Fertility, hatchability and occurrence of chick malformations in Muhoroni, Kisumu County.
Received: 29-Jun-2022, Manuscript No. PULJVRP-22-5118; Editor assigned: 01-Jul-2022, Pre QC No. PULJVRP-22-5118(QC); Reviewed: 14-Jul-2022 QC No. PULJVRP-22-5118; Revised: 29-Aug-2022, Manuscript No. PULJVRP-22-5118(R); Published: 05-Sep-2022
Citation: Wyckliff N, Sharon OB. Fertility, hatchability and occurrence of chick malformations in Muhoroni, Kisumu County. J Vet Res Med 2022;4(3): 1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Occurrence of malformations in incubated chicks possesses serious health risks and also economic losses including reduction in egg production, death, and losses when doing veterinary care. One of the major effects of chick malformation to the farmer is a great reduction in the quality of the chick. The visual scoring system relating to chick defects including navel closure, legs, beak and residual yolk abnormalities have a high correlation to the early chick mortality and general chick performance. Chickens with deformed beaks have reduced feed intake, growth rate and impaired normal behaviors like preening, poor mental condition and social contact with their mate. Due to these production losses, the following objectives were formulated; determination of fertility and hatchability rates of incubated eggs, to determine and characterize the different chick malformations/deformities and the associated risk factors in Muhoroni Sub-County, Kisumu, Kenya. It was found out that the fertility rate was 96.97%, whereas hatchability rate was at 86.49%. Similarly, prevalence rate of chick malformations was at 1.22% with several risk factors associated with their occurrence. Mis-management of the incubator was reported to be significantly related with chick deformities. This is by poor manipulation of the incubator settings orientation, turning during incubation, faulty incubators and poor sanitation of incubators. Other factors include breeder strain, breeder age, and pre incubation egg storage. It was concluded that fertility and hatchability of incubated chicks in the study area was optimal though chick deformities such as crossed beak, one eye, bowlegs, scattered feather, one ear, unhealed navel, one nose, bent neck were reported that mainly occurred due to human error during incubation. It is therefore recommended that poultry farmers should get some basic training on egg incubation, egg storage, selection and handling, incubator hygiene and proper poultry feeding.
Keywords
Fertility; Hatchability; Malformations; Incubated chicks; Muhoroni
Introduction
Agriculture is at the center of the global economy with 1.3 billion out of 1.7 billion people in the world directly or indirectly involved in farming. The World Bank notes that most middle income economies rely on agriculture as the main driver of economic development with countries such as India, China and Brazil investing more in the sector to create wealth. In sub-Saharan Africa, agriculture has been the main economic activity among rural communities which practice subsistence farming targeted to survival of families with little or no surplus trade was reported by FAO [1].
The global poultry population is approximately 16.2 billion, of which 71.6% is found in developing countries. Chickens are the most popular poultry grown worldwide. In sub-Saharan Africa, rural chicken production makes up about 60% of poultry with indigenous chickens accounting for 70% of the total chicken population as reported by [2].
Chicken production contributes significantly to the socio economic development and nutritional requirements of rural and peri rural households. Generally, cereals lack the most important amino acids for humans; lysine, threonine, sulphur bearing amino acids (methionine and cysteine) and occasionally tryptophan which are found in eggs and chicken meat [3]. Similarly, these products (meat and eggs) are also preferred for their good quality, texture, taste and health characteristics. This has resulted into an increased demand, which is an indicator of their great potential of generating higher income. Eggs are also high in lutein which lowers the risk of cataracts and muscular degeneration, particularly among people living in developing countries. In the least developed countries, the projected increase in egg consumption between 2005 and 2015 was 26%, compared with only 2.4% in the most developed countries [4].
However, this cycle has been interrupted by occurrence of conditions such low genetic potential, diseases, poor management practices during production (affecting the nutritional requirement of the chick and overall chick output) and chick malformations [5]. Malformation is an abnormal developmental feature or an abnormal formation of a part of or the whole body of an animal. This may occur in scattered legs, runts, impaired feathers, unhealed navel, disoriented beaks, extra legged chicks, and eye issues. In some cases, a combination of more than one deformity has been witnessed [6]. Possible causes of these malformations can either be due to genetic effects or environmental factors. Higher prevalence rate has been reported in incubated chicks. The extra case in incubated chicks is because of poor handling of the eggs before incubation or incubational factors such as poor hygiene, lack of turning, incubating temperatures and humidity, which increases the chance of malformation in the chicks [7-9].
Malformation in incubated chicks manifest as an economic impediment due to the cost incurred from massive reduction in the quality of the chick and eventual mortalities. Chick quality is a very crucial element considered by the buyers as it is the outward sign of the survival and future performance of the chick. Therefore, small and deformed chicks have a lower chance of being sold, and if they are sold it will be lower than the market value. It’s also an animal welfare problem because the chicks are subjected to pain and discomfort. For instance, a crippled chick is in a lot of both physical and emotional pain as it tries to walk and interact with the other normal chicks. A crossed beak chick cannot eat comfortably thus its freedom from hunger is violated [10,11].
Many farmers in the rural areas practice poultry farming as one of their sources of income in which they use various techniques to improve their production. Most of them use incubators as their artificial brooders. One of the major problems they are facing in poultry production is occurrence of chick malformations which greatly reduce their income. A lot of work has been carried out on factors affecting poultry production such as management practices, hygiene and diseases but little on chick malformations. To the best of the authors knowledge, the is no documented report on chick malformation in Muhoroni, Kenya, therefore the need for this project. This project sought to determine the fertility and hatchability rate of incubated eggs, prevalence and associated risk factors to chick malformations in Muhoroni sub county [12-15].
Materials and Methods
The study was carried out in Muhoroni sub county, Kisumu County, Kenya with a total population of 31,148 persons. The area is located 50 kilometers east of Kisumu city. Some of the economic activities carried out in the area include crops production (sugarcane, agro forestry, horticulture, maize), livestock keeping (dairy, poultry, goat, sheep and pig) and agro-processing. The weather condition in Muhoroni is warm favoring sugarcane farming as well as poultry farming mostly during the hatching period of the chicks. Data collection strategies included direct observation and administration of structured questionnaires to poultry farmers. The farmers were purposively selected provided there was an adult at the homestead during the data collection session and the household having an egg incubator. Questionnaires were structured to capture information on farmer demographics, poultry production practices, breeding (incubation and brooding) and occurrence of chick malformations [16-20].
Data analysis
Data from the questionnaires and notes from direct observation was entered to Microsoft excel and exported to R statistical package for analysis. Descriptive statistics were done to generate the proportion, percentages and rates for different variables. The hatchability rate was arrived at by direct observation of the number of both alive and dead chicks removed from the incubator. Calculation done by taking both the live and dead chicks divided by the total number of eggs incubated times a hundred. The prevalence rate was determined by observation of the number of malformed chicks over the total number of chicks hatched multiplied by a hundred. The risk factors were determined through correlation of malformation and the various independent variables [21,22].
Results
Respondents demographics
A total of 36 questionnaires were administered to different farmers from five wards where by 67% (24/36) of them were males and 33% (12/36) female respondents (Table 1).
| Variable | Characteristics | ||||
|---|---|---|---|---|---|
| Gender | Male | Female | |||
| 66.7% (24/36) | 33.3% (12/36) | ||||
| Age | 18-29 | 30-39 | 40-49 | 50-59 | Above 59 |
| 33.3% (12/36) | 38.9% (14/36) | 25% (9/36) | 0 | 2.8% (1/36) | |
| Level of education | None | Primary | Secondary | Degree/Diploma | Post graduate |
| 0 | 5.6% (2/36) | 58.3% (21/36) | 25% (9/36) | 11.1% (4/36) | |
| Ward | Muhoroni | Fort Tenan | God Nyithindo | Koru | Owage |
| 16.7% (6/36) | 22.2% (8/36) | 16.7% (6/36) | 30.6% (11/36) | 13.8% (5/36) | |
Table 1: Female respondents
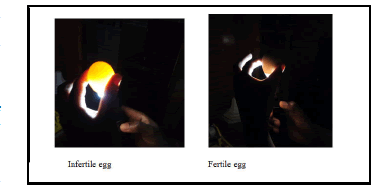
To determine the fertility rate of the incubated eggs, a total of 330 eggs that had been incubated for approximately 10 days were randomly picked and candled. This was done by shining a light through the egg to observe embryo development. In a dark room, the egg was held to the light of a candler (small flashlight) to observe the contents of the egg. A developing embryo can be seen as it blocks the light rays from passing through the egg (Figure 1). The fertility rate was arrived at by: taking the (number of eggs set in the incubator number of infertile eggs to get the fertile eggs)/divided by the number of eggs set and multiplied by a hundred to give a percentage. The fertility rate for eggs in Muhoroni sub county was 96.97% (Table 2).
| Ward | Eggs incubated | Eggs fertile | Fertility rate | Eggs incubated | Total hatches | Hatchability rate |
|---|---|---|---|---|---|---|
| Fort Ternan | 96 | 95 | 98.96% | 1800 | 1480 | 82.22% |
| God nyithindo | 30 | 28 | 93.33% | 138 | 112 | 81.16% |
| Koru | 126 | 124 | 98.42% | 1750 | 1558 | 89.03% |
| Muhoroni Town | 30 | 27 | 90% | 1000 | 909 | 90.90% |
| Owaga | 48 | 46 | 95.83% | 60 | 48 | 80% |
| Tamu | - | - | - | 78 | 67 | 85.90% |
| Total | 96.97% | 4826 | 4174 | 86.49% |
Table 2: Fertility and hatchability rate for incubated chicks in Muhoroni sub county.
The hatchability rate was arrived at by direct observation of the number of both live and dead chicks removed from the incubator. Calculation done by taking both the live and dead chicks divided by the total number of eggs incubated times a hundred to give a percentage (Table 1).
The hatchability rate for incubated chicks in the sub county was 86.49%
Prevalence of chick malformations
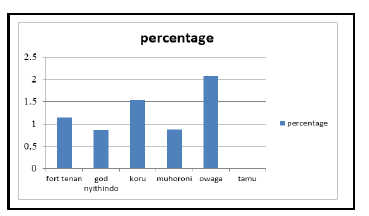
A total of 51 chicks were identified to have at least one form of malformation. The highest number (2.08%) was recorded in Owaga ward, followed by Koru (1.54%), then Fort Tena (1.15%), and 0.89% and 0.88% in God Nyithindo and Muhoroni wards respectively. There was no case of malformation reported in Tamu ward (Figure 2). The observed malformations include crossed beak, one eye, bowlegs, scattered feather, one ear, unhealed navel, one nose, bent neck which attributed to an average of 1.09% prevalence of malformed chicks. Unhealed navel was the highest at 27.5% while head and nose deformities had the least prevalence of 2% each (Table 3).
| Body part | Number | Percentage (%) |
|---|---|---|
| Beak deformity | 5 | 9.8 |
| Leg deformity | 11 | 21.6 |
| Head deformity | 1 | 2 |
| Unhealed navel | 14 | 27.5 |
| Neck deformity | 2 | 3.9 |
| Eye deformity | 2 | 3.9 |
| Impaired feathers | 3 | 5.9 |
| Nose deformity | 1 | 2 |
| Toe deformity | 12 | 23.5 |
| Wing deformity | 0 | 0 |
| Total | 51 | 100 |
Table 3: A table showing the number of chick deformities per body part affected.
Risk factors associated with chick malformation at hatching
When respondents were asked about their opinion on the possible causes of chick malformation, most of them 68.6% mentioned that faulty incubators as the main cause. Such faults included poor temperature regulation, unhygienic conditions, inaccessibility of the incubated eggs, power outage and mechanical breakdowns. There was a significant association between chick deformities and faulty incubators (p-value <0.05). Some other respondents also reported time taken to store the eggs before incubation (15%), setting and handling of eggs in the incubator (20%) and the age of the breeder hen (12%) as possible causes of chick deformities. They reported that eggs from older hens would have higher incidences of chick deformities compared to those from young and middle-aged breeder hens. Similarly, most of them thought that eggs that are stored for at most one week before incubation had lower incidence of deformities compared to eggs that were stored longer before incubation even though there was no significant correlation between the two (p-value>0.05). A few (8%) respondents reported that the deformities were inherited from either the cock or the hen and passed to the chick. Some (8%) farmers also thought that malformations occur when chicks hatch early and therefore not fully developed. Feeding of the breeder hen with some feed also was mentioned by 5% of the respondents as a possible cause of deformity though the respondents could not tell the specific feed product responsible for mal formations. Forty two percent of the respondents thought that diseases from the breeder hens and contamination of the eggs before storage could lead to hatching of chicks with deformities.
Discussion
Chicken production is an enterprise that is lucrative and farmer with low to middle income can venture into it as an alternative source of revenue. However egg fertility and hatchability rate are the two factors that should be considered to ensure optimum returns from chicken farming. On egg fertility, Ogunmoyo, et al. reported 81.3% fertility rate in eggs which is lower than the findings of this study of 96.97%. The difference could be attributed to differences in study sites and design of the studies. Ogunmoyo, et al. conducted their research at Oyo State, Southwest Nigeria where they bought, incubated and carried out the fertility test in a controlled environment unlike this study where farmers recall and direct observations were employed. Similarly Magothe TM reported a fertility rate of 61.8% in their study in different agro ecological zones (highland, midland and lowland) in Kenya sourcing eggs from the nearby hatcheries or large poultry farms with different management and incubation protocols. Much lower fertility rates of 35.7% was reported in hatcheries in Northern Jordan from 1,000 un hatched eggs through microscopic examination. However, Mauldin reported 92.75% whereas Fayeye, et al. reported 94.8% fertility rate in eggs. These findings are not significantly different from the findings of this study, and this could be attributed to the similarities in study designs, eggs handling and incubation procedures.
In terms of egg hatchability, Butcher, et al. reported a hatchability rate of between 78-88% while Hussain, et al. rates of between 82.8-86.9% which are within the range of the findings of this study of 86.49%. This could be due to exposure of the incubators to the same range of temperatures and humidity. On the other hand, Ogunmoyo, et al. reported a hatchability rate of 82.5% from 117,168 incubated eggs which are slightly lower than the findings of this study. The difference could be attributed to the different sample size and fluctuations in the incubator environmental factors. From different parts of Kenya, 74.2% hatchability rate has been reported Magothe, et al. Walgus, et al. reported a hatchability rate of 67% from their study in Fort Collins, Colorado which is significantly lower than the findings of this study. However, Roy, et al. reported a hatchability rate of 96.67% which is higher than 86.49% reported in this study. The difference could be attributed to the designs where the study in Colarado initially sorted eggs based on the size before incubation which was not the case in the current study because eggs were sourced from the nearby poultry farmers.
Mauldin, et al. reported that there are numerous factors that have pronounced influence on the hatchability of chicken eggs. Many of the factors are before the eggs are placed in the incubator. For example, breeder flock health, nutrition, breed, age of breeders and breeder flock management can result in tremendous variation in hatchability. Equally important is the micro environment surrounding the eggs prior to incubation. Egg collection, storage and handling must be optimum to maintain embryonic viability before and during incubation. After setting in the incubator, temperature, turning, humidity, ventilation in the incubator and incubator rooms, sanitation, and general hatchery management are all critical factors to ensure embryonic survival and hatchability. Roy, et al. reported that medium sized eggs are ideal for setting in order to obtain good hatchability and best result of body weight gain.
The late stage of embryonic development has particular importance due to its dramatic effect on life after hatching. Hananeh, et al. reported on 1,000 un hatched eggs out of which 357 (35.7%) were fertile. Approximately 50.5% of the dead embryos displayed abnormalities, including neck muscles with subcutaneous petechial haemorrhages (44.3%), beak abnormalities (3.8%), eye deformities (1.9%) and anencephaly (0.5%). This was contrary to the findings of this study that reported 9.8% beak deformity and 2.0% head deformity due to difference in the breeder gene that could have caused the malformations. Butcher, et al. reported a prevalence rate of between 0.22 to 0.30% whereas Markarian, et al. reported a prevalence rate of 0.53% used the histo pathological examination to determine the malformation of incubated chicks which is lower than 1.22% reported in this study. The differences could be due to farming practices and farmer level of awareness as well as the purpose of poultry keeping.
Hussain, et al. a prevalence rate of 0.22%-0.3%. Where brain hernia (29%), beak deformity (27%), failure to develop eye (25%), 4 legs (10%), lack of upper beak (8%) and twisted legs (1%). This was in contrary to the finding of this study which was 1.22%, this difference is could be due to the difference in the genes of the breeder flock because Hussain, et al. used ross 308 broiler breeder while this project observed the local improved indigenous breed.
Hussain, et al. reported a chick malformation prevalence rate of 0.22%-0.3%. Where brain hernia (29%), beak deformity (27%), failure to develop eye (25%), 4 legs (10%), lack of upper beak (8%) and twisted legs (1%), which were in contrast with the finding of this study which was beak deformity was 9.8%, eye deformity was 3.9% and leg deformity was 21.6%.
Butcher, et al. reported incidence of mal positions including head between thighs, head in the small end of egg, head under left wing, feet over head and beak above right wing. They also reported deformities such as exposed brain, anophthalmia, four legs, deformed beak, no upper beak and twisted leg. However, Ogunmoyo, et al. reported different types of malformations including scattered legs, runts, impaired feathers, unhealed navel, disoriented beaks, extra legged chicks and eye issues that were also found in this study.
Spontaneous development error such as misplaced thighs, monsters, multiple heads and beaks, exposed viscera, splayed beak, winglessness, absence of both upper and lower limbs, or digits of the feet had also been reported elsewhere Wannop though none of these were found in this study. Magothe, et al. reported incidence of malformations such as monstrosities primarily affecting the trunk of the body presented as sole leg deformity or multiple monstrosities involving legs, cranium, beaks and viscera. Crippled chicks, rough or unhealed navel, short down on chicks, malformed toes, malposition of the vent and small chicks have also been reported as the common chick malformations.
Smith JR, et al. reported that incubation distress easily led to splayed legs. They pointed out that the incidence of splayed legs in chicks at hatch ranges from 0.35 to 0.50%. From the findings of this study, some incidences of splayed legs were found but this was attributed to the slippery surface that the newly hatched chicks were kept. In a study by Markarian, legs and feet (curved legs and feet micromelia, achondroplasia) were ranked as the leading form of chick deformity, followed by head deformities (acrania) however, 64% of the affected chick had single defect, 29.9% having two defects and 5.2% having multiple deformities. Janikovicova, et al. reported macroscopic malformations such as open body cavity, beak deformities, upstretched wing and limbs, two headed embryos and hemorrhages some of which were also reported in this study.
The risk factors associated with incidence of chick malformation may include bird genotype, management practices, technology, incubator factors and breeder stock management. Ogunmoyo, et al. reported that some of the risk factors associated with chick malformation in incubated eggs are several. The first is sporadic abnormalities of unknown cause that occasionally occur as spontaneous events. In one of these the thighs and feet grow back to front for example monsters with multiple heads or beaks or even twin embryos sharing a common yolk sac. Some deformities were also reported as a result of incubator factors that may result in spina bifida or complete duplication of the vertebral column, exposed viscera and early embryonic mortalities may be induced by changing the incubation temperature.
The effects on hatchability, chick weight at hatch and hatching time were examined in two broiler breeder lines from 33 to 58 weeks of age. Incidence of early embryonic death increased, and incubation time decreased for eggs stored at 16.5°C as compared to those stored at 10°C. Chicks from morning eggs were heavier than those from afternoon eggs irrespective of storage conditions. Hatchability and chick weight varied with hen age irrespective of storage conditions. During long storage, hatching time varied with hen age independently on breeder line, storage, temperature or egg lying time was reported by Ruiz, et al.
Some abnormalities are genetically determined such as shortening of the upper beak with exposure of the brain, splayed beak, and winglessness, absence of one or both limbs or digits due to a recessive gene. However, other abnormalities could be induced by nutritional deficiencies though these may be seen in commercial production of birds. A deficiency of vitamin A in the parent stock may lead to a reduction in the number of chickens at hatching and to dystrophy of the limbs. A condition known as parrot beak, sometimes associated with limb dystrophy, may result from manganese deficiency. Riboflavin deficiency causes clubbed down, in which the feather follicles fail to develop properly so that the feathers are curled up. This abnormality is occasionally associated with incomplete closure of the abdomen and used to be quite common on commercial farms. Like the parrot beak deformity, it is quite easily induced by depleting the parent stock of this vitamin for a short period of time.
Wannop reported that embryonic defects can be induced by drugs or formaldehydes. Various drugs are used at therapeutic levels for the control of diseases of poultry breeding stock, and some may affect the embryos. For example, the folic acid antagonists may lead to an 80% reduction in hatchability. Death then occur at all stages in embryogenesis and in most cases the head or one or more limbs may fail to develop. Fumigation of eggs with formaldehyde vapour at the incorrect time of incubation (24th-84th hours) leads to production of embryos with beak and eye abnormalities. Some chick deformities have been reported to have been induced by micro organisms such as viruses. New castle disease has been reported to cause death of the embryo which takes on a hemorrhagic appearance. Avian encephalomyelitis virus, which is similar to poliomyelitis virus, causes dwarfing and paralysis associated with muscular dystrophy and contracted feet. Infection with avian bronchitis virus gives rise to dwarfing and death of the embryo. Apart from the pathogenic viruses, the presence of orphan viruses has also to be considered in the context of experimental studies. Thus, eggs may contain the agent that causes avian lymphoid leucosis, and that may contaminate vaccines. Adenoviruses and mycoplasma organisms may also be present in orphans Wannop, Olkowski, et al. reported that the high risk of anatomical anomalies has been associated with maternal exposure to common environmental pollutants, chlorination disinfection by products in drinking water and electromagnetic fields. Common pollutants include PCBs, dioxins, heavy metals, hydrocarbons and pesticides. Similarly, Archer, et al. reported that egg quality and embryo survival are influenced by hen and sire’s age, health, nutrition, ratio, genetics and stress. Walgus, et al. reported that the rate and direction of air flow over the over the eggs during incubation influence gaseous exchange through the shell and shell membranes thus greatly influencing the rate of hatching. Oviedo R, et al. reported that incubation distress easily led to splayed legs. This condition has been associated with high humidity during incubation, but the results of their results indicated that higher temperature conditions during the last phase of embryo development may have a bigger impact. Splayed legs are also observed when newly hatched chicks are placed on slippery floors. Higher temperatures during incubation may have the bigger impact on sprayed leg incidence because they affect bone, tendon and muscle development and thyroid metabolism. Embryos can be heat stressed in commercial hatcheries due to incubator and hatcher design that prevents adequate air flow around the eggs in some locations. Occasionally reduced ventilation during incubation and extended length of hatching time (12-24) hours may cause the heat stress that triggers higher incidence of splayed legs.
Grochowska, et al. reported on the factors affecting egg weight loss during incubation, early embryonic mortality, hatchability and chick mortality in the first seven days of life with the use of classification tree technique. The study was performed on 20,817,600 hatching eggs originating from 7 different breeder flocks. The study has showed that the 2 main factors i.e., breeder flock age and egg storage time that affects hatchability also influences chick mortality. Moreover, adjusting the management decisions at commercial hatcheries would increase production results in different egg sets. This varies in different egg sets in respect to breeder flock age, egg storage time, setter and hatcher type and genotype.
Cold stress has a significant effect on percentage of egg weight loss. The percentage of exploders and early hatched chicks and chick weight is higher in below 3°C. Cold stress has played a vital effect upon chick length, hatchability and hatching of fertile eggs. It has considerable effect on early middle and late mortality, total embryo mortality and exposure of brain. Ectopic viscera and most mortality are observed in below 4°C. Cold stress has an impact on the number of cull chicks; string navel, button navel, omphalitis, full body cavity, red hocks, dehydration, dirty chicks and stubby down. Cold stress affects performance during incubation and overall chick quality.
Other potential risk factors may include undernourished males, improper egg handling, warm and cool spots in the incubator due to faulty design, old or improperly stored eggs, high incubation humidity, lethal genes, inadequate ventilation, improper fumigation of eggs, heredity, slick hatching trays and also navel infection (omphalitis).
Conclusion
From this study, fertility and hatchability rates of incubated chicks was reported at 96.97% and 86.49% respectively. Similarly, 1.22% prevalence of chick malformation was also reported during the study period. The observed malformations include crossed beak, one eye, bowlegs, scattered feather, one ear, unhealed navel, one nose, bent neck which attributed to an average of 1.09% prevalence of malformed chicks. Unhealed navel was the highest at 27.5% while head and nose deformities had the least prevalence of 2% each. Several risk factors were identified to have been associated with the chick malformations such as poor manipulation of the incubator settings, faulty incubators, poor sanitation of incubators, source of incubated eggs, storing of eggs before incubation.
It is therefore recommended that poultry farmers should get some basic training on egg incubation, egg storage, selection and handling, incubator hygiene and proper poultry feeding.
References
- Njuguna, Kabuage, Bett, et al. Economic analysis of indigenous chicken production: The case of small holder farmers in Makueni and Kakamega counties, Kenya. Int J Ag Ext Rural Dev. 2017;5(5):564-70.
- Roy BC, Ranvig H, Chowdhury SD, et al. Production of day old chick from crossbred chicken eggs by broody hens, rice husk incubator and electric incubator, and their rearing up to 6 weeks. Lives Res Rural Dev. 2004;18(16):18-25.
- Windhorst. A projection of the regional development of egg production until 2015. Worlds Poult Sci J. 2008;64(3):356-76.
- Hananeh, Al-Natour, Alaboudi, et al. Congenital abnormalities in dead-in-shell chicks associated with mixed bacterial infections. J Avian Dis. 2021;68(5):667-93.
[Crossreff] [Googlescholar] [ Indexed]
- Ogunmoyo, Odunsi. Incidence of abnormalities in hatched day-old chicks of commercial hatchery in Ogbomoso, Oyo state Southwest Nigeria. Nigeria J Animal Sci. 2018;20(3):156-61.
- King’ori. Review of the factors influencing egg fertility and hatchability in poultry. Int J Poult Sci. 2011;10:483-92.
- Magothe TM, Okeno TO, Muhuyi WB, et al. Indigenous chicken production in Kenya: I. Current status. Worlds Poult Sci J. 2012;68(1):119-32.
- Mauldin. Factors affecting hatchability. Poultry Sci. 2002;7:105-17.
- Fayeye, Olapade. Hatch out analysis and repeatability estimates of common hatchability problems in ISA-Brown breeder stock. Agrosearch. 2013;13(2):51-8.
- Hussain A, Bilal M, Habib F, et al. Effects of low temperature upon hatchability and chick quality of Ross-308 broiler breeder eggs during transportation. J Anim Feed Sci. 2019;9(2):59-67.
- Walgus, Sadler. Incubation factors affecting hatchability of poultry eggs. Poultry Sci. 2003;79:497-503.
- Tona K, Decuypere E, Coucke W. The effect of strain, hen age and transferring eggs from turning to stationary trays after 15 to 18 days of incubation on hatch- ability. Br Poult Sci. 2001;42:663-7.
- Markarian. Type and frequency of malformation in chick embryos. Vet Med Nauki J. 1978;15(4):40-4.
[Crossreff] [Googlescholar] [Indexed]
- Wilson, Neuman, Eldred, et al. Embryonic malpositions in broiler chicken and Bobwhite quail. Appl Poult Res J. 2003;12:14-23.
- Wannop. Some abnormalities of the chick embryo. Lab Anim. 1968;2:191-4.
- Olkowski, Laarveld, Wajnarowicz, et al. Trends in developmental anomalies in contemporary broiler chickens. Int hatch pract. 2013; 28:1-2.
- Oviedo-Rondon, Wineland. Incubation distress easily leads to splayed legs.Poultry Science. 2011;97(9):1332-6.
- Ruiz J, Lunam CA. Effects of pre incubation storage conditions on hatchability, chick weight at hatch and hatching time in broiler breeders. Poult Boil Sci. 2002;43(3):374-83.
[Crossreff] [Googlescholar] [ Indexed]
- Bai H, Zhu J, Sun Y, et al. Identification of genes related to beak deformity of chickens using digital gene expression profiling. PloS one. 2014;9(9):107050.
[Crossreff] [Googlescholar] [ Indexed]
- Grochowska E, Kinal A, Sobek Z. Field study on the factors affecting egg weight loss, early embryonic mortality, hatchability and chick mortality with the use of classification tree technique. Poult Sci. 2019; 98(9):3628-36.
[Crossreff] [Googlescholar] [ Indexed]
- Tona K, Bruggeman V, Onagbesan O. Day old chick: Relationship to hatching egg quality, adequate incubation practice and prediction of broiler performance. Avian Biol Res. 2005;16:109-19.
- Abiola, Meshioye, Oyerinde, et al. Effect of egg size on hatchability of broiler chicks. Arch Zootec. 2008;57:83–6.