of the reflecting partition in the molecule. The rotation occurs in the forms D and L in different directions because of the opposite arrangement of the charges of the hydrogen ions H (+) and the hydroxyl group OH (-) in these forms. Thus, the reason for the different rotation of the plane of polarization of light lies not in the different forms of the crystals of D -tartaric acid and L-tartaric acid, as Pasteur suggested, but in differentforms of molecules, clearly visible in the image in the space 5D. Itshould be noted that a characteristic feature of tartaric acidmolecules, which leads to rotation of the light polarization plane, ischaracteristic of organic compounds of course, organic compoundsare present in living matter, but organic compounds themselves arenot living matter. Therefore, it is incorrect to say that the rotation ofthe plane of polarization is an innate property of living matter.
States of amino acid molecules in protein structures
The simplest structure of a protein is a chain of amino acid molecules. Amino acid molecules exist in two enantiomorphic species L and D. The dimension of these forms is 4. The works were widely used to describe the chains of amino acid molecules. However, these works do not take into account the higher dimension of amino acid molecules, which leads to contradictions, and the methods developed in these works cannot be taken into account. Then the polypeptide chain of two molecules of L-amino acids, taking into account the four-dimensionality of amino acid molecules. From Linear polypeptide chain of L–molecules amino acids and figure of. Linear polypeptide chain of D–molecules amino acids, it can be seen that in order for the corresponding vertices of tetrahedrons with the center (i.e., specific atoms or functional groups) could lie on a system of parallel lines (i.e., form a linear polypeptide chain), it is necessary that the amino acid molecules in the chain alternate with their mirror image relative to the edge in the tetrahedron connecting the bond centers of the polypeptide chain (i.e., relative to the segment CO-NH). The chains differ by the mirror reflection of the molecules relative to the perpendicular to the segment CO–NH (L and D molecules). The translational symmetry of the circuits can be observed. Moreover, the elementary translational element in this case is a group of two linked amino acid molecules, one of which is a mirror image of another amino acid molecule relative to the segment. From these two linked amino acid molecules, you can create a convex polytope by connecting the edges of each vertex of any of the molecules with all other vertices in the group. This is how a simplex polytope of dimension 9 is formed, since the dimension of a simplex polytope is one less than the number of vertices in a simplex, and the number of vertices in two tetrahedra with a center is 10. Therefore, a polypeptide linear amino acid chain has translation symmetry, in which an elementary translation element is a simplex polytope of dimension 9.
The polypeptide chain can change its direction. This, in particular, is required in the formation of globular proteins. In this case, loops of the polypeptide chain are formed. Since the polypeptide chain, as we have seen, is a chain of polytopes of higher dimension, it is necessary to verify the possibility of such turns in the chain of polytopes of higher dimension, which are a chain of amino acid molecules. It shows a possible implementation of such a rotation. It is interesting to note that when the polypeptide chain is rotated, the enantiomorphic shape of the amino acid molecules changes.
This follows from the geometric analysis of the rotation presented. If the upper part of the polypeptide chain is considered as the initial one, then it is a sequence of D-molecules of the amino acid from left to right. When the chain is rotated, a polypeptide chain is formed, going from right to left (right turn), while the amino acid molecules look like L-molecules. If the lower part of the polypeptide chain is considered the initial one, then the chain is a sequence of L- molecules going from left to right. When turning in this case (left turn), the molecules take the form of D-molecules going in sequence from right to left. The change in the shape of amino acid molecules during the rotation of the polypeptide chain can be considered, in a sense, the justification for the existence of enantiomorphic forms of amino acid molecules.
Changes in the enantiomorphic forms of amino acid molecules during chain rotations have not previously been paid attention to. Thus, statements about the chiral purity of proteins in living organisms, which are characterized by the formation of globules with a turn of amino acid chains by 180°, are without foundation.
States of the saccharide molecules in the monosaccharide chains
As shown in the works, all monosaccharides with a closed cycle have the highest dimensions (monosaccharides furanoses have a dimension of 12, monosaccharides pyranoses have a dimension of 15). For example, a molecule of α –D -glucose monosaccharide has the form of a polytope in. Pyranose monosaccharide α –D –glucose. Based on this image, you can get a simplified three - dimensional image, taking into account all the features of the image of dimension 15.So, two images of the α –D -glucose β -anomer and α -anomerwere obtained.
Monosaccharide molecules combine with each other thanks to the combination of two hydroxyl groups with the release of a water molecule. The remaining oxygen atom connects the remaining part of the monosaccharides. The most common chains of α -D -glucose monosaccharide residues. Here, residual α - D - glucose molecules can be joined through an oxygen atom, connecting carbon atoms 1 -1, 1 -4, 4 -4, 4 -1. In some cases, the carbon atom C(2) in the cycle will be involved in the compound. Branching chains of glucose molecules occurs at the sixth carbon atom in the functional group CH2OH. Monosaccharide chains have different forms depending on the type of these molecules, i. e. depending on whether these molecules are - anomers or α - anomers. Using the obtained simplified three - dimensional models of α - D - glucose molecules, we consider in more detail these compounds in a metric image, taking into account the angles between the valence bonds. Let two - anomers of the α -D -glucose molecule join together, linking the carbon atom C(1) of the first α -D -molecule to the carbon atom C(4) of the second α -D -molecule (lactose).
‘It is easy to see that the same attachment of the third α -D -glucose molecule leads to a linear arrangement of molecules when viewed from above on this compound. Linearity will not change with the following similar connections. However, given the three - dimensionality of the image, you should look at this connection from a different view. Projections of anomer disaccharides shows an image of the junction of two α - D - glucose molecules when viewed from the front. The black segments in Projections of anomer disaccharides are the traces of the intersection of the upper bases of the prism with a plane passing through the valence bonds of the oxygen atom and carbon atoms. According to the obtained solution, the angle between the base of the prism and the valence bond is 36°. both the first α -D -glucose molecule and the second α -D -glucose molecule. The valence angle at the oxygen atom in compounds with two carbon atoms in the chain of α -D - glucose molecules, as you know, is slightly larger than the normal valence angle and is about 112°. Thus, the angle 1ÃÂ?2 in. Projections of - anomer disaccharides is 176°. This means that the base of the second α -D - glucose molecule has a slope to the base of the first α -D -glucose molecule other than zero. More precisely, this slope is 4°, therefore the second α -D -glucose molecule, when viewed in full view, turns to the left relative to the first α -D -glucose molecule. It is clear that such an addition of a third α -D -glucose molecule will increase the angle of inclination of this molecule relative to the first molecule. Thus, the statement about the linearity of the chain of α -D -glucose molecules when connecting them in the case of anomers is not entirely accurate. One can say that this statement is somewhat one - sided.
Let two α - anomers of the α -D -glucose molecule join together, linking the carbon atom C(1) of the first α -D - molecule to the carbon atom C(4) of the second α -D -molecule (maltose). Based on the geometric image, you can imagine a top view of this connection.
It is easy to see that the same attachment of the third α -D -glucose molecule leads to a nonlinear arrangement of molecules when viewed from above on this compound. Nonlinearity will save with the following similar connections. Thus, the chain of molecules turns to the right. However, given the three - dimensionality of the image, you should look at this connection from a different view) shows an image of the junction of two α -D -glucose molecules when viewed from the front. The black segments in figures are the traces of the intersection of the upper bases of the prism with a plane passing through the valence bonds of the oxygen atom and carbon atoms. According to the obtained solution, the angle between the base of the prism and the valence bond is 36° both the first α - D - glucose molecule and the second α -D -glucose molecule. Thus, on the angle 1H C(1) is 36°and the angle C(1)OC(4) is 112°. Since the angle C(4)O2 is 36° too so the angle between bases 1H and O2 is 14°. This means that the base of the second α -D -glucose molecule has a consequent slope to the base of the first α -D -glucose molecule to the left. It is clear that such an addition of a third α -D -glucose molecule will increase the angle of inclination of this molecule relative to the first molecule.
In the chains of - anomers, one of the projections is linearly transmitted along the chain, and the projection perpendicular to it along the chain along a curved line. In chains of α - anomers (spirals), it is observed that when moving along a chain of projections in mutually perpendicular planes, the pyranose molecules rotate in opposite directions. Thus, chains of α - anomers of α -D -glucose molecules rotate simultaneously in opposite directions in perpendicular planes.
Formation of "right" and "left" nucleic acids
Nucleic acid is a chain of D –ribose (α - D - ribose or 2 – deoxy – D – ribose. Further analysis refers to the case α - D – ribose. In the case 2 –deoxy – D –ribose of an analysis similar to, it is given in molecules between which are the phosphoric acid residues that connect these molecules. Let us construct this circuit, taking into account the concept of repulsion of electron pairs. According to this concept, tetrahedral coordination of atoms and functional groups in these compounds is carried out around the phosphorus atom in the phosphoric acid residue, and in the carbon atoms in the D -ribose molecule also. It is this concept that leads to the higher dimensionality of the remainder of phosphoric acid and the molecule of D -ribose. When a phosphoric acid residue is added to the D - ribose molecule from the hydroxyl group of the phosphoric acid residue and the hydroxyl group of the D - ribose molecule, a water molecule that leaves the compounds forms and an oxygen atom that binds the phosphoric acid residue and the D - ribose molecule. The concept of repulsion of electron pairs also operates here, according to which two valence electrons of the oxygen atom maximally repel each other, forming electron pairs with electrons of the phosphorus atom and the carbon atom. Therefore, taking into account that tetrahedral coordination is carried out around the atoms of phosphorus and carbon already, it can assume that the valence bonds of the oxygen atom are located linearly with respect to the oxygen atom. When the second D - ribose molecule is added to the phosphoric acid residue, the second hydroxyl group of the phosphoric acid residue with the compound CH2OH of this D - ribose molecule also forms molecules of water that leaves the compound and an oxygen atom connecting the phosphorus atom and the functional group CH2 of the D - ribose molecule. By virtue of the concept of repulsion of electron pairs, the linear arrangement of the valence bonds of the oxygen atom can be considered here also. In addition, as the first step within the framework of the notions of the functional dimension of molecules, can shall assume the linear arrangement of the valence bonds also in the neighborhood of the functional group CH2 of the D - ribose molecule. One will take into account this when constructing a chain of D - ribose molecules and phosphoric acid residues. To construct the sequence of D - ribose molecules and the phosphoric acid residue in the chain can will use a simplified image of the D - ribose molecule of dimension 12. Let us leave only the outer contour and valence bonds necessary for interaction with the environment from the D - ribose molecule. At the same time, one will not depict the hydrogen atoms inside the contour of carbon atoms. We approximately to consider that the lengths of chemical bonds carbon - carbon, carbon - oxygen close to each other. The simplified images of the D – ribose molecules of the enantiomer form A and of the enantiomer form B under specific conditions.
The absence of edges and atoms in Figures the enantiomer B of D - ribose molecule in chain of DNA does not mean that they really are not. This is only the conventionality of the image, the full image these molecules of dimension 12. It should be noted that under the assumed projection conditions, the D - ribose of molecules on a two - dimensional plane in which a closed carbon ring is located ha one - sided s a number of properties that are convenient for further constructions. The enclosed carbon ring is a regular pentagon with an internal angle at the vertices 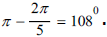 The angles in the three trapezoids formed by the valence bonds and the outer contour, because of the symmetry and parallelism of the bases, are 54° and 126°. In two obtuse - angled isosceles triangles formed by valence bonds and the outer contour, the angles are 126° and 27°.
The angles in the three trapezoids formed by the valence bonds and the outer contour, because of the symmetry and parallelism of the bases, are 54° and 126°. In two obtuse - angled isosceles triangles formed by valence bonds and the outer contour, the angles are 126° and 27°.
Now, in a projection onto a two - dimensional plane, one shall depict a sequence of the simplified images of D -ribose molecules and phosphoric acid residues, taking into account the separation of water molecules at the junction of phosphoric acid residues and D - ribose molecules and the linear arrangement of the valence bonds in vicinity of these compounds. In Structure of the nucleic acid molecule for D – ribose molecules in form A. In Structure of the nucleic acid molecule with sequence structure of the nucleic acid molecule with for D – ribose molecules in form B. The projection of the nucleic acid molecules depicted in structure of Two D –ribose molecules of the form B in the structure of the EB can be described mathematically.
The vertex b in this figure corresponds to the phosphorus atom, and the two edges emanating from it correspond to the projections of two valence bonds of the atom phosphorus with two oxygen atoms. They are at some angle γ on the projection. This angle characterizes the location of the residual phosphoric acid in space with respect to the D - ribose molecule B at the site of their connection (vertex k). It can be shown that the angle γ is linearly related to the angle between two neighboring nuclei in the spiral chain. Indeed, the smallest distance between two corresponding points of two neighboring D -ribose molecules B in the chain Δh corresponds to the segment OC. The smallest angle Δα characterizing the helical surface corresponds to the angle between the straight line that is the extension of the OC segment and the next segment CC1 between two molecules of D - ribose, originating from the vertex C. The angle 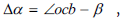 where
where 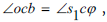 β is the angle between segment the helical surface corresponds to the angle between the straight line that is the extension of the OC segment and the next segment CC1 between two molecules of D - ribose, originating from the vertex C. The angle β = 54° - δ where 54° is the angle between edge OS and edge OK. The angle
β is the angle between segment the helical surface corresponds to the angle between the straight line that is the extension of the OC segment and the next segment CC1 between two molecules of D - ribose, originating from the vertex C. The angle β = 54° - δ where 54° is the angle between edge OS and edge OK. The angle 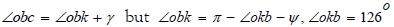 by construction, therefore
by construction, therefore 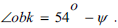 On the other hand,
On the other hand, 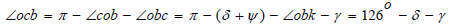 Therefore,
Therefore, 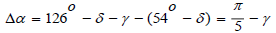
An amazing result was obtained: the rotation angle between two molecules of α - D - ribose in the chain depends linearly only on the angle between the projections of the valence bonds in the phosphoric acid residue, that is, the orientation of the phosphoric acid residue in the space with respect to the α - D - ribose molecule,

Concordantly with model of Watson and Crick, in period of nuclei chain included 10 nuclei. In this chain the angl Δα = 36o . Therefore, in this case, according to (1), the angle γ is also equal to 36°. If in the period of nuclei chain include 12 nuclei, so Δα =36o and γ = 42o.
It is possible to calculate the minimum distance between the corresponding points of neighboring α - D - ribose molecules in the nucleic chain Δh . It is understood that 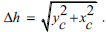
Where, Values yc,xc are from the system,
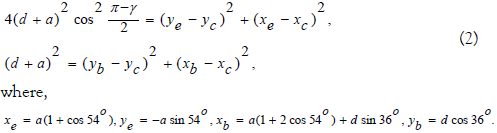
The first equation of system (2) can obtain from the equality of the square of the length of the segment ec to the sum of the squares of the differences of the coordinates of its vertices, on the one hand. On the other hand, the square of the length of the segment ec is the square of the doubled product of the cosine of the angle at the base of the isosceles triangle ecb by the length of the force side. The second equation of system (2) is obtained from the equality of the square of the side length cb to square of the sum of the segments a and d, on the one hand, and to the sum of the squares of the differences of the coordinates of its vertices, on the other hand. Solving system (2) for given a, d, one can find the value of Δh . To find the solution, the squares of the coordinate differences in the right - hand sides of the equations of system (2) are opened and the second equation is subtracted from the first. The linear connection of the coordinates of the vertex c is obtained. The equation of this connection is substituted into the second equation of system (2) and a quadratic equation is obtained for one of the coordinates of vertex c. Thus, for each value of the angle γ for given the values of a, d are the coordinates of the vertex c.
It is easy to verify that by mirroring the image with respect to the bisector bϑ of the angle γ passing through the vertex b, one can obtain an image of two coupled nucleotides corresponding to another enantiomeric form of nucleic acid. In this way, the calculation of the parameters of the nucleic acid structure according to the system (2) corresponds directly to two enantiomeric forms of the nucleic acid structure. In the beginning, in order to find out the properties of the solutions of the system (2) corresponding to possible nucleic acid structures, it can numerically study these solutions, arbitrarily varying the value of the angle γ, which is the defining parameter of this system. Note that in this study it confines ourselves to analyzing one chain of a nucleic acid molecule, although, as is known a DNA molecule consists of two or three chains of the DNA molecules connected to each other by hydrogen bonds. Such a connection of chains can be carried out in the future, relying on the obtained regularities of one chain.
Since, the lengths of chemical bonds between carbon - carbon and carbon - oxygen are ≈ 0.15 nm, can will adopt a = 0.15 nm. The length of chemical bond phosphor – oxygen can adopt d =0.18 nm. The results of calculating the characteristics of the nucleic acid structure from the equations of system (2) are shown in the numerical values of some parameters are given in (Table 1).
| Γ degree |
Δα degree |
N | Δh Nm | R Nm |
| 36 | 36 | 10 | 0,4677 | 0,7447 |
| 42 | 30 | 12 | 0,501 | 0,9575 |
| 64,5 | 7,5 | 48 | 0,607 | 46,397 |
| 72 | 0 | ∞ | 0,6394 | ∞ |
| 84 | 12 | 30 | 0,6815 | 32,557 |
| 90 | 18 | 20 | 0,7 | 2 |
| 102 | 30 | 12 | 0,7337 | 1,4 |
| 108 | 36 | 10 | 0,7639 | 12,163 |
TABLE 1 The characteristic dimensions of the right and left nucleic acid helices
This occurs up to an angle γ = 110°, at which the spiral turns into a closed ring since there is no rise of nucleotides with the help of phosphoric acid residues. In cases where the angle is greater than 72° Δα = γ - 72.
It can be seen from this figure that when γ is greater than 72°, the angle Δα between two adjacent segments is laid to the left of the initial segment. This leads to the rotation of the spiral to the left.
Then the radius of the nucleic acid helix is nm. The same nucleic acid radius was observed in experiments for 10 nucleotides in the period. This form of nucleic acid structure was designated B. From Table 1 can see that for γ = 42° the distance between nucleotides in structure nucleic acid Δh =0,5 nm. Wherein the number of nucleotides in the period is 12 and the radius of the spiral r =0,957 nm. Then the radius of the nucleic acid helix is r + Δr ≈ 1 , 35 nm. These data roughly correspond to the form of the nucleic acid structure, designated A. It is also known from experiments the existence of a nucleic acid structure with 12 nucleotides in a period with left rotation. This form of nucleic acid structure was designated Z. From Table 1 it follows that the value of the angle γ = 102° corresponds to this form. The existence of nucleic acid structures in the form of a closed ring has also been experimentally confirmed. This form of nucleic acid structure corresponds to the angle γ = 110° in (Table 1).
Thus, from the variety of solutions of the system (2) corresponding to the possible nucleic acid structures, four forms of the nucleic acid structure were experimentally confirmed. This does not mean that other forms of nucleic acid structure that correspond to other solutions of system (2) do not exist. It should be assumed that these solutions have not yet been found in experiments, and in the future, when creating the appropriate conditions, they will be found. The received decisions should be considered also at consideration of forms of structures of nucleic acid consisting of three connected spirals. In addition, must not forget that each solution of the system (2) corresponds simultaneously to two enantiomeric forms of the nucleic acid structure.
Conclusion
A geometric analysis of the behavior of molecules of living matter (amino acids, sugars, nucleic acids) in a higher-dimensional space has shown that their movements and shapes of molecules obey only the conditions of their possible existence, and do not obey the imperatives set in advance by someone. Thus, amino acids, contrary to the principles of chiral purity, can simultaneously coexist with both dextrorotatory (polarized light) and levorotatory molecules, forming, for example, the most important globular proteins for life. Sugar molecules can form spirals simultaneously rotating both to the right and to the left in different planes of multidimensional space. Nucleic acids form spirals rotating both to the right and to the left. The statements of some authors about the existence of only one of the types of biomolecules are erroneous, based on the observed property of tartaric acid to rotate polarized light in one direction. This property is associated not with some obscure ideas about living things, but with the geometric characteristics of the tartaric acid molecule. All properties of the molecules of living matter obey only the previously defined general principle of the phenomenon of life.
References
- Frank-Kamenetskii M. H-form DNA and the hairpin-triplex model. Nature. 1988:19;214.
- Frank–Kamenetskiy MD. Queen of a living cell. From the structure of DNA to the biotechnological revolution. Moscow: Act – Press. 2010.
- Gillespie RJ. Molecular Geometry. New York: Van Nostrand Reinold Company. 1972.
- Ha SC, Lowenhaupt K, Rich A, et al. Crystal structure of a junction between B-DNA and Z-DNA reveals two extruded bases. Nature. 2005:20;1183-86.
- Paster L. Selected words. Aca Sci USSR; 1960.
- Roimers NF. Ecology, Theory, laws, rules, principles and hypotheses. Russ young. 1994.