Framework for the cost-effectiveness of secondary prevention strategies in cardiovascular diseases: A Canadian theoretical modelbased analysis
2 Centre de Recherche du Centre Hospitalier de l’, Université de Montréal, Montreal, QC, Canada, Email: fiorellafa@gmail.com
3 Pfizer Canada Inc., Kirkland, QC, Canada, Email: fiorellafa@gmail.com
4 Department of Medicine, Centre Hospitalier de l’, Université de Montréal, Montreal, QC, Canada, Email: alexismatteatu@gmail.com
5 Department of Social and Preventive Medicine, Université Laval, Quebec City, QC, Canada
6 Centre de Recherche du CHU de Québec, Université Laval, Axe Santé des Populations et Pratiques Optimales en Santé, Hôpital du St-Sacrement, Quebec City, QC, Canada
7 Beaulieu-Saucier Pharmacogenomics Centre, Montreal Heart Institute, Université de Montréal, Montreal, QC, Canada, Email: arickdubois@gmail.com
Received: 22-Aug-2017 Accepted Date: Aug 31, 2017; Published: 07-Sep-2017
Citation: Fiorella Fanton-Aita, Alexis Matteau, Brian J. Potter, et al. Framework for the cost-effectiveness of secondary prevention strategies in cardiovascular diseases: A Canadian theoretical modelbased analysis. J Pharmacol Res 2017;1(1):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
To estimate the relative risk reduction threshold for adding a novel expensive lipid-lowering therapy (NT) to standard therapy, in a secondary prevention population intolerant to high doses of statins, using a cost-effectiveness analysis from the Canadian Provincial Ministries of Health perspective (single-payer system).
Keywords
Pharmacoeconomic evaluation; Theoretical model; Cardiovascular diseases; Secondary prevention; Lipid-lowering therapy
Abbreviations
A10: Atorvastatin 10 mg; CVD: Cardiovascular Disease; CVE: Cardiovascular Events; ECVD: Established Cardiovascular Disease; ICER: Incremental Cost-Effectiveness Ratios; LDL-C: Low-Density Lipoprotein Cholesterol; MI: Myocardial Infarction; NT: Novel Add-On Lipid-Lowering Therapy; PCSK9: Proprotein Convertase Subtilisin/Kexin Type 9; QALY: Quality-Adjusted Life Years; RT: Reference Treatment; SD: Standard Deviation; WTP: Willingness To Pay
Introduction
Despite medical advances, cardiovascular disease (CVD) remains one of the leading causes of death in Canada [1]. An estimated 80% of these cardiovascular deaths are caused by heart attacks or stroke [2]. Statin lipid-lowering agents are considered the standard of care in both primary and secondary prevention [3-5]. Statins decrease low-density lipoprotein cholesterol (LDL-C) [6], and each reduction of 1 mmol/L (39 mg/dL) has been associated with a 20-25% reduction of non-fatal myocardial infarction (MI) and cardiovascular death [7]. In Canada, CVD carries a large economic burden with an estimated total cost of CAD$12.1 billion [8]. Allocation of limited healthcare resources must be constantly optimized to ensure the sustainability of the system and timely access to costeffective care. Economic evaluation of health technologies provides “value for money” information that is critical to policy-makers [9].
The efficacy [10] and cost-effectiveness [11] of statins have been well documented. While generally well-tolerated, cases of intolerance due to myalgias may lead to statin discontinuation, nonadherence [12] or suboptimal dosages [13]. Approximately 20% of patients treated with the maximal tolerated dose of statins do not reach the recommended LDL-C target [14]. Moreover, when patients cannot tolerate high doses of statins necessary to reach target LDL-C levels, lower doses of statins are recommended [3]. Other classes of lipid-lowering medication have long been available, either as add-on therapy or as an alternative to statins, such as cholesterol absorption inhibitors (ezetimibe), fibrates, niacin-based preparations, and bile acid sequestrants [15], but they are less effective in reducing LDL-C and do not provide comparable benefit in terms of clinical outcomes [16]. Novel classes of medication for the prevention of CVD through LDL-C reduction have been developed and some have recently become commercially available, such as the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, alirocumab and evolocumab. However, the cost-effectiveness of such novel agents in the Canadian context is not well established.
We therefore performed a theoretical pharmacoeconomic evaluation of a novel add-on lipid-lowering therapy (NT) for a secondary prevention population intolerant to high-doses of statins to determine the threshold costs and effectiveness at two different incremental cost-effectiveness ratios (ICER) that could be considered for reimbursement within the Canadian healthcare context. The objective of this study was to establish a paradigm for the rapid evaluation of the cost-effectiveness of new lipid-lowering therapies recently approved or currently under clinical evaluation.
Methods
Model structure
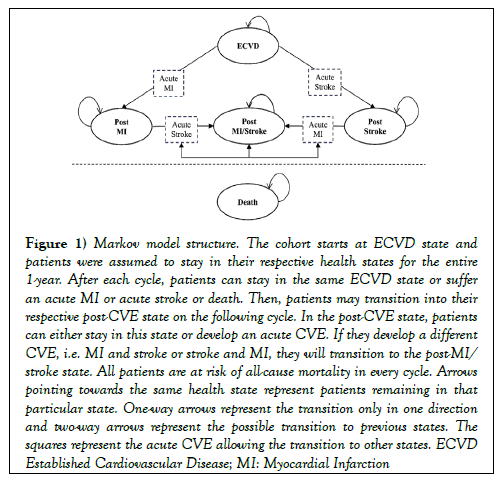
A Markov cohort model (Figure 1) was developed using TreeAge Pro software 2016 (TreeAge Software, Williamstown, MA, USA) to assess the relative risk reduction of the NT added to RT compared to RT alone when fixing two different cost-effectiveness thresholds from the perspective of a Canadian single-payer provincial health system. The population considered consisted of patients with established CVD (ECVD) intolerant to high doses of statins with a baseline age of 60 years old. The model had a lifetime horizon (up to a maximum of 100 years old) and evaluated the impact of the novel therapy on acute cardiovascular events (CVE): myocardial infarction (MI) and stroke, and death. Post-CVE health states were also created to capture higher cardiovascular risk profiles, higher costs, and reduced quality of life for the patients experiencing adverse outcomes. The model included five health states: ECVD, post-MI, post-stroke, postMI/stroke and death. The cohort entered the model in the ECVD health state and patients were assumed to stay in their respective health state for the entire 1-year cycle. After each cycle, patients could either stay in the same ECVD state or suffer an acute CVE, MI or stroke, allowing the transition to a post-CVE state on the following cycle. In the post-CVE states, patients could either stay in this state or develop an acute CVE and transition into any other post-acute state. All patients were at risk of all-cause mortality in every cycle.
Figure 1: Markov model structure. The cohort starts at ECVD state and patients were assumed to stay in their respective health states for the entire 1-year. After each cycle, patients can stay in the same ECVD state or suffer an acute MI or acute stroke or death. Then, patients may transition into their respective post-CVE state on the following cycle. In the post-CVE state, patients can either stay in this state or develop an acute CVE. If they develop a different CVE, i.e. MI and stroke or stroke and MI, they will transition to the post-MI/stroke state. All patients are at risk of all-cause mortality in every cycle. Arrows pointing towards the same health state represent patients remaining in that particular state. One-way arrows represent the transition only in one direction and two-way arrows represent the possible transition to previous states. The squares represent the acute CVE allowing the transition to other states. ECVD Established Cardiovascular Disease; MI: Myocardial Infarction
Reference treatment
The RT chosen for this model was atorvastatin 10 mg (A10), a low-dose, highpotency statin proven to be efficacious in reducing CVE [17].
Model inputs
Transition probabilities
The transition probabilities were obtained from the TNT Study [17,18] for the first year. For the subsequent years, annual transition probabilities were obtained from well-known randomized control trials, IMPROVE-IT [19], PEGASUS [20] and SPARCL [21] (Table 1).
| Base | Range for DSA | Source | ||
|---|---|---|---|---|
| case | Low | High | ||
| Initial events (first year) | ||||
| Acute MI | 0.012 | 0.011 | 0.014 | La Rosa, 2005 (17); Wagner, 2009 (18) |
| Acute stroke | 0.006 | 0.005 | 0.006 | La Rosa, 2005 (17); Wagner, 2009 (18) |
| Subsequent events | ||||
| Post MI followed by | ||||
| Acute MI | 0.026 | 0.023 | 0.028 | Cannon, 2015 (19) |
| Acute stroke | 0.007 | 0.006 | 0.008 | Cannon, 2015 (19) |
| Post stroke followed by | ||||
| Acute MI | 0.007 | 0.006 | 0.008 | Amarenco, 2006 (21) |
| Acute stroke | 0.024 | 0.022 | 0.026 | Amarenco, 2006 (21) |
| Post MI /stroke followed by | ||||
| Acute MI | 0.018 | 0.016 | 0.02 | Bonaca, 2015 (20) |
| Acute stroke | 0.024 | 0.022 | 0.026 | Amarenco, 2006 (21) |
DSA: Deterministic Sensitivity Analysis; MI: Myocardial Infarction
Table 1: Model input parameters: Annual event probabilities.
Costs and utilities
All costs are presented in Table 2. Acute event costs included the cost of hospitalization for each CVE considered in the model. All costs were obtained from the literature.
| Base | Range for DSA | Standard | Distribution | Source | ||
|---|---|---|---|---|---|---|
| case | Low | High | deviation | |||
| Costs | ||||||
| RT (generic A10) | $115 | $86 | $143 | $17 | Gamma | RAMQ (22) |
| NT | $7,000 | $5,250 | $8,750 | $1,089 | Gamma | Assumption |
| MI | $23,400 | $17,550 | $29,250 | $3,510 | Gamma | Cohen, 2014 (40) |
| Stroke | $62,422 | $46,817 | $78,028 | $9,363 | Gamma | Mittman, 2012 (41) |
| Post-MI | $1,491 | $1,118 | $1,864 | $224 | Gamma | Cohen, 2014 (40); RAMQ (42) |
| Post stroke | $2,000 | $1,500 | $2,500 | $300 | Gamma | Assumption |
| Utilities | ||||||
| ECVD | 0.778 | 0.661 | 0.895 | 0.117 | Beta | Sullivan, 2005 (27) |
| MI | 0.651 | 0.553 | 0.749 | 0.098 | Beta | Sullivan, 2005 (27) |
| Stroke | 0.512 | 0.435 | 0.589 | 0.077 | Beta | Sullivan, 2005 (27) |
| Post-MI | 0.685 | 0.582 | 0.788 | 0.024 | Beta | Ara, 2010 (26) |
| Post-stroke | 0.641 | 0.545 | 0.737 | 0.037 | Beta | Ara, 2010 (26) |
| Post-MI/stroke | 0.612 | 0.52 | 0.704 | 0.092 | Beta | Sullivan, 2005 (27) |
| Mortality rates | ||||||
| Non-CV | Non-CV * 2.0 | NA | NA | NA | NA | Statistics Canada (29) |
| 1st MI | Age-specific | NA | NA | NA | NA | Johansen, 2002 (43) |
| Post MI | Non-CV * 3.7 | NA | NA | NA | NA | Statistics Canada (29) |
| 1st Stroke | Age-specific | NA | NA | NA | NA | Holroyd-Leduc.2000 (44) |
| Post Stroke | Non-CV * 2.1 | NA | NA | NA | NA | Statistics Canada (29) |
| Post MI-Stroke | Non-CV * 3.7 | NA | NA | NA | NA | Statistics Canada (29) |
| Mortality multiplier | ||||||
| ECVD | 2 | 1.39 | 2.61 | 0.306 | Gamma | Lampe, 2000 (30) |
| MI | 3.7 | 2.68 | 4.72 | 0.25 | Gamma | Lampe, 2000 (30) |
| Stroke | 2.1 | 1.45 | 2.75 | 0.326 | Gamma | Dennis, 1993 (31) |
| Discount rate | 0.015 | 0 | 0.03 | NA | NA | CADTH (28) |
A10: Atorvastatin 10 mg; CADTH: Canadian Agency for Drugs and Technologies in Health; DSA: Deterministic Sensitivity Analysis; ECVD: Established Cardiovascular Disease; MI: Myocardial Infarction; NA: Not Applicable; NT: Novel Therapy; OCCI: Ontario Case Costing Initiative; RAMQ: Régie de l’assurance maladie du Québec; RT: Reference Treatment
Table 2: Model input parameters: Annual costs, utilities, mortality and mortality multipliers
The acquisition cost of the RT, defined by generic A10, was obtained from the List of Medications [22] of the Régie de l’assurance maladie du Québec. This List provides the acquisition cost of all medications covered by the basic prescription drug insurance plan in the province of Quebec [23]. The acquisition cost of the NT was set at $7,000 per patient per year in the base case analysis. NT cost was established within the same order of magnitude of PCSK9 inhibitors therapies cost [24,25] currently available in Canada, but not reimbursed by the provincial health insurance plans. All cost are presented in Canadian dollars.
Health state-specific utilities were defined based on two studies performed by Ara et al. [26] and Sullivan et al. [27], where a utility of 1 indicates full health and a utility of 0 is assigned to the death health state (Table 2). As all patients in this model began in the ECVD state, the baseline utility upon entering the model was determined at 0.872. As recommended by the 2017 Canadian Agency for Drugs and Technologies in Health guidelines, all costs and utilities were discounted at 1.5% [28].
Novel lipid-lowering therapy relative risk reduction
The NT relative risk reduction was defined as the degree of cardiovascular protection provided by the NT when added to the RT compared to the RT alone for a composite outcome of MI, stroke or death. The NT relative risk reduction ranging from 0.40 to 0.90 was considered.
Mortality
Statistics Canada life tables were used to estimate all-cause mortality rates [29]. The mortality rates for patients who did not suffer from an acute CVE in the model, the ECVD state, were calculated based on Canadian general population, minus the MI and stroke mortality. Acute CVE mortality probabilities were obtained from cardiovascular trials. Mortality multipliers were used to reflect the higher probability of death when compared to general population given their past cardiovascular condition (Table 2) [30,31]. For post-MI/stroke followed by a MI or a stroke, the mortality probability attributed was the worst case scenario.
Sensitivity analyses
Both deterministic and probabilistic sensitivity analyses were performed. The deterministic sensitivity analyses included variations in all costs (± 25%), utilities (± 15%), CVE probabilities (± 10%) and mortality multipliers (± 2 SD). In addition, variations were considered in the relative risk reductions and the annual cost of the NT was varied from $1,000 to $11,000. The discount rate, set at 1.5% in the base case analysis, was varied from 0% to 3%. The probabilistic sensitivity analyses were addressed through second order analyses using 10,000 iterations, gamma distributions for nontherapy costs and mortality multipliers, RT and NT costs and beta distributions for utilities.
Results
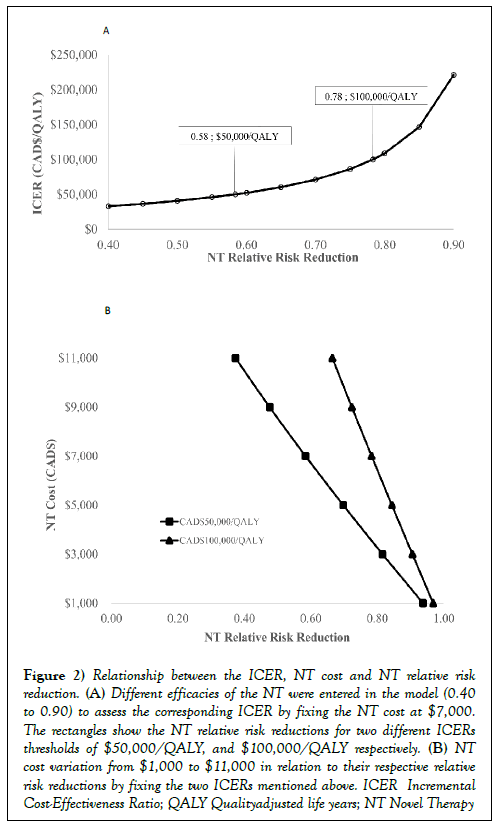
The baseline model results, considering a baseline NT acquisition cost of $7,000 per patient per year and two willingness to pay (WTP) thresholds, are presented in Figure 2A. The NT minimal relative risk reduction required to satisfy a payer WTP threshold of $50,000/quality-adjusted life years (QALY) is projected to be 0.58, but 0.78 for a WTP of $100,000/QALY. The NT relative risk reductions in relation to the ICER show a positive exponential relationship, which approximates a critical minimal relative risk reduction of 0.80 above which the ICER increases dramatically. The NT relative risk reductions as well as the NT costs were varied to establish their relationship by fixing two different ICER thresholds (Figure 2B). There is a clear negative linear relationship between NT relative risk reduction and NT costs at each of the WTP thresholds.
Figure 2: Relationship between the ICER, NT cost and NT relative risk reduction. (A) Different efficacies of the NT were entered in the model (0.40 to 0.90) to assess the corresponding ICER by fixing the NT cost at $7,000. The rectangles show the NT relative risk reductions for two different ICERs thresholds of $50,000/QALY, and $100,000/QALY respectively. (B) NT cost variation from $1,000 to $11,000 in relation to their respective relative risk reductions by fixing the two ICERs mentioned above. ICER Incremental Cost-Effectiveness Ratio; QALY Qualityadjusted life years; NT Novel Therapy
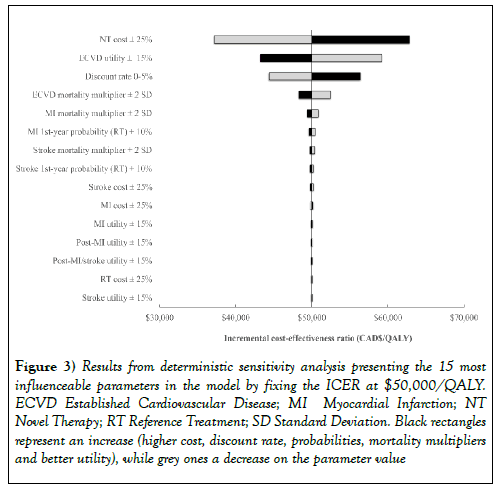
At a WTP of $50,000/QALY, assuming minimal relative risk reduction of an add-on NT and keeping the acquisition cost fixed a baseline values, the total cost for the RT arm alone is $8,615 for 6.06 QALY gained and $77,091 for the NT added to RT for a gain of 7.43 QALY. The equivalent calculations were also made for WTP threshold of $100,000/QALY, resulting in a total cost of $71,605 for 6.69 QALY (Table 3). Deterministic sensitivity analyses results are presented in the Tornado Diagram (Figure 3).
Figure 3: Results from deterministic sensitivity analysis presenting the 15 most influenceable parameters in the model by fixing the ICER at $50,000/QALY. ECVD Established Cardiovascular Disease; MI Myocardial Infarction; NT Novel Therapy; RT Reference Treatment; SD Standard Deviation. Black rectangles represent an increase (higher cost, discount rate, probabilities, mortality multipliers and better utility), while grey ones a decrease on the parameter value
| Strategy | WTP | Relative risk reduction | Total | Incremental | Total | Incremental |
|---|---|---|---|---|---|---|
| ($/QALY) | Cost ($) | Cost ($) | QALY | QALY | ||
| RT | 50,000 | 0.58 | 8,615 | 68,476 | 6.06 | 1.37 |
| RT + NT | 77,091 | 7.43 | ||||
| RT | 1,00,000 | 0.78 | 8,615 | 62,990 | 6.06 | 0.63 |
| RT + NT | 71,605 | 6.69 |
NT: novel therapy; QALY: quality-adjusted life years; RT: reference treatment; WTP: willingness to pay.
Table 3: Theoretical costs and benefits of add-on NT based on an acquisition cost of $7,000 and a minimal relative risk reduction for cost-effectiveness at each of three predefined WTP thresholds.<
This diagram displays the impact on the ICER of the 15 most important parameters at a WTP fixed at $50,000/QALY. The three most important parameters affecting the model are the NT cost ($5,250 to $8,750), the ECVD utility (0.741 to 1.0) and the discount rate (0% to 3%). The probabilistic sensitivity analysis was performed, but the results are not presented, as the scatter plots were all essentially symmetrically distributed around the WTP threshold line due to the nature of the analysis.
Discussion
Our model allowed the estimation of two relative risk ratios that a NT, efficacious in lowering CVE but expensive, would need to have by fixing two different cost-effective WTP thresholds in a population intolerant to high doses of statins in secondary prevention of CVD. The relative risk ratios obtained were 0.58 for an ICER of $50,000/QALY and 0.78 for an ICER of $100,000/QALY. Our cost-effectiveness model showed to be sensitive to the cost of the NT, the utility of ECVD and the discount rate.
In a previous study done by our group, we estimated the maximal clinical benefit predicted using cardiovascular risk assessment models [32]. The risk ratio of a novel agent was assumed to vary between 0.46 and 0.66. We have estimated a relative risk reduction for a novel efficacious, but costly therapy at 0.58 for a WTP of $50,000/QALY, which falls within the range previously estimated. Several post-hoc studies showed a reduction on composite cardiovascular endpoints. The Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM) trial estimated a hazard ratio of 0.52 (0.31-0.90) when comparing alirocumab versus placebo [33]. The composite endpoint considered was a combination of death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. The OSLER trials estimated a hazard ratio of 0.47 (0.28-0.78) when comparing evolocumab versus standard-therapy group [34]. This study considered a composite outcome of major adverse CVE, such as death (cardiovascular and noncardiovascular); coronary events (MI, UA and coronary revascularization); cerebrovascular events (stroke, transient ischemic attack) and heart failure requiring hospitalization as cardiovascular endpoint. However, these posthoc studies are now confirmed by the FOURIER study, a long-term phase 3 randomized control trial [35]. Evolocumab showed a hazard ratio of 0.85 for the primary endpoint (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) and 0.80 for the secondary end point (cardiovascular death, MI and stroke) when compared to placebo.
We have chosen two different WTP thresholds to label an intervention as cost-effective. It could be argued that a fixed threshold might not represent our reality considering that, a WTP of $50,000/QALY, for example, is an arbitrary estimate derived from the cost-effectiveness ratio for dialysis in the 1970s [36]. Even though it is hard to find a threshold that could represent the WTP of a society, fixing a threshold is a powerful tool when informing decisions [37]. Therefore, we chose to set two ICER values at $50,000/ QALY representing the lower bound of a cost-effective threshold value, and $100,000/QALY representing an intermediate threshold value. According to the American College of Cardiology/American Heart Association [38] and the World Health Organization [39] reports, an ICER set at <US$ 50,000 (CAD$ 62,925; Bank of Canada exchange rate of 1.2585 on July 20th 2017) per QALY gained represent a threshold value of an option identified as highly cost-effective.
Our study allowed the estimation of theoretical relative risk reductions for two WTP thresholds, allowing different scenarios to be considered. Our study has several limitations. First, the Markov cohort approach is useful to assess different options for a given scenario in a stochastic manner, but it lacks of memory (phenomenon also referred as Markovian assumption or Markov property) and assumes risk is constant over time. Therefore, our results should be interpreted considering this limitation as well as the following model assumptions. Second, we have supposed that the maximal tolerate dose of statins was the equivalent of A10. However, statins are nowadays generic, and their cost by type and dosage do not differ significantly in Canada. Third, the NT cost was assumed to approximate the cost of PCSK9 inhibitors, an available costly add-on therapy for patients who do not reach target LDL-C levels recommended by the Canadian Cardiovascular Society Guidelines [3]. The NT cost was the parameter influencing the most the model, increasing the ICER considerably in the two fixed-WTP scenarios. Fourth, we chose several studies to populate the probabilities of all cardiovascular events. Nevertheless, the population studied in all the chosen trials were similar. Fifth, we have assumed utilities from an English and US population to be the same as for Canadians. However, sensitivity analyses for all utilities were performed, and the ECVD utility was the second parameter influencing the most the model. Utilities from other health states did not impacted the model significantly. Lastly, we have assumed different mortality multipliers for each event (ECVD, acute MI and stroke). Nonetheless, the mortality multipliers have been used by others in another cost-effectiveness study, and sensitivity analysis were performed for all mortality multipliers [18].
In summary, we have estimated a relative risk reduction of 0.58 and 0.78 for a WTP of $50,000/QALY and $100,000/QALY respectively. We assessed a composite outcome of acute MI, stroke and death for a novel efficacious but costly therapy in patients intolerant to high doses of statins in secondary prevention of CVD. Currently, there is an unmet need in lowering LDL-C. Considering the high cost of new available therapies, their cost-effectiveness relies on a high degree of efficacy. Long-term clinical studies as well as economic evaluations are needed for the new arising therapies to draw concise decisions about their efficacies, costs and potential reimbursement by healthcare plans. The present study could be used as a template for further exploratory analyses.
Funding
This study was funded by Génome Québec and Genome Canada through the large-scale genomics applied research project “Personalized medicine strategies for molecular diagnostics and targeted therapeutics of cardiovascular diseases”. Authors’ contributions: All authors contributed to the design of the study and approved the manuscript.
Disclosures
F.F.-A., A.C.I. and D.M. have disclosed that they each received a PhD grant from Genome Quebec and Genome Canada. F.F.-A. became a Pfizer employee after the completion of this study. Her work does not influence any of the methods, results or interpretation reported in this paper. A.M. has disclosed that he has received speaker fees from AstraZeneca, Bayer and Pfizer. D.M. has disclosed that he is a consultant for Amgen Canada, Pfizer Canada, Triton, Dymaxium, Pharmascience, JAMP, Leo Pharm, Athena Research, Baxter, AbbVie, Gilead, Roche, Sanofi, BristolMyers Squib, and Medicus Economics LLC. B.J.P. has received grants from Medtronic, Edwards Lifesciences and AstraZeneca as well as speaker fees from Pfizer and Bayer. He was an advisory board member for the companies Bayer, Servier and AstraZeneca. J.R.G. has disclosed that he has received a Pfizer Canada Inc. Post-Doctoral Mentoree Award and the 2015–2016 Bernie O’Brien Post- Doctoral Fellowship Award for work unrelated to this paper. He has also received honoraria from Sanofi-Aventis and Bio-K International Inc. A.D. and M.-P.D. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article. J.-C.T. has disclosed that he has received research grants and honoraria from Servier. J.L.L. has disclosed that he has acted as a consultant and is involved in research projects. He has also received remuneration or is expecting it from the following companies: AstraZeneca, Bio-K International Inc., Campbell Alliance Group Inc., CUBIST Pharma Inc., GSK, Lundbeck Canada Inc., Merck Canada Inc., Novartis Canada Inc., Pfizer Canada Inc., Sanofi, and ZS Associates.
REFERENCES
- Statistics Canada. CANSIM table 102-0561. Leading causes of death, by sex (Both sexes). Last modified 2015; 12-10. (Accessed October 15, 2016).
- World Health Organization. Cardiovascular disease. Accessed October 17, 2016.
- Anderson TJ, Gregoire J, Pearson GJ, et al. Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol 2016;32:1263-82.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ACC/AHA guideline on the assessment of cardiovascular risk. a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49-73.
- Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular. Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315-81.
- Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152:1490–500.
- Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease. meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581-90.
- Public Health Agency of Canada. Economic burden of illness in Canada (2005–2008), 2014 (Accessed January 10, 2016).
- Guidelines for the economic evaluation of health technologies. Canada. 3rd edn. Ottawa: Canadian. Agency for Drugs and Technologies in Health. 2006.
- Chan DK, O'Rourke F, Shen Q, et al. Meta-analysis of the cardiovascular benefits of intensive lipid lowering with statins. Acta Neurol Scand 2011;124:188-95.
- Deano RC, Pandya A, Jones EC, et al. A look at statin cost-effectiveness in view of the 2013. ACC/AHA cholesterol management guidelines. Curr Atheroscler Rep. 2014;16:438.
- McGinnis B, Olson KL, Magid D, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41:1805-11.
- Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins. the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14:1-10.
- Ahmed S, Cannon CP, Murphy SA, et al. Acute coronary syndromes and diabetes: Is intensive lipid lowering beneficial? Results of the PROVE IT-TIMI 22 trial. Eur Heart J. 2006;27:2323-29.
- Saxon DR, Eckel RH. Statin Intolerance. A Literature Review and Management Strategies. Prog Cardiovasc Dis 2016;59:153-64.
- Ara R, Tumur I, Pandor A, et al. Ezetimibe for the treatment of hypercholesterolaemia. a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-212.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35.
- Wagner M, Goetghebeur M, Merikle E, et al. Cost-effectiveness of intensive lipid lowering therapy with 80 mg of atorvastatin, versus 10 mg of atorvastatin, for secondary prevention of cardiovascular disease in Canada. T Can J Clin Pharmacol. 2009;16:e331-445.
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary. Syndromes. N Engl J Med. 2015;372:2387-97.
- Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-800.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549-59.
- Régie de l’assurance maladie du Québec. Liste des médicaments. Last update 3 October 2016 (Accessed October 14, 2016).
- Régie de l’assurance maladie du Québec. List of Medications. (Accessed July 29, 2016).
- PCSK9 inhibitor monoclonal antibodies for the treatment of hypercholesterolemia. Ottawa: CADTH; 2015 Dec (CADTH issues in emerging health technologies; Issue 145).
- Canadian Agency for Drugs and Technologies in Health. Notice of Final Recommendation Alirocumab. July 2016 (Accessed November 24, 2016).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13:509-18.
- Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care 2005;43:736-49.
- Guidelines for the economic evaluation of health technologies: Canada. 4rd edn. Ottawa: Canadian. Agency for Drugs and Technologies in Health, 2017.
- Statistics Canada. CANSIM Table 102-0551. Deaths and mortality rate, by selected grouped causes, age group and sex, Canada (Accessed October 15, 2016).
- Lampe FC, Whincup PH, Wannamethee SG, et al. The natural history of prevalent ischaemic heart disease in middle-aged men. Eur Heart J. 2000;21:1052-62.
- Dennis MS, Burn JP, Sandercock PA, et al. Long-term survival after first-ever stroke: the Oxfordshire. Community Stroke Project. Stroke. 1993;24:796-800.
- Fanton-Aita F, Matteau A, Iliza AC, et al. Maximal expected benefits from lowering cholesterol in primary prevention for a high-risk population. Curr Med Res Opin. 2016:1-4.
- Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-99.
- Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-9.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713-22.
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165-78.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000 per-QALY threshold. N Engl J Med. 2014;371:796-97.
- Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures. a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-22.
- World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE) (Accessed November 21, 2016).
- Cohen D, Manuel DG, Tugwell P, et al. Direct healthcare costs of acute myocardial infarction in Canada’s elderly across the continuum of care. J Econ Ageing. 2014;3:44-9.
- Mittmann N, Seung SJ, Hill MD, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci. 2012;39:793-800.
- Régie de l’assurance maladie du Québec. Manuel des médecins spécialistes – Rémunération à l'acte (Accessed April 1, 2017).
- Johansen H, Nair C, Mao L, et al. Revascularization and heart attack outcomes. Health Rep. 2002;13:35-46.
- Holroyd-Leduc JM, Kapral MK, Austin PC, et al. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833-37.