Highlight of new agents inducing capacitation-related changes in stallion spermatozoa
2 Department of Emergency and Organ Transplantation (DETO), Section of Veterinary Clinics and Animal P, University of Bari Aldo Moro, S.P. Casamassima Km 3, 70010 Valenzano, Bari, Italy, Email: g.accogli@arpa.puglia.it
3 INRA Val de Loire, UMR1282 Infectiologie et Santé Publique, Cytometry Platform Nouzilly, France, Email: carla.moros-nicolas@inra.fr
Received: 29-Jun-2018 Accepted Date: Aug 03, 2018; Published: 10-Aug-2018
Citation: Moros-Nicolás C, Accogli G, Douet C, et al. Highlight of new agents inducing capacitation-related changes in stallion spermatozoa. J Reprod Biol Endocrinol. 2018;2(3):61-70.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: To fertilize the oocyte, the sperm undergoes physiological and biochemical modifications known as “sperm capacitation”. The aim of this study was to analyze by flow cytometry and immunofluorescence analysis the changes induced by several molecules on stallion sperm viability, plasma membrane fluidity, cholesterol content, calcium influx and tyrosine phosphorylation. Moreover, the modifications induced on glycocalyx sperm sugars were studied by means of lectin-based cell microarray analysis. Frozen sperm samples were incubated with calcium ionophore, MβCD, α-Lfucosidase, neurotensin, HSPA8, cAMP and 4-AP. Flow cytometry analysis showed that membrane fluidity was affected by MβCD, cAMP, neurotensin, HSPA8 and 4-AP; cholesterol depletion was induced by MβCD; an increase of intracellular calcium was produced by calcium ionophore and cAMP; and sperm viability was affected by MβCD. Tyrosine phosphorylation increased when sperm was incubated with MβCD and cAMP. Sperm microarray analysis revealed that i) MβCD and cAMP treatment increased α2,3-linked sialic acid and T antigen, whereas decreased α1,3-linked fucose signals; ii) cAMP alone increased α1,6- and α1,2-linked fucoses; iii) MβCD alone reduced sialylated T antigen, lactosamine, αGal, GalNAc, terminating glycans as well as α1,6fucose in high-mannose glycans. The combination of MβCD + cAMP treatment preserved the α2,3-linked sialic acid increasing, determined the GalNAc, GlcNAc, α1,2-linked fucose enhancement, whereas reduced T antigen and high-mannose glycans. Our results determine the specific effect of each molecule in order to establish a combination of several agents to achieve an efficient in vitro capacitation technique for stallion sperm.
Keywords
Stallion sperm capacitation, Lectin-based cell microarray analysis, Flow cytometry, Equine in vitro fertilization
Several domestic and wild species of horses and donkeys are in a high risk of extinction [1-4]. The development of an efficient In Vitro Fertilization (IVF) technique would be interesting for embryo production for the preservation of endangered species. The embryo production is the fastest method to restore a breed allowing the preservation of both, the female and the male genetics [5]. Intracytoplasmic Sperm Injection (ICSI) is well developed in equines [6,7], but this technique is expensive, time-consuming and requires well-trained personal. Thus, the development of the IVF in this species could reduce the costs of embryo production for the horse industry. To date, in the equine species, no efficient IVF technique is available [8]. For years, several teams tried to develop a reliable technique; however, to this day only two foals were born in 1991 by IVF [9]. In that study, in vivo matured oocytes were inseminated with fresh sperm treated with calcium ionophore A23187 [9]. But, since then, no more foals were born by means of this technique [10].
Since the 90s to 2007 the equine IVF rate varied between 2% and 40%. Oocytes were matured by means of in vitro and the sperm was treated with different agents such as caffeine [10-13], calcium ionophore [7,9,12,14,15] or heparin [12,15-18]. Two years after, in 2009, McPartlin reported an IVF rate of 60% using procaine to induce sperm hyperactivation [19]. An IVF rate of 62% was reported in 2013 using this molecule and along with the pre-incubation of the oocytes with porcine Oviductal Fluid (pOF) [20]. But later in 2015 it was reported that procaine induced cytokinesis rather than fertilization [21]. To this date no capacitation treatment for stallion spermatozoa has been successfully developed; therefore, the identification of new capacitating agents for equines is crucial To fertilize the oocyte the sperm undergoes physiological and biochemical changes known as sperm capacitation [22]. Some of the modifications associated with capacitation in other species imply an increase in plasma membrane fluidity, a depletion of cholesterol content, an increase of intracellular calcium, a rise of cAMP concentration and the phosphorylation of tyrosine residues [23-27]. Different molecules have proven to induce modifications similar to in vitro; for instance, α-L-fucosidase was tested on boar increasing the spermatozoal intracellular calcium concentration and the tyrosine phosphorylation [28], neurotensin increases the intracellular calcium concentration in mice [29] and induces sperm protein tyrosine phosphorylation and the acrosome reaction in mice and bull spermatozoa [29,30], and Heat Shock Protein 8 (HSPA8) increases the sperm membrane fluidity on boar spermatozoa [31]. In stallion spermatozoa, some of the molecules previously tested include calcium ionophore A23187 as inductor of the acrosome reaction [9,12], methyl-β-cyclodextrine (MβCD) as cholesterol acceptor [32,33], dibutyryl cAMP (db cAMP) as mediator of the protein tyrosine phosphorylation [12,34] and 4-aminopyridine (4-AP) to induce sperm hyperactivation [35]. On the other hand, it has been demonstrated the effect of pH on protein tyrosine phosphorylation [36] and over prostasomes recruitment, which are extracellular vesicles that could transfer molecular components that induce hyper motility and the acrosome reaction [37]. Sperm glycocalyx represent the interface between the male gamete and the extracellular environment, and it is composed by many different glycoconjugates including glycoproteins, glycolipids, and Glycol-Phosphatidylinositol (GPI)-anchored glycoproteins [38]. These glycolconjugates play many crucial roles in reproductive biology as they are involved in sperm survival in the oviduct, transport in the oviduct, capacitation, spermegg interaction, acrosome reaction, zona binding and fertilization, and immuneprotection in the female tract [38,39]. Glyco-conjugates changes have been often connected to capacitation reaction/events as well as to the inhibition of premature capacitation in many species [38,40].
There are several techniques available for the decoding of glycoconjugate structure and most of them are lectin based. Due to their specific glycan binding ability they were often used in the detection of linkage, and terminal modifications of complex glycans [41]. In particular lectins have been used for sperm glycocalyx analysis in several techniques such as mass spectrometry [42], western blotting [43], histochemistry [44] and microarray [45].
Considering this, the aim of this study was to evaluate the effect on stallion sperm of molecules that were previously described as capacitating agents in different species, and furthermore to analyze the different parameters related to sperm capacitation by means of flow cytometry [46-48] and immunofluorescence analysis [49,50]. Moreover, their effect on sperm surface glycocalyx by means of cell microarray has been investigated [51,45]. Frozen sperm has been used as means to study these parameters, as the goal of this work is to develop an efficient IVF technique that may be used for equine breeders.
Methods
All reagents were obtained from Sigma-Aldrich® (France) unless otherwise indicated
Stallion Semen Collection
This experiment included three mature Welsh stallions of proven fertility. The stallions were housed at INRA in Nouzilly, France. Semen was collected on a regular basis by using a closed artificial vagina (INRA model). After collection, raw semen was filtered through gauze to exclude the gel fraction of the ejaculate and immediately processed.
Freezing Procedure
The freezing procedure was performed as previously described [52]. Filtered semen of each ejaculate was divided into different aliquots and diluted in INRA96® extender (IMV-Technologies, France) at 37°C. Ejaculates were then cooled (22°C for 10 min) and centrifuged (600 g x 10 min). The pellet was resuspended in INRA Freeze® (IMV-Technologies) to obtain 100 x 106 sperm cells/mL. Each tube of diluted semen was cooled to 4°C and maintained at this temperature over 75 min. The cooled semen was loaded into 0.5 mL polyvinyl chloride straws (IMV-Technologies) sealed with polyvinyl alcohol sealing powder. Freezing was performed with a programmable freezer (Nitrogen freezer, automatic Mini-Digitcool, IMV-Technologies) (60°C/min until -140°C), then plunged and stored in liquid nitrogen.
Thawing Procedure
Straws were thawed for 30 seconds in a water bath at 37ºC. The content was diluted in Hank’s salts solution (1.26 mM CaCl2, 5.36 mM KCl, 0.44 mM KH2PO4, 0.80 mM MgSO4 (7H2O), 136.89 mM NaCl, 0.33 mM Na2HPO4 (12H2O), 4.16 mM NaHCO3, 5.50 mM Glucose (anhydrous), 20 mM Hepes, at pH 7.1 and 300 mOsm) supplemented with 1% (w/v) BSA (HH-BSA 1%) (2 mL/straw) previously warmed at 37ºC.
Sperm Treatment
Frozen-thawed spermatozoa were centrifuged for 5 min at 500 x g at 37ºC and re-suspended at 20 x 106 spermatozoa/ml in HH-BSA 1%. Then, sperm was cultured without or with the different molecules; spermatozoa were incubated at 37ºC for 5 min with calcium ionophore A23187 (6 μM), 15 min with HSPA8 (1 and 10 μg/mL), 30 min with MβCD (1 mM), α-L-fucosidase (0.845 IU and 3.38 IU), neurotensin (10 and 100 μM), cAMP (1.2 mM), 4-AP (4 and 10 mM), or the combination of cAMP (1.2 mM) + MβCD (1 mM). Different doses were chosen according to previous experiments published elsewhere by other authors. Moreover preliminary experiments using similar and higher doses were carried out to determine the optimal dose in stallions. Samples from three stallions were analyzed for each molecule.
Sperm Analysis
➢ Flow cytometry analysis
After the incubation with the different molecules (as explained above), sperm samples were centrifuged for 5 min at 500 × g and re-suspended in HGLL medium (1.26 mM CaCl2, 5.36 mM KCl, 0.44 mM KH2PO4, 0.80 mM MgSO4 (7H2O), 21.38 mM NaCl, 0.33 mM Na2HPO4 (12H2O), 4.16 mM NaHCO3, 73.38 mM Glucose (anhydre), 20 mM Hepes, 126.08 mM lactose at pH 7.1 and 300 mOsm). In order to analyze the different parameters related to sperm capacitation spermatozoa were divided in three different aliquots and labelled separately with the fluorescent probes: i) 30 min at 37ºC with fluo3-AM (Molecular Probes) (5 μL/mL) diluted in DMSO to evaluate the intracellular Ca2+, which was associated to propidium iodide (Molecular Probes, France) (10 μg/mL) in distilled water during 10 min at 37ºC to distinguish the membrane integrity, ii) 5 min at 37ºC with merocyanine 540 (Molecular Probes) (1.54 μg/mL) diluted in DMSO to analyze plasma membrane fluidity, iii) to evaluate cholesterol depletion after centrifugation samples were fixed with 2.5% glutaraldehyde (Electron Microscopy Sciences, France) diluted in 0.1 M sodium cacodylate trihydrate (Merck, France) and labelled with filipin III (50 μg/mL) diluted in DMSO for 40 min at room temperature, then spermatozoa were centrifuged and resuspended in 0.1 M sodium cacodylate trihydrate (Merck) medium. Finally, 1 x 106 spz/mL from each treatment were analyzed in a flow cytometer MoFlo® Legacy (Beckman Coulter, Fort Collins, CO, USA) equipped with two lasers operating at 488 nm and 100mW and in UV and 100mW. The flow cytometer was calibrated daily with Flow-check fluorospheres (Beckman Coulter). For blue fluorescence from filipin III a Band-pass (BP) filter 450 nm was used, detection width: 410 to 490 nm; for green fluorescence from fluo3-AM a BP filter: 530 nm, detection width: 515 to 545 nm; for orange fluorescence from merocyanine 540 a BP filter: 575 nm, detection width: 562 to 588 nm; and for red fluorescence from propidium iodide a BP filter: 670 nm, detection width: 655 to 685 nm. The threshold was adjusted on the forward-scattered light (FSC) to exclude small debris, FSC vs Side- Scattered Light (SSC) dot plot was used to exclude the rest of the debris and the aggregate cells. On double staining compensation was used to minimize spillover of green fluorescence into the red channel. Summit software (Beckman Coulter) was used to analyze the samples. Sperm was labelled with the different probes in the same experiment analysing one molecule with its control group each time. Percentages related to the different populations of spermatozoa after the staining with the different fluorescent probes were compared, and the percentage of positive sperm was determined by comparison with unstained spermatozoa prepared in the same way using regions positioned on the different sub-populations.
➢ Localization of proteins phosphorylated in tyrosine residues
The localization of tyrosine phosphorylated proteins was studied by indirect immunofluorescence as previously described (50, 53). After the incubation with MβCD 1mM, cAMP 1.2mM or the combination of these two molecules (MβCD 1mM + cAMP 1.2mM), samples were centrifuged for 3 min at 700 × g and fixed in 2% paraformaldehyde solution (Santa Cruz Biotechnology, Germany) in phosphate buffered saline (PBS) (Oxoid, France) for 60 min at 4ºC. The samples were then washed in PBS and blocked with 4% (w/v) BSA-PBS overnight at 4ºC. Spermatozoa were washed, re-suspended in PBS and smeared onto a microscope slide, previously treated with poly- L-lysine, and allowed to air dry. Slides were then incubated for 2 h with anti-phosphotyrosine monoclonal antibody at 4ºC (1:100 in 1% (w/v) BSAPBS, clone 4G10, Millipore, Spain) rinsed with PBS, and incubated for an additional 2 h with fluorescent-conjugated goat anti-mouse antibodies (1:300 in 1% (w/v) BSA-PBS, Dylight549 KPL, France). After rinsing with PBS, samples were mounted on the slides with glycerol (Prolabo, France) and kept at 4ºC in darkness until observation under an epifluorescent microscope (Zeiss).
➢ Cell Microarray Fabrication
Incubation of sperm was carried out with the molecules MβCD 1mM, cAMP 1.2mM or the combination of both of them (MβCD 1mM + cAMP 1.2mM), and centrifuged twice in 0.01 M PBS (pH 7.4) at 800 g for 5 min. Then it was fixed in 4% (v/v) buffered paraformaldehyde for 45 min at room temperature (RT), centrifuged at 800 g for 5 min, washed twice with PBS and stored at 4°C until use. Cell microarray was prepared according to Accogli et al. (2017) (45). Shortly after centrifugation of the sperm containing buffered solution the pellet was re-suspended in 50 μL of PBS (about 1x106 of spermatozoa) and transferred by spotting in three replicates onto three-dimensional thin film coated glass slides (Nexterion Slide H) (Schott, Germany). Spotting was performed with a non-contact microarray printing robot sciFLEXARRAYER S1 (Scienion, Germany). The spermatozoa were spotted into 12 identical arrays on the slide. Each sample was spotted in 10 replicates per line within each array.
Approximately 500 pL of cell suspension was spotted for each spot. The transfer efficiency and reproducibility of the printing process was assessed with a standard quality control test array performed by the microarrayer and 400 replicates (20 × 20 spots) were generated in a single run. Printing of the 0.01 M PBS buffer was also performed to check the background signal produced by the lectins incubation. The slides were then placed in a humidity chamber (50-70%) at 37°C for 1 h to ensure sufficient attachment of the spermatozoa to the slide surface. The unoccupied surface of slides was blocked with 1 M ethanolamine dissolved in 0.01 M PBS with 0.05% Tween 20 (PBST) at RT for 1 h. Blocked slides were gently washed with PBST (rinsed twice 2 min each).
➢ Cell microarray - lectin binding procedure
A multi-well incubation chamber was applied onto the surface of the spotted slides in line with the subarrays that were created during the printing process. The used biotinylated lectins, their concentration and sugar specificity are listed in Table 1. Each lectin (Vector Laboratories, USA) was diluted in PBST at optimized concentrations to have the highest specific signal with the lowest background (Table 1) and loaded directly onto the samples for all 12 subarrays. Samples were allowed to react with 50 μL of lectins solution at RT for 1 h. Lectins were then gently removed and slides were immediately washed in PBST for 5 min. Subsequently, each subarray was incubated with Cy3-conjugated Streptavidin (Jackson ImmunoResearch Laboratories, USA) at 0.5 μg/mL in PBST for 15 min. Redundant Cy3-conjugated streptavidin was removed and the slides were washed with PBST and then with distilled water (4 min each). Lastly, residual water was removed using a slides centrifuge at 6000 revolutions per minute (RPM) (Arrayit Corporation, USA).
| Lectin Abbreviation | Source of lectin | µg/ml | Sugar specificity | Inhibitory sugar |
|---|---|---|---|---|
| MAL II | Maackia amurensis | 15 | NeuNAcα2,3Galβ1,3GalNAc | NeuNAc |
| SNA | Sambucus nigra | 15 | NeuNAcα2,6Gal/GalNAc | NeuNAc |
| Jacalin | Artocarpus | 15 | (NeuNacα2,3)Galβ1,3GalNAc | Gal |
| PNA | Arachis hypogaea | 25 | Galβ1,3GalNAc | Gal |
| RCA120 | Ricinus communis | 20 | Galβ1,4GlcNAc | Gal |
| GSA I-B4 | Griffonia simplicifolia | 20 | αGal | Gal |
| DBA | Dolichos biflorus | 25 | GalNAcα1,3(LFucα1,2)Galβ1,3/4GlcNAcβ1 | GalNAc |
| SBA | Glycine max | 20 | α/βGalNAc | GalNAc |
| s-WGA* | Triticum vulgaris | 15 | βGlcNAc | GlcNAc |
| GSA II | Griffonia simplicifolia | 20 | D-GlcNAc | GlcNAc |
| Con A* | Canavalia ensiformis | 15 | αMan>αGlc | Man |
| PSA | Pisum sativum | 20 | L-Fucα1,6GlcNAc | Fuc |
| UEA I | Ulex europaeus | 20 | L-Fucα1,2Galβ1,4GlcNAcβ | Fuc |
| LTA | Lotus tetragonolobus | 15 | αL-Fuc | Fuc |
Fuc:Fucose; Gal:galactose; GalNAc:N-acetylgalactosamine; Glc:Glucose; GlcNAc:N-acetylglucosamine; Man:Mannose; NeuNAc:N-acetyl neuraminic (sialic) acid; s-WGA:succinylated WGA. * The used lectins bind terminal sugars except for Con A and s-WGA, which also bind internal residues.
Table 1: Lectins used, their sugar specificities and the inhibitory sugars used in control
➢ Scanning and data analysis
Images of the stained cell microarray slides were taken using the InnoScan®710 fluorescent scanner (Innopsys, France) set at the appropriate excitation wavelength for Cy3 (532 nm). The resolution was set at 3 μm for quantification purposes. The slide images obtained in lectin-based cell microarray analysis were evaluated using the Mapix 5.5.0 software (Innopsys) and the fluorescence intensity signals of each spot was measured with background subtraction. The raw numeric values corresponding to the detected intensity of spots from cell microarrays were normalized and reported as the value of fluorescence intensity relative to the average number of cells per spot. In addition, the normalized values from each detected sugar residue were grouped to analyze its expression.
Statistical Analysis
For flow cytometry analysis, sperm percentages for the three stallions were compared by a non-parametric Kruskal & Wallis test. Differences were considered statistically significant at p <0.05. Data are expressed as mean +/- S.E.M.
For tyrosine phosphorylation analysis, number of spermatozoa was compared between control and treated sperm by a non-parametric Chi2 test. Differences were considered statistically significant at p <0.01.
For microarray analysis, analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test was performed on data in order to assess the significant intensity differences between samples. The statistical significance was set at p <0.01.
Results
Flow Cytometry
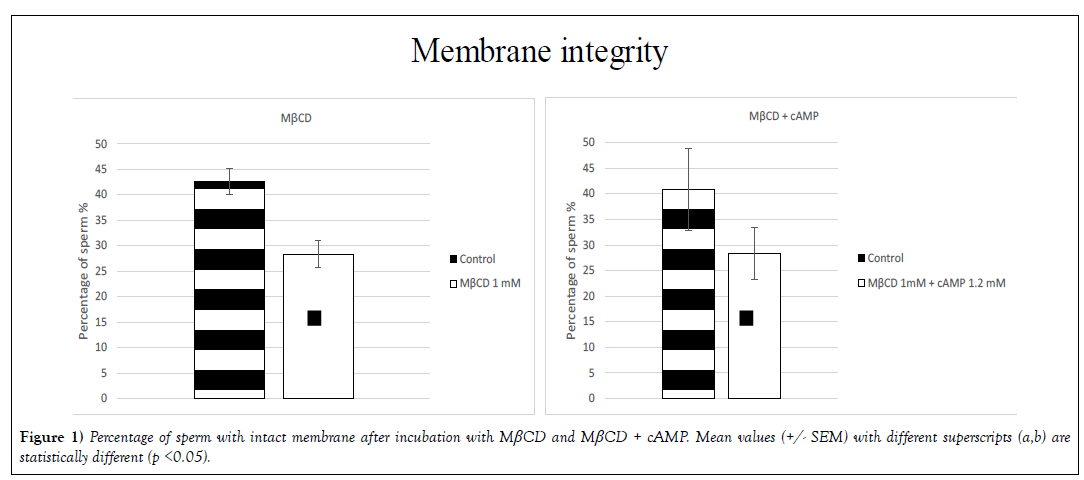
➢ Membrane integrity
Membrane integrity (p< 0.05) was affected by the presence of MβCD. The percentage of intact sperm was 43% (+/- 2.6) in the control group and 28% (+/- 2.6) in the treated group. In the treatment with MβCD + cAMP the percentage of sperm with an intact membrane was 41% (+/- 8.0) in the control group and 28% (+/- 5.1) in the treatment group (Figure 1). The remaining molecules did not affect membrane integrity with statistical significance.
Their mean age was 29.3 ± 4.9 years and mean Body Mass Index (BMI) was 26.9 ± 6.7 Kg/m2. Baseline hormonal characteristics of the patients are given in Table 1.
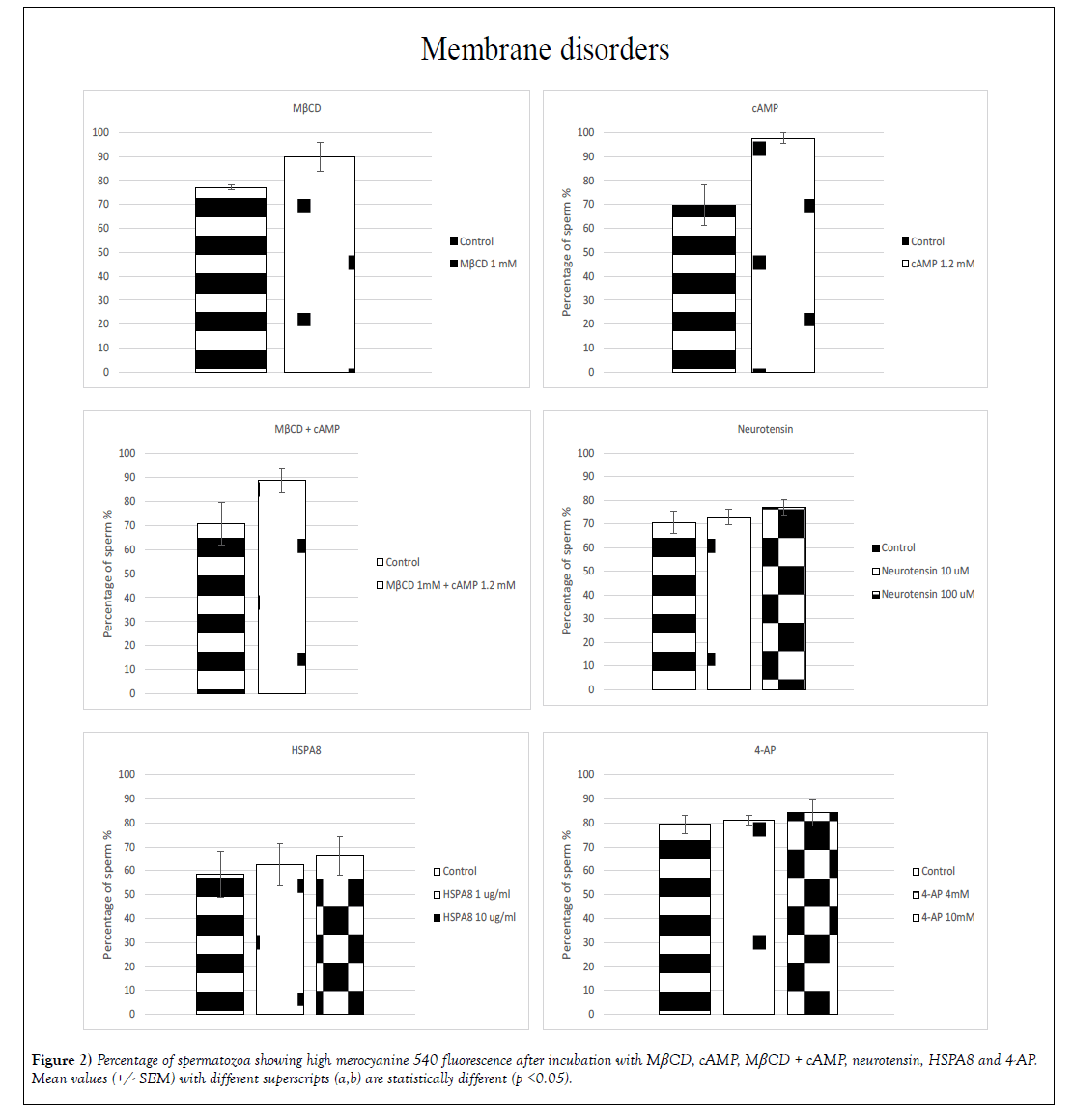
➢ Membrane disorders
A significant (p< 0.05) increase in membrane fluidity was induced by: MβCD, cAMP, neurotensin, HSPA8 and 4-AP (Figure 2). In the treatment with MβCD and cAMP the percentage of spermatozoa exhibiting high-intensity merocyanine fluorescence showed an increase of 13% for MβCD (77% +/- 0.9 for the control group and 90% +/- 6.0 for the treated group) and 28% for cAMP (70% +/- 8.4 for the control and 98% +/- 2.2 for the treated group) whereas the incubation with cAMP + MβCD showed an increase of 18% of the population exhibiting high-intensity fluorescence (71% +/- 8.8 for the control and 89% +/- 5.0 for the treated group). With neurotensin, HSPA8 and 4-AP significant differences were found when sperm was incubated with the highest doses, increasing 6% in the group treated with 100 μM neurotensin (71% +/- 4.5 for the control and 77% +/- 3.2 for the treated group), 8% for the sperm treated with 10 μg/mL HSPA8 (58% +/- 9.6 for the control and 66% +/- 7.9 for the treated group) and 5% for the sperm treated with 4-AP 10mM (79% +/- 3.9 for the control and 84% +/- 5.5 for the treated group) (Figure 2).
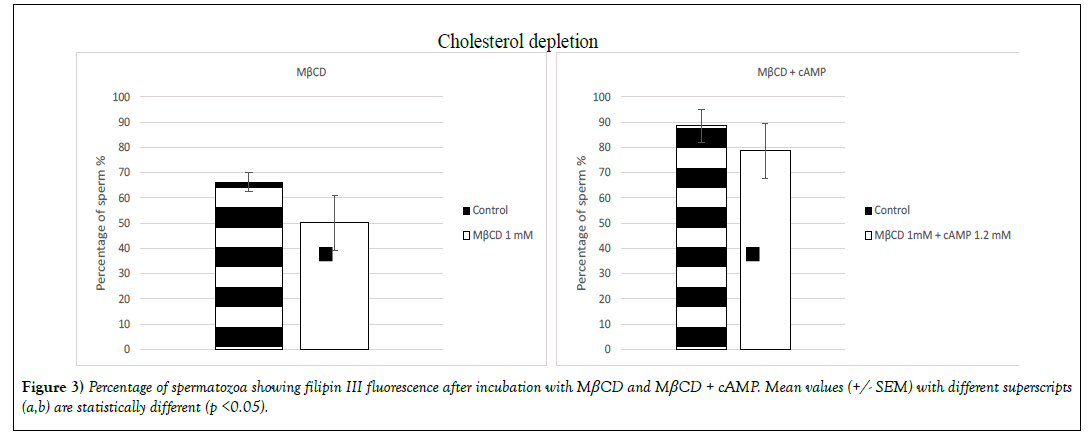
➢ Cholesterol depletion
Cholesterol content reduction (p< 0.05) was induced only by the molecule MβCD and the combination of cAMP with MβCD (Figure 3). On sperm that had been incubated with MβCD the percentage of population labelled with the probe filipin III suffered a decrease of 16% (66% +/- 3.7 for the control group and 50% +/- 10.9 for the treated group) and 9% when sperm was incubated with MβCD + cAMP (89% +/- 6.4 for the control group and 79% +/- 10.8 for the treated group) (Figure 3).
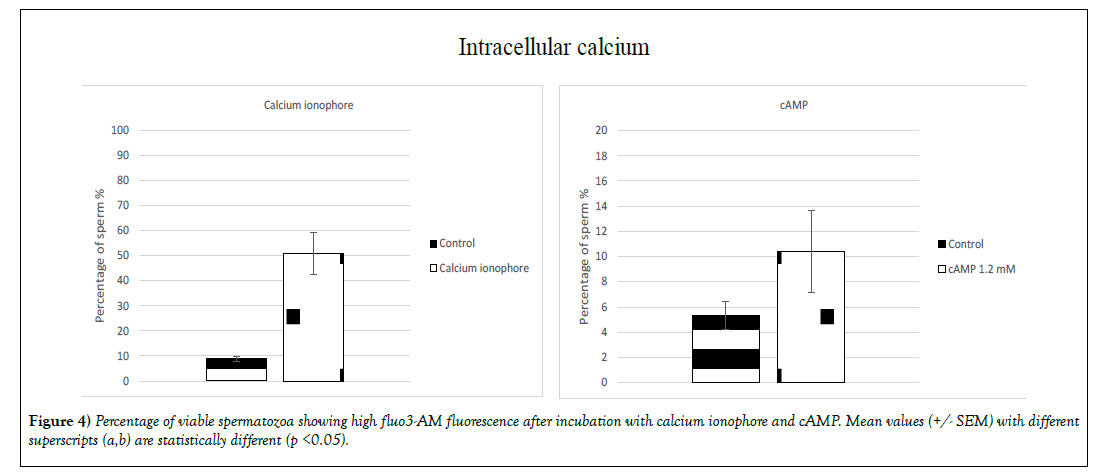
➢ Intracellular calcium
A significant (p<0.05) increase of the intracellular calcium concentration for sperm with intact membrane was induced by two molecules: calcium ionophore and cAMP (Figure 4). The percentage increase exhibiting highintensity fluo3-AM fluorescence was 42% when sperm was treated with calcium ionophore (9% +/- 0.9 for the control group and 51% +/- 8.5 for the treated group) and 5% for the sperm treated with cAMP (5% +/- 1.1 for the control group and 10% +/- 3.2 for the treated group). Surprisingly the treatment MβCD + cAMP did not significantly increase the percentage of population exhibiting high-intensity fluorescence (Figure 4).
Tyrosine Phosphorylation
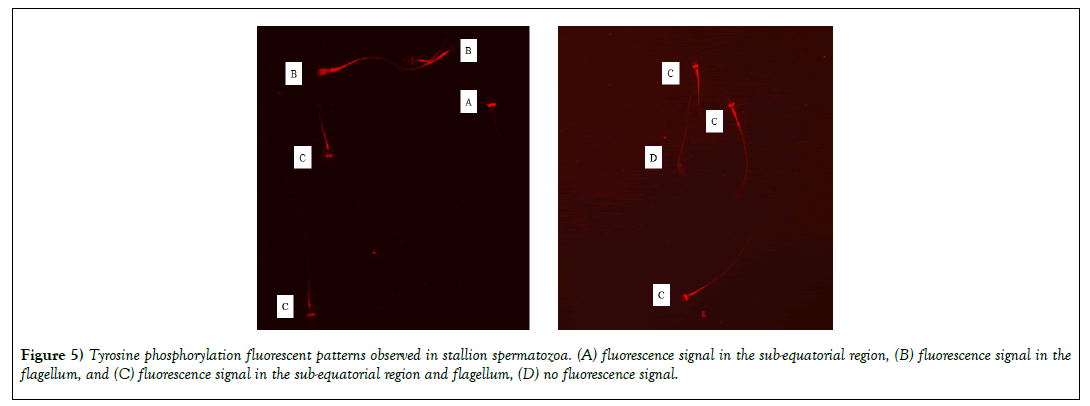
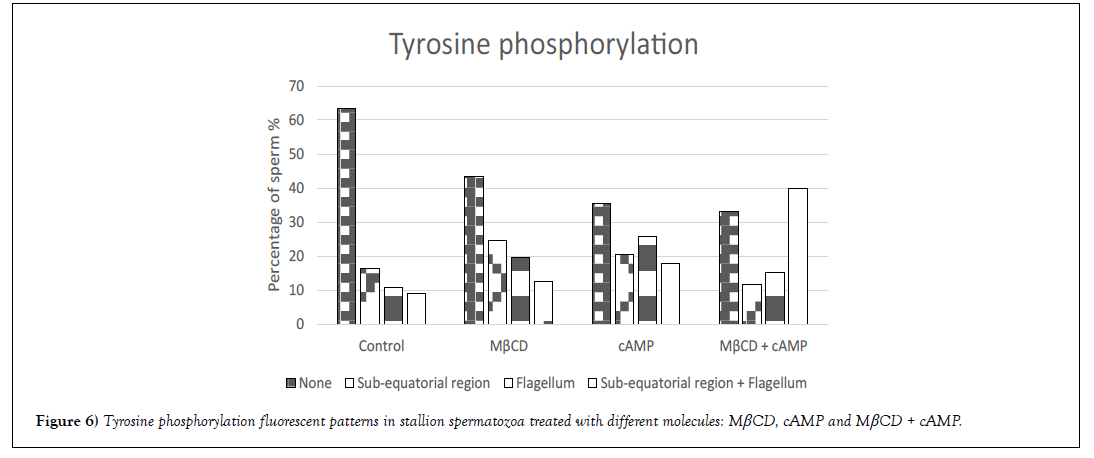
Tyrosine phosphorylation was evaluated after sperm incubation with MβCD, cAMP or the combination of both molecules. Different fluorescent patterns were observed in the different experimental groups (Figure 5): (A) fluorescence signal in the sub-equatorial region, (B) fluorescence signal in the flagellum, and (C) fluorescence signal in the sub-equatorial region and flagellum. A significant increase (p<0.01) in the percentage of fluorescent sperm was observed after incubation with the different molecules being 36%, 56%, 64% and 67% for control, MβCD, cAMP and MβCD + cAMP groups respectively. Moreover, different patterns were found to be more common depending on the molecule - for instance, the most common pattern for MβCD was in the sub-equatorial region, followed by the flagellum and the sub-equatorial region and flagellum (A>B>C), for cAMP the most common fluorescent pattern was found in the flagellum, followed by the subequatorial region and the sub-equatorial region and flagellum (B>A>C); and for MβCD + cAMP it was the sub-equatorial region and flagellum followed by the flagellum and by the sub-equatorial region(C>B>A) (Figure 6).
Lectin Binding
Glycoprofiling performed in this work consisted in the analysis of the lectin binding pattern of semen samples treated with two different molecules: MβCD, cAMP and MβCD + cAMP. Untreated semen (control) was also analyzed.
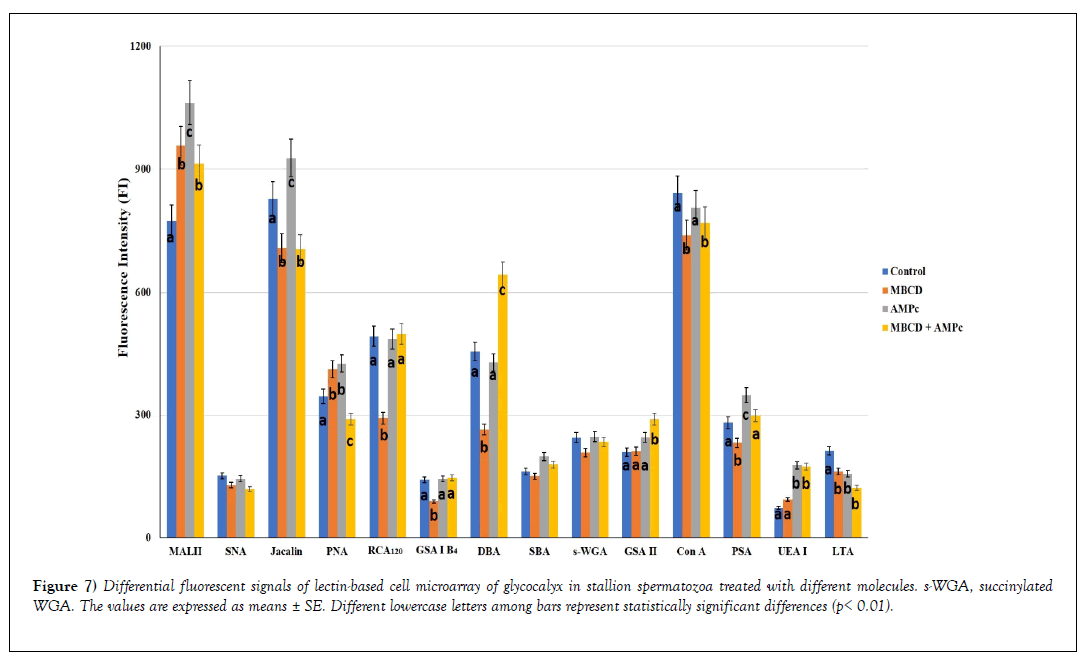
Figure 7 shows the lectin binding patterns from all the analyzed samples expressed as mean ± SE of Fluorescent Intensity (FI).
Figure 7: Differential fluorescent signals of lectin-based cell microarray of glycocalyx in stallion spermatozoa treated with different molecules. s-WGA, succinylated WGA. The values are expressed as means ± SE. Different lowercase letters among bars represent statistically significant differences (p< 0.01).
On the basis of FI revealed in untreated sperm (control), three range values were identified: 1) strong binding (FI ≥ 600) (MAL II, Jacalin and Con A affinity), 2) moderate binding (FI: 300-600) (PNA, RCA120 and DBA reactivity), 3) weak binding (FI: <300) (SNA, GSA I-B4, SBA, succinylated WGA, GSA II, PSA, UEA I, and LTA binding).
The comparison of lectin binding intensity between the control sperm vs treated one, as well as among treated sperm, revealed significant differences (decrease or increase), except for SNA, SBA and succinylated WGA.
The effect of these treatments on the binding pattern was as follows:
1. MβCD increased the reactivity of MAL II and PNA, whereas it reduced the affinity for the remaining lectins (Jacalin, RCA120, GSA I-B4, DBA, Con A, PSA, LTA);
2. cAMP increased the affinity with MAL II, Jacalin, PNA, PSA, and UEA I, whereas it only reduced the LTA binding;
3. MβCD + cAMP
a) decreased the effect of cAMP on MAL II, Jacalin, Con A, and PSA affinity;
b) increased the cAMP effect on DBA and GSA II reactivity;
c) maintained the increased reactivity of RCA120, GSA I-B4, and UEA I due to cAMP;
d) neutralized the increased affinity for PNA when MΒCD and cAMP were separately used.
Discussion
To fertilize the oocyte the sperm undergoes physiological and biochemical modifications known as “sperm capacitation” [22]. Some of these changes are related to an increase of intracellular calcium, an augmentation of membrane fluidity, the loss of cholesterol content or the phosphorylation of tyrosine residues [23-27] and surface glycan remodeling [38,40]. Previous studies have demonstrated the utility of flow cytometry [46-48], and immunofluorescence [49,50] to analyze these modifications on stallion sperm.
In this study, several molecules described as “capacitating molecules” have been incubated with stallion sperm in order to study capacitation-like changes and were analyzed by means of flow cytometry, immunofluorescence and lectin-based cell microarray. Frozen-thawed sperm was used because the use of frozen sperm for equine IVF is a good choice to apply this technique in the reproductive field.
During sperm capacitation an increase of the membrane fluidity is produced [54]. This modification has been studied in this work, labelling sperm with merocyanine 540 as previously published for stallion sperm by other authors [32,46,55]. It was previously published that HSPs have an action over membrane fluidity [56]. Specifically, HSPA8 has been tested on boar, bull and ram spermatozoa enhancing the in vitro sperm survival [31,57,58], and increasing the sperm membrane fluidity in pigs [31]; that effect was achieved with a concentration of 0.5 μg/mL. However, under our conditions only a higher dose (10 μg/mL) had an effect over membrane fluidity on stallion sperm. In this work other molecules, such as neurotensin (100 μM) and 4-AP (10 mM), have been demonstrated to produce a slightly effect over membrane fluidity. Whereas MβCD and cAMP induced higher modifications, similar results were previously reported for MβCD on stallion [32] and bull sperm [59]. In contrast, cAMP did not induce significant changes over membrane fluidity on Rhesus macaque sperm [60].
To study the concentration of intracellular calcium, sperm was labelled with fluo3-AM. To induce this effect in vitro, authors have used calcium ionophore A23187, as it increases the intracellular calcium levels in intact cells [61]; it has also been used to induce the acrosome reaction in several species [62-66]. Furthermore, this molecule was used to treat the sperm that originated the only two foals that were born by IVF in 1991 [9]; however, it also produces sperm immobilization although it does not affect to sperm fertility [26]. Among the different parameters studied on this work it only induced changes over the intracellular calcium concentration. Other molecule, cAMP has also induced significant changes over calcium concentration. On bull spermatozoa MβCD induces a rise over calcium concentration [59], however the same dose did not have an effect over stallion sperm. On the other hand, α-L-fucosidase and neurotensin increase the intracellular calcium concentration on boar and mice spermatozoa respectively [28,29], an equal and a higher dose of these molecules were used over stallion sperm; however, no differences on calcium concentration were found between control and treatment groups under our conditions. To the best of our knowledge there is no information available about the concentration of these molecules in the oviduct of the mare. 4-AP induces sperm hyperactivated motility on stallion spermatozoa [35], as demonstrated by Loux et al. Its effect is not mediated by a high rise in the intracellular calcium concentration; no effect over calcium entrance was found under this conditions.
Capacitation involves substantial reduction in membrane cholesterol [67]. To analyze the cholesterol depletion, stallion sperm was labelled with filipin III. The molecule MβCD is a cholesterol acceptor that has been extensively used for sperm capacitation in different species, for instance in mice, horses or bulls [32,33,59,68]. In our experiment 30 min of sperm incubation with 1mM of MβCD induced significant cholesterol depletion; this same result was demonstrated by Macías-García in stallions with a longer incubation [33], in bulls, mice [69] and boars [70]. However, it should be considered that stallion sperm viability is affected by this molecule as demonstrated for bull spermatozoa [59].
Thus, the aim of this study was to study capacitation like-changes on stallion sperm to test later on equine IVF. Considering that the more interesting effects were obtained with MβCD and cAMP, which induce modifications over calcium concentration, cholesterol depletion and membrane fluidity, a study of the tyrosine phosphorylation and the sperm surface glycopattern was also performed on sperm treated with these two molecules.
As previously demonstrated cAMP/PK-A regulates sperm capacitation and tyrosine protein phosphorylation [25,71]. Pommer and collaborators in 2003 [34] described four different patterns for tyrosine phosphorylation on stallion sperm - same patterns were also found under our conditions (none, sub-equatorial region, flagellum, and sub-equatorial region + flagellum). The percentage of fluorescent sperm increased after incubation with the different molecules (MβCD, cAMP or MβCD + cAMP) compared to the control group. Similar results after the incubation with MβCD were demonstrated by Western-blot analysis in stallions [34] bulls, mice [69] (Visconti et al. 1999b) or boars [70], whereas the study of tyrosine phosphorylation in bulls by means of flow cytometry did not induce significant differences [59]. The incubation of mice [24] and human sperm [67] with cAMP also induces an increase of tyrosine phosphorylation. Thus, sperm incubation with these molecules induces tyrosine phosphorylation on stallion sperm, being an important marker for sperm capacitation.
The lectin-binding signals indicated that sperm surface of the investigated specimens contained a complex glycan pattern constituted of N- and O-linked glycans. A previous lectin histochemistry study on the equine sperm surface glycoprofiling did not detect DBA, RCA120, and LTA binding sites [44]. This demonstrates that the microarray approach provides more information about the cell surface glycans than the lectin histochemistry, which is conventionally used for in situ profiling of mammalian cell glycocalyx. The spermatozoa used in this study reacted with all used lectins with different signal intensities, although the presence of glycans terminating with NeuNAcα2, 6Gal/GalNAc (SNA) and GalNAc (SBA) and containing GlcNAc (succinylated WGA) were not affected by the experimental design. It has been reported that during the capacitation WGA reactive glycoproteins are removed from boar [72–74] and bovine [75] sperm surface. This suggests that species-specific glycan changes occur during sperm capacitation.
Sperm glycocalyx N-glycans were high mannose and biantennary complex types (Con A) terminating with α2,6-linked sialic acid (SNA), lactosamine (RCA120), and fucose (PSA, UEA I, LTA). These sugar residues have been detected on the boar, bovine and goat spermatozoa glycocalyx in a lectin microarray study [76]. PSA binding sites have also been reported in a lectin histochemistry study on the buffalo bull sperm glycocalyx [77]. The N-glycans are characterized by very good flexibility, microheterogeneity, and due to these features they are responsible for the physicochemical properties of the glycocalyx [78]. In previous studies, it has been reported that oligomannosidic chains could have a recognition signal role in the sperm selection during their transit through the female reproductive tract [79] as well as they could be involved in the binding of sperm to zona pellucida in humans [80]. Con A reactivity has been related to the presence of pro/acrosin in swine spermatozoa [81].
Sperm glycocalyx O-glycans contained terminal α2,3-linked sialic acid, core 1 disaccharide (Galβ1,3GalNAc) (named T antigen) (Jacalin, PNA), Tn antigen (the simplest mucin O-glycan made a single GalNAc linked to serine or threonine) (SBA), and GalNAcα1,3(L-Fucα1,2)Galβ1, 3/4GlcNAcβ1 (DBA). The latter sugar sequence constitutes the glycocalyx of several mammal spermatozoa [76]. It has been reported that O-linked (mucin-type) glycans are involved in sperm-zona binding during the fertilization process and altered glycosylation of MUC1 mucin occurring in pathological conditions [82].
Concerning sialoglycans, as above mentioned, we found both α2,3- and α2,6-linked sialic acids (MAL II, SNA). Sialic acids are implicated in the sexual selection by female immunity against paternal antigens [83], protect sperm from immune recognition in the female reproductive tract [39] and constitute a component of reproductive compatibility in vivo [38].
The used capacitating agents induced glycan profile changes in horse spermatozoa glycocalyx. During the in vitro capacitation changes in the lectin binding patterns have been observed in some domestic animal species such as guinea pig [84], bovine [75], boar [72,73] and other mammals [40].
In the present study, MβCD and cAMP treatments increased O-linked glycans terminating with α2,3-linked sialic acid (MAL II) and T antigen (PNA), whereas decreased α1,3-linked fucose signals (LTA) cAMP increased Galβ1,3GalNAc (Jacalin) as well as α1,6- and α1,2-linked fucosides (PSA and UEA I). Furthermore, MβCD reduced Jacalin signals, indicating a decrease of T antigen penultimate to sialic acid which is not detected by PNA. The incubation with this medium also produced lower signals of lactosamine (RCA120), αGal (GSA I-B4), and GalNAcα1,3(LFucα1,2) Galβ1,3/4GlcNAcβ1 (DBA) terminating glycans as well as α1,6fucose (PSA) glycans. The combination of MβCD + cAMP treatment determined the increase of GalNAcα1,3(LFucα1,2)Galβ1,3/4GlcNAcβ1 (DBA), GlcNAc (GSA II), and α1,2-linked fucose (UEA I) signals, whereas reduced T antigen and high-mannose glycan signals (PNA, Con A). A reduction of Con A- and PNA-binding sites has been observed in capacitated boar spermatozoa [72]. Reports indicate that capacitation corresponds with an overall decrease in sialic acid content in mouse [85], and human [86]. Our results seem to disagree with the literature because we observed an significantly increase of α 2,3-linked sialic acid (MAL II). However, it is noteworthy that all our experiments induced a α2,6-linked sialic acid (SNA) decrease, although with no statistical significance. Concerning PSA reactivity, we did not consider contradictory the different PSA binding signals produced by MβCD and cAMP capacitating media. It has been reported a reduction of PSA binding in buffalo bull spermatozoa capacitated in modified Krebs-Ringer bicarbonate buffered medium [77]. This suggests that the glycan pattern expressed on sperm capacitated glycocalyx could depend on the capacitating media used. As lectin affinity is strongly affected by the stearic hindrance of the ligand oligosaccharide structure [87], the observed difference in lectin signals could be related to the molecular remodeling occurring during the capacitation. Lastly, it is important to note that glycosylation is four-dimensional, rather than three-dimensional, with the added dimension varying recognition by specific receptor type molecule such as lectins.
To sum up, the lectin based sperm microarray procedure demonstrated that the capacitation procedure induces changes in the glycosylation pattern of stallion sperm glycocalyx which could be affected by the capacitating media used.
Finally, in this study some capacitation-related modifications have been investigated on stallion sperm; however, it would be interesting to analyze some other criteria. To this end, the effect of these molecules on acrosome reaction will be investigated further.
CONCLUSION
In this work several molecules have been tested over stallion sperm to investigate their effect on capacitation by means of flow cytometry and immunofluorescence; moreover, modifications induced on glycocalyx sperm sugars were studied by means of the lectin-based cell microarray analysis. MβCD and cAMP induce changes over membrane fluidity, intracellular calcium concentration, cholesterol depletion, tyrosine phosphorylation and the sperm glycosylation pattern. Thus, these molecules must be considered as good agents to be tested on equine IVF.
Source of Funding
Carla Moros-Nicolás has been supported by a post-doctoral fellowship from “Ministry of Education and Universities of the CARM, Seneca-Agency Science and Technology Foundation of the Region of Murcia “. This work was financially supported by the “French Institute of the Horse and Riding” (IFCE) and by grants from Development and Cohesion Fund 2007-13-APQ Research Puglia Region ‘Regional program to support smart specialization and social sustainability and environmental – Future In Research’
Acknowledgements
Authors want to acknowledge Dr. Alireza Fazeli and Dr. Sarah Elliott for supplying us with the HSPA8. We also thank Dr. Rebeca López-Úbeda and Dr. Luis Vieira for their advices about immunofluorescence analysis, Pierre Milon for the sperm freezing and Fabrice Reigner and his team at the Unité Expérimentale de Physiologie Animale de l’Orfrasière (UEPAO) for their support with the stallions. This work has benefited from the facilities and expertise of the “Platform Cell Imaging” (PIC) of the UMR 85 “Physiology of Reproduction and Behavior”.
Following sperm-oolemma binding, fusion between the two gamete plasma membranes marks the last step in the fertilization process. One of the first proteins that were shown to be important for gamete fusion was the membrane protein CD9 [24]. CD9-deficient oocytes show normal sperm binding but impaired fusion and hence CD9 appears to be important, however not essential for fusion. Together with CD9, also tetraspanins CD81 and CD151 were reported to play complementary roles in sperm-egg fusion [2]. Tetraspanins are well known to form extensive intermolecular interaction networks and hence it appears very likely that these molecules do not directly facilitate gamete fusion but are essential to form oolemma raft -like microdomain structures. In this way, fusogens can not only be enriched on either side but can also be brought into close spatial proximity to ultimately enable fusion between the two gametes. In addition to integral membrane proteins, GPI-anchored proteins were shown to be important for gamete fusion. Oocyte- specific disruption of the synthesis of GPI-anchored proteins was demonstrated to result in a phenotype similar to CD9-deficient mice [25]. On the sperm side, the plasma membrane protein DE, also known as cysteine-rich secretory protein 1 (CRISP1), was shown to be important for gamete fusion. Crisp1-deficient male mice are fertile, but sperm of these mice showed impaired fusion [26]. In addition, sperm proteins ADAMs are not only important for oocyte binding, but may also be essential for fusion [27].
In summary, the beginning of new life is a complex and highly regulated multi-step process involving several levels of oocyte-sperm interaction. Despite extensive research, we still face a lot of open questions. Therefore, improving our mechanistic understanding of fertilization could provide new opportunities to improve assisted fertilization, not only in human medicine, but for example also in stockbreeding or endangered species. Furthermore, additional discoveries could lead to novel explanations for so far idiopathic infertility.
REFERENCES
- Alderson GLH. The Numerical and Genetic Status of Native Horse and Pony Breeds in Britain. In: Bodó I, Alderson L, Langlois B, Conservation Genetics of Endangered Horse Breeds. Bled, Slovenia: Wageningen Academic Publishers, 2005;116: 91-98.
- Adams GP, Ratto MH, Collins CW, et al. Artificial Insemination in South American Camelids and Wild Equids. Theriogenology 2009;71: 166–175.
- Smits K, Hoogewijs M, Woelders H, et al. Breeding or Assisted Reproduction? Relevance of the Horse Model Applied to the Conservation of Endangered Equids. Reprod Domest Anim. 2012; 47:239-248.
- Goudet G, Douet C, Kaabouba-Escurier A, et al. Establishment of Conditions for Ovum Pick up and IVM of Jennies Oocytes toward the Setting up of Efficient IVF and in vitro Embryos Culture Procedures in Donkey (Equus asinus). Theriogenology. 2016; 86:528-535.
- Gandini G, Pizzi F, Stella A, et al. The Costs of Breed Reconstruction from Cryopreserved Material in Mammalian Livestock Species. Genet Sel Evol .2007; 39:465-479.
- Choi YH, Varner DD, Love CC, et al. Production of Live Foals via Intracytoplasmic Injection of Lyophilized Sperm and Sperm Extract in the Horse. Reproduction. 2011;142:529-538.
- Hinrichs K. Assisted Reproduction Techniques in the Horse. Reprod Fertil Dev. 2012;25:80-93.
- Leemans B, Gadella BM, Stout TAE, et al. Why Doesn’t Conventional IVF Work in the Horse? The Equine Oviduct as a Microenvironment for Capacitation/fertilization. Reproduction. 2016; 152:233-245.
- Palmer E, Bézard J, Magistrini M, Duchamp G. In vitro fertilization in the Horse. A Retrospective Study. J Reprod Fertil. 1991;44:375-384.
- Mugnier S, Kervella M, Douet C, et al. The Secretions of Oviduct Epithelial Cells Increase the Equine in vitro fertilization Rate: Are Osteopontin, Atrial Natriuretic Peptide A and Oviductin Involved? Reprod Biol Endocrinol .2009;7:129.
- Del Campo MR, Donoso MX, Parrish JJ, e al. In vitro fertillization of in-vitro matured equine oocytes. J Equine Vet Sci .1990;10:18–22.
- Zhang JJ, Muzs LZ, Boyle MS. In vitro fertilization of Horse Follicular Oocytes Matured in vitro. Mol Reprod Dev. 1990;26:361-365.
- Choi YH, Okada Y, Hochi S, et al. In vitro fertilization Rate of Horse Oocytes with Partially Removed Zonae. Theriogenology. 1994; 42:795-802.
- Gr0ndahl C, Hst T, Brck I, et al. In vitro production of equine embryos. In: Sharp DC, Bazer FW (eds.), Equine Reproduction VI. Madison, WI: Society for the Study of Reproduction 1995;1:299-307.
- Alm H, Torner H, Blottner S, et al. Effect of Sperm Cryopreservation and Treatment with Calcium Ionophore or Heparin on in vitro fertilization of Horse Oocytes. Theriogenology,. 2001;56:817-829.
- Dell’Aquila ME, Fusco S, Lacalandra GM, et al. In vitro Maturation and Fertilization of Equine Oocytes Recovered during the Breeding Season. Theriogenology .1996;45:547-560.
- Dell’Aquila ME, Cho YS, Minoia P et al. Intracytoplasmic Sperm Injection (ICSI) versus Conventional IVF on Abattoir-Derived and in vitro-Matured Equine Oocytes. Theriogenology. 1997;47:1139-1156.
- Roasa LM, Choi YH, Love CC, et al. Ejaculate and Type of Freezing Extender Affect Rates of Fertilization of Horse Oocytes in vitro. Theriogenology. 2007;68:560-566.
- McPartlin LA, Suarez SS, Czaya CA, et al. Hyperactivation of Stallion Sperm Is Required for Successful in vitro fertilization of Equine Oocytes. Biol Reprod 2009;81 :199-206.
- Ambruosi B, Accogli G, Douet C, et al. Deleted in Malignant Brain Tumor 1 Is Secreted in the Oviduct and Involved in the Mechanism of Fertilization in Equine and Porcine Species. Reproduction 2013;146:119–133.
- Leemans B, Gadella BM, Stout TAE, et al. Procaine Induces Cytokinesis in Horse Oocytes via a pH-Dependent Mechanism. Biol Reprod. 2015;93:23.
- Yanagimachi R. Mammalian Fertilization. In: Physiology of Reproduction. New York: Knobil and Neil J. 1994.
- Visconti PE, Bailey JL, Moore GD, et al. Capacitation of Mouse Spermatozoa. I. Correlation between the Capacitation State and Protein Tyrosine Phosphorylation. Development. 1995;121:1129-1137.
- Visconti PE, Stewart-Savage J, Blasco A, et al. Roles of Bicarbonate, cAMP, and Protein Tyrosine Phosphorylation on Capacitation and the Spontaneous Acrosome Reaction of Hamster Sperm. Biol Reprod.1999;61:76-84.
- Flesch FM, Gadella BM. Dynamics of the Mammalian Sperm Plasma Membrane in the Process of Fertilization. Biochim Biophys Acta. 2000;1469:197-235.
- Tateno H, Krapf D, Hino T, et al. Ca2+ Ionophore A23187 Can Make Mouse Spermatozoa Capable of Fertilizing in vitro without Activation of cAMP-Dependent Phosphorylation Pathways. Proc Natl Acad Sci U S A.2013;110:18543-18548.
- Buffone MG, Wertheimer EV, Visconti PE, et al. Central Role of Soluble Adenylyl Cyclase and cAMP in Sperm Physiology. Biochim Biophys Acta. 2014;1842:2610-2620.
- Romero-Aguirregomezcorta J, Matás C, Coy P. α-L-Fucosidase Enhances Capacitation-Associated Events in Porcine Spermatozoa. Vet J. 2015;203:109-114.
- Hiradate Y, Inoue H, Kobayashi N, et al. Neurotensin Enhances Sperm Capacitation and Acrosome Reaction in Mice. Biol Reprod.2014;91:53.
- Umezu K, Hiradate Y, Oikawa T, et al. Exogenous Neurotensin Modulates Sperm Function in Japanese Black Cattle. J Reprod Develop. 2016;62:409-414.
- Moein-Vaziri N, Phillips I, Smith S, et al. Heat-Shock Protein A8 Restores Sperm Membrane Integrity by Increasing Plasma Membrane Fluidity. Reproduction 2014;147:719-732.
- Bromfield EG, Aitken RJ, Gibb Z, et al. Capacitation in the Presence of Methyl-β-Cyclodextrin Results in Enhanced Zona Pellucida-Binding Ability of Stallion Spermatozoa. Reproduction. 2014;147:153-166.
- Macías-García B, González-Fernández L, Loux SC, et al. Effect of Calcium, Bicarbonate, and Albumin on Capacitation-Related Events in Equine Sperm. Reproduction. 2015;149:87-99
- Pommer AC, Rutllant J, Meyers SA. Phosphorylation of Protein Tyrosine Residues in Fresh and Cryopreserved Stallion Spermatozoa under Capacitating Conditions. Biol Reprod. 2003;68:1208-1214.
- Loux SC, Crawford KR, Ing NH, et al. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol Reprod. 2013;89:123.
- González-Fernández L, Macías-García B, Velez IC, et al. Calcium-calmodulin and pH regulate protein tyrosine phosphorylation in stallion sperm. Reproduction.2012;144:411-22.
- Aalberts M, Sostaric E, Wubbolts R, et al. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta. 2013;1834:2326-2335.
- Tecle E, Gagneux P. Sugar-Coated Sperm: Unraveling the Functions of the Mammalian Sperm Glycocalyx. Mol Reprod Dev. 2015;82:635-650.
- Tollner TL, Bevins CL, Cherr GN. Multifunctional Glycoprotein DEFB126--a Curious Story of Defensin-Clad Spermatozoa. Nat Rev Urol. 2012;9:365-375.
- Liu M. Capacitation-Associated Glycocomponents of Mammalian Sperm. Reprod Sci.2016;23:572-594.
- Sharon N, Lis H. Glycobiology. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53-62.
- Calvete JJ, Raida M, Sanz L, et al. Localization and Structural Characterization of an Oligosaccharide O-Linked to Bovine PDC-109. Quantitation of the Glycoprotein in Seminal Plasma and on the Surface of Ejaculated and Capacitated Spermatozoa. FEBS Lett. 1994;350:203-206.
- Chandra A, Srinivasan KR, Jamal F, et al. Post-translational Modifications in Glycosylation Status during Epididymal Passage and Significance in Fertility of a 33 kDa Glycoprotein (MEF3) of Rhesus Monkey (Macaca mulatta). Reproduction. 2008;135 :761-770.
- Desantis S, Ventriglia G, Zizza S, et al. Lectin-binding Sites on Ejaculated Stallion Sperm During Breeding and Non-breeding Periods. Theriogenology. 2010;73:1146-1153.
- Accogli G, Lacalandra GM, Aiudi G, et al. Differential Surface Glycoprofile of Buffalo Bull Spermatozoa during Mating and Non-Mating Periods. Animal .2017; 1-9.
- Barrier-Battut I, Kempfer A, Becker J, et al. Development of a New Fertility Prediction Model for Stallion Semen, Including Flow Cytometry. Theriogenology. 2016;86:1111-1131.
- Peña FJ, Martín-Muñoz P, Ortega-Ferrusola C. Flow Cytometry Probes to Evaluate Stallion Spermatozoa. J Equine Vet Sci. 2016;33:23-28.
- Peña FJ, Ortega-Ferrusola C, Martín-Muñoz P. New Flow Cytometry Approaches in Equine Andrology. Theriogenology. 2016;86:366–372.
- González-Fernández L, Macías-García B, Loux SC, et al. Focal Adhesion Kinases and Calcium/calmodulin-Dependent Protein Kinases Regulate Protein Tyrosine Phosphorylation in Stallion Sperm. Biol Reprod. 2013;88:138.
- Vieira LA, Gadea J, García-Vázquez FA, et al. Equine Spermatozoa Stored in the Epididymis for up to 96h at 4°C Can Be Successfully Cryopreserved and Maintain Their Fertilization Capacity. Anim Reprod Sci.2013;136:280-288.
- Accogli G, Desantis S, Martino NA, et al. A lectin-based Cell Microarray Approach to Analyze the Mammalian Granulosa Cell Surface Glycosylation Profile. Glycoconj J. 2016;33:717-724.
- Pillet E, Labbe C, Batellier F, et al. Liposomes as an Alternative to Egg Yolk in Stallion Freezing Extender. Theriogenology. 2012;77:268-279.
- Matás C, Vieira L, García-Vázquez FA, et al. Effects of Centrifugation through Three Different Discontinuous Percoll Gradients on Boar Sperm Function. Anim Reprod Sci.2011;127:62-72.
- Wolf DE, Hagopian SS, Lewis RG, et al. Lateral Regionalization and Diffusion of a Maturation-Dependent Antigen in the Ram Sperm Plasma Membrane. J Cell Biol. 1986;102:1826-1831.
- da Silva C, Balao da M, Macías-García B, et al. Melatonin Reduces Lipid Peroxidation and Apoptotic-like Changes in Stallion Spermatozoa. J Pineal Res .2011;51:172-179.
- Horváth I, Multhoff G, Sonnleitner A, Vígh L. Membrane-Associated Stress Proteins: More than Simply Chaperones. Biochim Biophys Acta.2008;1778:1653-1664.
- Elliott RM, Lloyd RE, Fazeli A, et al. Effects of HSPA8, an Evolutionarily Conserved Oviductal Protein, on Boar and Bull Spermatozoa. Reproduction.2009;137:191-203.
- Lloyd RE, Elliott RMA, Fazeli A, et al. Effects of Oviductal Proteins, Including Heat Shock 70 kDa Protein 8, on Survival of Ram Spermatozoa over 48 H in vitro. Reprod Fertil Dev.2009;21:408-418.
- Águila L, Arias ME, Vargas T, et al. Methyl-β-Cyclodextrin Improves Sperm Capacitation Status Assessed by Flow Cytometry Analysis and Zona Pellucida-Binding Ability of Frozen/Thawed Bovine Spermatozoa. Reprod Domest Anim.2015;50:931-938
- Baumber J, Meyers SA. Changes in Membrane Lipid Order with Capacitation in Rhesus Macaque (Macaca mulatta) Spermatozoa. J Androl.2006;27:578-587.
- Reed PW, Lardy HA. A23187: A Divalent Cation Ionophore. J Biol Chem.1972.247:6970-6977.
- Talbot P, Summers RG, Hylander BL, et al. The Role of Calcium in the Acrosome Reaction: An Analysis Using Ionophore A23187. J Exp Zool.1976;198:383-392.
- Reyes A, Goicoechea B, Rosado A. Calcium Ion Requirement for Rabbit Spermatozoal Capacitation and Enhancement of Fertilizing Ability by Ionophore A23187 and Cyclic Adenosine 3’:5’-monophosphate Fertil Steril.1978;29:451-455.
- Suarez SS, Vincenti L, Ceglia MW. Hyperactivated Motility Induced in Mouse Sperm by Calcium Ionophore A23187 Is Reversible. J Exp Zool.1987;244:331-336.
- Uçar O, Parkinson TJ. In vitro Induction of the Acrosome Reaction in Ovine Spermatozoa by Calcium Ionophore A23187. Acta Vet Hung.2003;51:103-109.
- Zhang J, Ding X, Bian Z, et al. The Effect of Anti-Eppin Antibodies on Ionophore A23187-Induced Calcium Influx and Acrosome Reaction of Human Spermatozoa. Hum Reprod.2010;25:29-36.
- Osheroff JE, Visconti PE, Valenzuela JP, et al. Regulation of Human Sperm Capacitation by a Cholesterol Efflux-Stimulated Signal Transduction Pathway Leading to Protein Kinase A-Mediated up-Regulation of Protein Tyrosine Phosphorylation. Mol Hum Reprod.1999;5:1017-1026.
- Takeo T, Hoshii T, Kondo Y, et al. Methyl-Beta-Cyclodextrin Improves Fertilizing Ability of C57BL/6 Mouse Sperm after Freezing and Thawing by Facilitating Cholesterol Efflux from the Cells. Biol Reprod.2008;78:546-551.
- Visconti PE, Galantino-Homer H, Ning X, et al. Cholesterol Efflux-Mediated Signal Transduction in Mammalian Sperm. Beta-Cyclodextrins Initiate Transmembrane Signaling Leading to an Increase in Protein Tyrosine Phosphorylation and Capacitation. J Biol Chem. 1999;274:3235-3242.
- Shadan S, James PS, Howes EA, et al. Cholesterol Efflux Alters Lipid Raft Stability and Distribution during Capacitation of Boar Spermatozoa. Biol Reprod.2004; 71:253-265.
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of Protein Tyrosine Phosphorylation during Bovine Sperm Capacitation by a Cyclic Adenosine 3’5’-monophosphate-Dependent Pathway. Biol Reprod.1997;56:707-719.
- Vazquez JM, Martinez E, Pastor LM, et al. Lectin Histochemistry during in vitro Capacitation and Acrosome Reaction in Boar Spermatozoa: New Lectins for Evaluating Acrosomal Status of Boar Spermatozoa. Acta Histochem.1996;98:93-100.
- Jiménez I, Gonzalez-Marquez H, Ortiz R, et al. Expression of Lectin Receptors on the Membrane Surface of Sperm of Fertile and Subfertile Boars by Flow Cytometry. Arch Androl.2002; 48:159-166.
- Garénaux E, Kanagawa M, Tsuchiyama T, et al. Discovery, Primary, and Crystal Structures and Capacitation-Related Properties of a Prostate-Derived Heparin-Binding Protein WGA16 from Boar Sperm. J Biol Chem.2015;290:5484-5501.
- Medeiros CM, Parrish JJ. Changes in Lectin Binding to Bovine Sperm during Heparin-Induced Capacitation. Mol Reprod Dev.1996;44:525-532.
- Xin AJ, Cheng L, Diao H, et al. Comprehensive Profiling of Accessible Surface Glycans of Mammalian Sperm Using a Lectin Microarray. Clin Proteomics.2014;11:10.
- Kaul G, Sharma GS, Singh B, et al. Capacitation and Acrosome Reaction in Buffalo Bull Spermatozoa Assessed by Chlortetracycline and Pisum Sativum Agglutinin Fluorescence Assay. Theriogenology.2001;55:1457-1468.
- Schröter S, Osterhoff C, McArdle W, et al. The Glycocalyx of the Sperm Surface. Hum Reprod Update.1999; 5:302-313.
- Nardone P, Cerezo AS, de Cerezo JM. Cytochemical Characterization and Localization of Ligomannosidic Oligosaccharide Receptors on the Normal Human Spermatozoa Using Fluorescent Lectins: Comparison of Different Fixation Procedures. Am J Reprod Immunol .1985;9:124-128.
- Chen JS, Doncel GF, Alvarez C, Acosta AA. Expression of Mannose-Binding Sites on Human Spermatozoa and their Role in Sperm-Zona Pellucida Binding. J Androl. 1995;16:55-63.
- Töpfer-Petersen E, Calvete J, Schäfer W, et al. Complete Localization of the Disulfide Bridges and Glycosylation Sites in Boar Sperm Acrosin. FEBS Lett.1990;275:139-142.
- Franke FE, Kraus S, Eiermann C, et al. MUC1 in Normal and Impaired Spermatogenesis. Mol Hum Reprod.2001;7:505-512.
- Ghaderi D, Springer SA, Ma F, et al. Sexual Selection by Female Immunity Against Paternal Antigens Can Fix Loss of Function Alleles. Natl Acad Sci USA.2011;108:17743-17748.
- Schwarz MA, Koehler JK. Alterations in Lectin Binding to Guinea Pig Spermatozoa Accompanying in vitro Capacitation and the Acrosome Reaction. Biol Reprod. 1979;21:1295-1307.
- Ma F, Wu D, Deng L, et al. Sialidases on mammalian sperm mediate deciduous sialylation during capacitation. J Biol Chem.2012;287:38073-38079.
- Focarelli R, Rosati F, Terrana B. Sialyglycoconjugates Release during in vitro Capacitation of Human Spermatozoa. J Androl.1990;11:97-104.
- Spicer SS, Schulte BA. Diversity of Cell Glycoconjugates Shown Histochemically: A Perspective. J Histochem Cytochem. 1992;40:1-38.