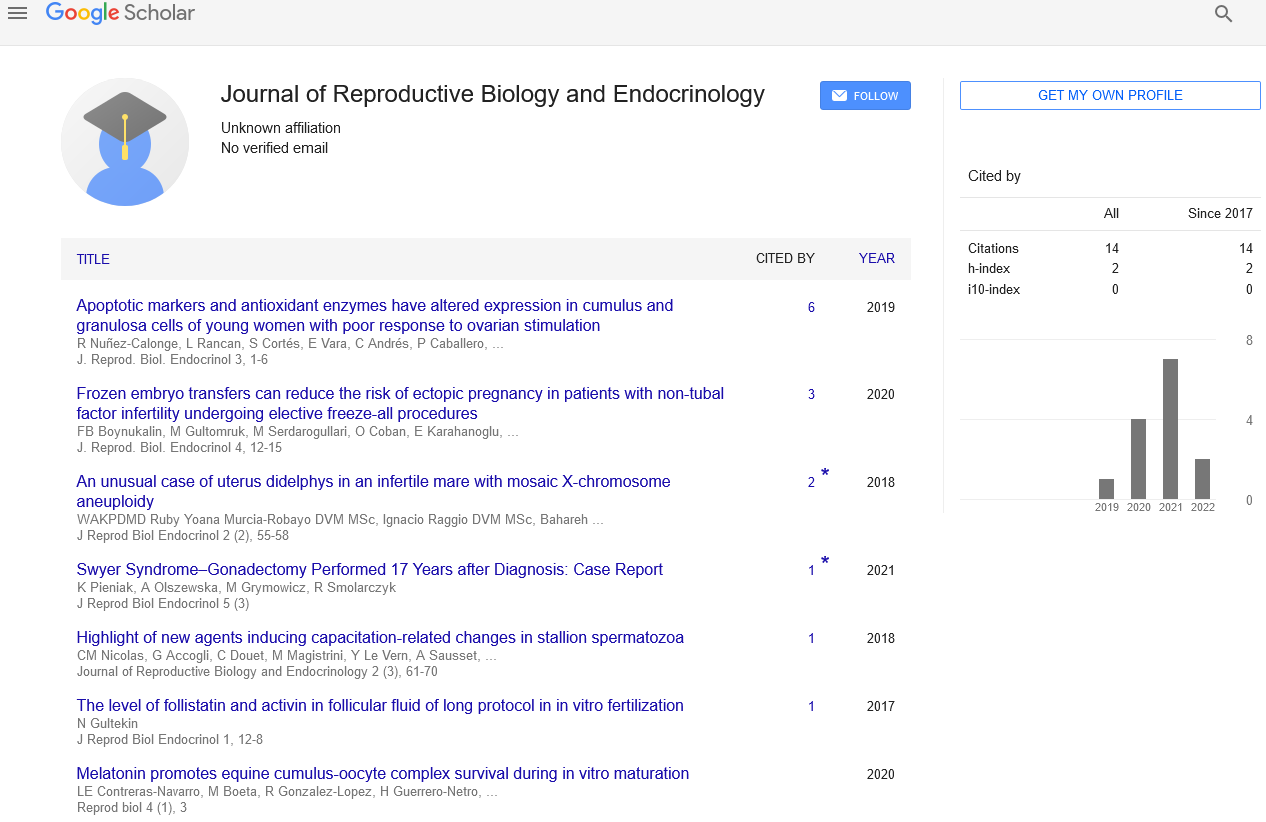
Sign up for email alert when new content gets added: Sign up
Utility of circulating cell-free DNA in assessing microsatellite instability and loss of heterozygosity in breast cancer using human identification approach
6th World Congress on GYNECOLOGY AND OBSTETRICS
June 20-21, 2022 | Paris, France
Norah Alsharhan
Saudi Food and Drug Authority, Saudi Arabia
Posters & Accepted Abstracts: J Reprod Biol Endocrinol
Abstract :
The diagnostic and prognostic utility of circulating cell-free DNA (cfDNA) in breast cancer (BC) patients was recently reported. Here, we investigated the use of cfDNA to examine microsatellite instability (MSI) and loss of heterozygosity (LOH) for early BC diagnosis. cfDNA and genomic DNA from 41 female BC patients and 40 healthy controls were quantified using NanoDrop spectrophotometry and real-time PCR. The stability of genomic and cfDNA was assessed using a high-resolution AmpFlSTR MiniFiler human identification kit. Significant increases in cfDNA plasma concentrations were observed in BC patients compared to controls. The genotype distribution of the eight autosomal short tandem repeat (STR) loci D7S820, D13S317, D21S11, D2S1338, D18S51, D16S539, FGA, and CSF1PO were in Hardy–Weinberg equilibrium. Significant differences in the allele frequencies of D7S820 allele-8, D21S11 allele-29, allele-30.2, allele-32.2, and CSF1PO allele-11 were seen between BC patients and controls. LOH and MSI were detected in 36.6% of the cfDNA of patients compared to genomic DNA. This study highlights the utility of plasma-derived cfDNA for earlier, less invasive, and cost-effective cancer diagnosis and molecular stratification. It also highlights the potential value of cfDNA in molecular profiling and biomarkers discovery in precision and forensic medicine. Reference 1. Messaoudi, S.A.; Al Sharhan, N.A.; Alharthi, B.; Babu, S.R.; Alsaleh, A.B.; Alasiri, A.M.; Assidi, M.; Buhmeida, A.; Almawi, W.Y. Detection of genetic mutations in patients with breast cancer from Saudi Arabia using Ion AmpliSeq Cancer Hotspot Panel v.2.0. Biomed. Rep. 2022, 16, 26. 2. Woodhouse, R.; Li, M.; Hughes, J.; Delfosse, D.; Skoletsky, J.; Ma, P.; Meng, W.; Dewal, N.; Milbury, C.; Clark, T. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS ONE 2020, 15, e0237802. 3. Assidi, M.; Yahya, F.M.; Al-Zahrani, M.H.; Elkhatib, R.; Zari, A.; Elaimi, A.; Al-Maghrabi, J.; Dallol, A.; Buhmeida, A.; Abu-Elmagd, M. Leptin protein expression and promoter methylation in ovarian cancer: A strong prognostic value with theranostic promises. Int. J. Mol. Sci. 2021, 22, 12872. 4. Rolfo, C.; Cardona, A.F.; Cristofanilli, M.; Paz-Ares, L.; Diaz Mochon, J.J.; Duran, I.; Raez, L.E.; Russo, A.; Lorente, J.A.; Malapelle, U.; et al. Challenges and opportunities of cfDNA analysis implementation in clinical practice: Perspective of the International Society of Liquid Biopsy (ISLB). Crit. Rev. Oncol./Hematol. 2020, 151, 102978.