Is your sterile equipment ready to be used? Really? Look again!
Received: 15-Jan-2018 Accepted Date: Jan 23, 2018; Published: 02-Mar-2018
Citation: Tolosa M, Fowler S. Is your sterile equipment ready to be used? Really? Look again! J Nurs Res Pract. 2018;2(1):20.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
For decades, anesthesia providers have been involved in performing invasive procedures for patients for a variety of surgical procedures. Some of these procedures may include placement of peripheral or central venous or arterial catheters, neuraxial anesthesia like spinal or epidural techniques, and peripheral nerve blocks for analgesia or anesthesia, just to name a few.
The rate of adverse outcomes is closely related to the functionality and sterility of the equipment as well as the knowledge and skills of the anesthesia providers. Often, anesthesia providers assume that the surgical-grade supplies are considered sterile, non-contaminated, and in perfect working conditions as long as the sterile package is unopened and undamaged. Most manufacturing companies are required to place labels on their products stating “STERILE EO in unopened, undamaged package”.
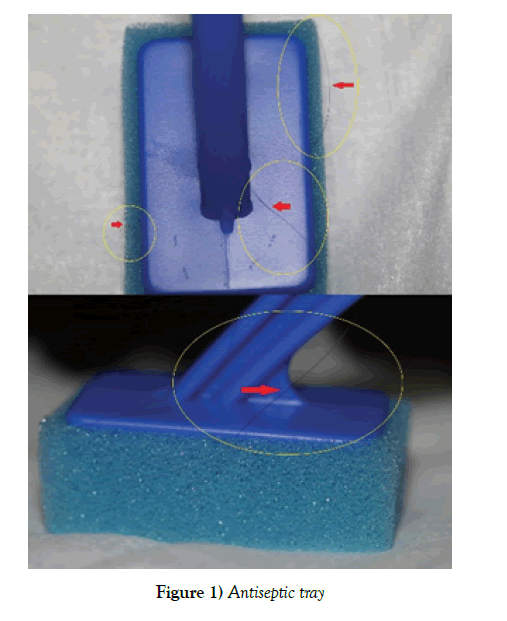
Recently, our team was preparing to perform an elective cesarean delivery on an otherwise healthy, term parturient. At our facility, we use Pencan Spinal Needle Trays by B. Braun Medical Inc. Bethlem, PA 18018-3524. As the Certified Registered Nurse Anesthetist (CRNA) was preparing the tray, she noticed something that stood out from the prep/antiseptic tray where the prep sponges, along with the Povidone-Iodine solution and the 4 in. X 4 in. gauze sponges were. Upon closer look, she noticed a foreign body (hair) wrapped in and around one of the prep sponges (Figure 1).
Initially, the provider thought that maybe one of her hairs had fallen into the tray. Closer observation of the sponge revealed that the hair was between the sponge and the plastic stick, which may suggest that the hair was glued in during the manufacturing process of the prep sponge. This prep sponge with the hair was then included as part of the spinal tray and sterilized as per the manufacturer standard specifications. Since the referred package was unopened and undamaged, would you, an anesthesia provider, have continued to use the “sterile” spinal tray? In our case, we discarded this spinal tray and used a new one. The cesarean section proceeded uneventful without complications. The patient was never harmed.
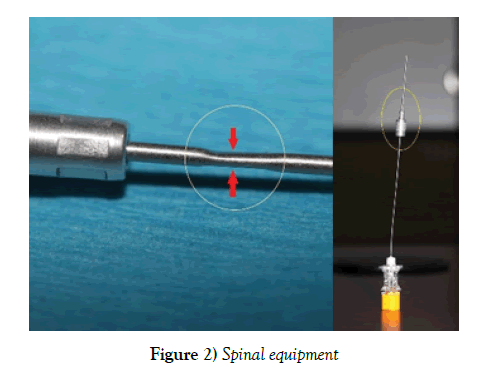
On a separate incident, one of our Student Registered Nurse Anesthetists (SRNA) was performing the required simulated graded demonstration of a spinal anesthetic technique using a new and unopened Pencan Spinal Needle Tray by B. Braun Medical Inc. Bethlem, PA 18018-3524. The simulated back model was prepped and draped using the standard sterile fashion. The “skin” was localized at the level of L3-L4, and the 20 Ga. X 1-1/4 in introducer needle was inserted without resistance. The SRNA inserted the Pencan 25 Ga. X 3-1/2 in. Spinal Needle through the introducer and verbalized hitting bone despite multiple attempts and redirection of the spinal needle and introducer. After careful examination, of the spinal equipment, we noticed a crimp located 3 mm on the introducer needle, proximal to the hub (Figure 2). The tray was discarded and the SRNA could perform the skill with a new tray.