Multiple Variation in the Upper Abdominal Aorta: A Unique Cadaveric Case Report and Clinical Implications
2 Division of Education, School of Biomedical Sciences, Faculty of Medicine, CUHK, Hong Kong
Received: 01-Apr-2024, Manuscript No. ijav-24-7014; Editor assigned: 04-Apr-2024, Pre QC No. ijav-24-7014 (PQ); Reviewed: 22-Apr-2024 QC No. ijav-24-7014; Revised: 26-Apr-2024, Manuscript No. ijav-24-7014 (R); Published: 30-Apr-2024, DOI: 10.37532/1308-4038.17(4).380
Citation: Mak YS. Multiple Variation in the Upper Abdominal Aorta A Unique Cadaveric Case Report and Clinical Implications. Int J Anat Var. 2024;17(4): 524-527.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The arterial vasculature supplying upper abdominal organs demonstrates a high degree of variation. Such variations pose considerable challenges to hepatic surgical procedures like transarterial chemoembolization (TACE), treating inoperable hepatocellular carcinoma (HCC) or trauma handling complicated. We, therefore, report a rare Chinese cadaveric case of coexistence of 4 anatomical variations branching in the foregut of the abdominal aorta, including (1) separate origins of inferior phrenic arteries from the celiac trunk (2) trifurcation of the common hepatic artery, (3) an aberrant right gastric artery origin, and (4) accessory right hepatic artery originating from the celiac trunk from a Chinese female donor body during the human dissection enhancement workshop carried out in CUHK. With such a special case discovered in a Chinese cadaver, this paper explores the related clinical significance and helps educate medical practitioners to minimize complications related to arterial variation.
Keywords
Celiac trunk; Anatomic variations; Panagouli classification; Right gastric artery; Common hepatic artery
INTRODUCTION
The upper abdominal aorta typically branches into the celiac trunk and superior mesenteric artery at the lesser omentum to supply the stomach, spleen, liver, and small intestine. The arterial vasculature supplying upper abdominal organs demonstrates a high degree of variation reported in various publications [1], thereby increasing the complexity and difficulty of diagnostic imaging and therapeutic procedures. The celiac axis, celiac artery or celiac trunk CT, the first abdominal branch of the aorta at the level of T12/L1 vertebrae passing below the median arcuate ligament of the aortic hiatus, serves as the primary arterial blood supply of the foregut. In 87.6% majority, the common hepatic artery (CHA), left gastric artery (LGA) and splenic artery (SA) share a common origin from the celiac trunk [2,3]. The inferior phrenic arteries (IPA) can arise from a common IPA trunk or independently from either the aorta or celiac trunk [4]. The CHA after giving branch to the gastroduodenal artery (GDA) continues as the hepatic artery proper (HAP). HAP gives rise to the right gastric artery (RGA) and bifurcates into left and right hepatic arteries [5]. The right accessory hepatic artery (aRHA) is an arterial branch of the right liver run in the portacaval space, which commonly derives from superior mesenteric artery (SMA) [6].
A major classification of CT branching variation was proposed by Panagouli et al (2013), upon a systematic review of 12,196 cases [1]. Trifurcation of the CT into LGA, CHA, and SA (Panagouli type I), bifurcation of the CT (Panagouli type II), and additional branches existing in the CT (Panagouli type III) have been recorded with a prevalence of 89.42%, 7.40%, and 1.06% respectively.
Concerning the blood supplies of the CT to the stomach, spleen, liver, gallbladder, and pancreas, the vascular variation of the celiac trunk and its branches are essential in planning surgical interventions. Medical educational trainings, radiologists and clinicians should be aware of the presence of any variations of abdominal aorta, particularly during liver transplantation, chemotherapy for hepatocellular carcinoma (HCC) and other abdominal surgeries, which minimize the treatments to lower risks and avoid complications [6,7]. We have investigated and aimed to report the rare from the CT co-existence of the four variant branches from the celiac trunk and its branches in a single body donor from a Chinese population as none of researchers have documented such variation patterns from the literature review present their importance.
MATERIALS AND METHODS
During a dissection enhancement workshop held between early June to early August 2023 in the Faculty of Medicine, The Chinese University of Hong Kong (CUHK), Hong Kong, we found a few variations in vascular pattern in the upper abdominal region on an 86-year-old Chinese female body donor, who was one of ‘silent teachers’ from the CUHK body donation program and signed up the consent form to express their voluntary commitment to contribute to anatomy education.
CASE REPORT
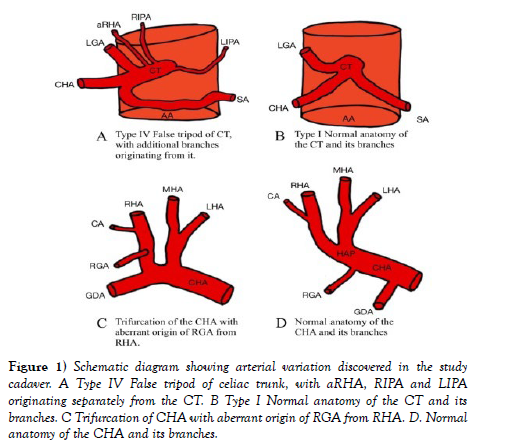
From the investigation of the dissection, the multiple variation branches were identified, and unseen of the arterial blood supply of the foregut as shown in schematic diagram (Figure 1). A false tripod was displayed in which the celiac trunk gave rise to the LGA before bifurcating into the CHA and the SA.
Figure 1) Schematic diagram showing arterial variation discovered in the study cadaver. A Type IV False tripod of celiac trunk, with aRHA, RIPA and LIPA originating separately from the CT. B Type I Normal anatomy of the CT and its branches. C Trifurcation of CHA with aberrant origin of RGA from RHA. D. Normal anatomy of the CHA and its branches.
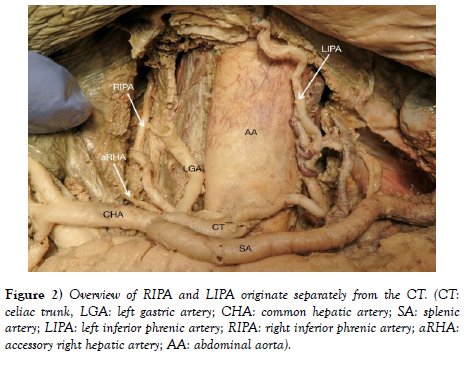
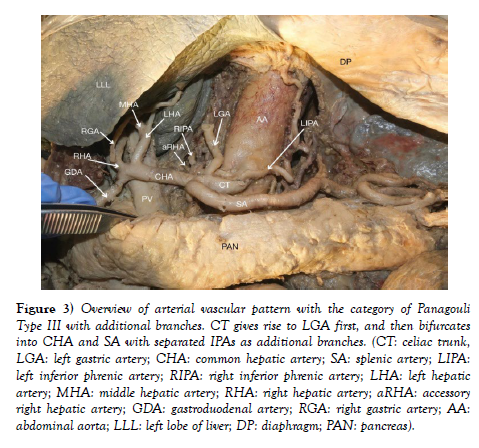
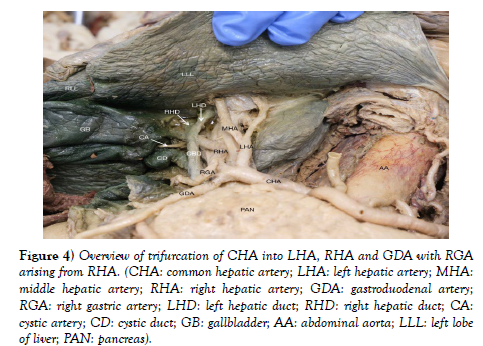
IPAs originated individually from the celiac trunk without a common trunk, demonstrating the variant pattern of Panagouli type III (Figures 2 and Figure 3). Trifurcation of the CHA was observed when it divided into the GDA, LHA and RHA, resulting in the absence of the hepatic artery proper. The RGA originates from the RHA instead of the hepatic artery proper (Figure 3 and Figure 4. The accessory right hepatic artery found incidentally branches from the celiac trunk (Figure 2 and Figure 3).
Figure 3) Overview of arterial vascular pattern with the category of Panagouli Type III with additional branches. CT gives rise to LGA first, and then bifurcates into CHA and SA with separated IPAs as additional branches. (CT: celiac trunk, LGA: left gastric artery; CHA: common hepatic artery; SA: splenic artery; LIPA: left inferior phrenic artery; RIPA: right inferior phrenic artery; LHA: left hepatic artery; MHA: middle hepatic artery; RHA: right hepatic artery; aRHA: accessory right hepatic artery; GDA: gastroduodenal artery; RGA: right gastric artery; AA: abdominal aorta; LLL: left lobe of liver; DP: diaphragm; PAN: pancreas).
Figure 4) Overview of trifurcation of CHA into LHA, RHA and GDA with RGA arising from RHA. (CHA: common hepatic artery; LHA: left hepatic artery; MHA: middle hepatic artery; RHA: right hepatic artery; GDA: gastroduodenal artery; RGA: right gastric artery; LHD: left hepatic duct; RHD: right hepatic duct; CA: cystic artery; CD: cystic duct; GB: gallbladder; AA: abdominal aorta; LLL: left lobe of liver; PAN: pancreas).
DISCUSSION
In Figure 1 of the case, additional branches arise from the CT. Therefore, it is classified as Panagouli type III, with the LIPA and RIPA arising independently from the CT, and the aRHA originates from the CT distal to the RIPA as additional branches; and the CT displays a false tripod with the LGA branching off proximal to the bifurcation of the CT into SA and CHA. It is crucial for surgeons and radiologists to take note of the variations of the celiac trunk branching pattern during procedures such as transplant surgeries, hepatobiliary and pancreatic laparoscopic surgeries, selective embolization treating pseudoaneurysm, and radiological procedures [8,9]. The complexity of the procedures and associated risk of inadvertent vascular injury could be high given the multiple variations presented in our case [10]. There is an increased amount of blood loss, operation time, and postoperative drainage for patients with celiac trunk variation [11].
Arterial supply of the liver normally depends on the right and left hepatic artery from hepatic artery proper. Different variations occur in approximately 20% of the population [7]. Reviewing studies based on observations on multidetector computed tomography (MDCT) angiography, the trifurcation of CHA into GDA, RHA, LHA are found in 1.8% to 8% of the cases [12- 15]. The presence of aRHA from the celiac trunk can be classified as superior accessory hepatic artery with prevalence of up to only 2% [8]. It provides an alternative arterial supply to the liver when major hepatic arteries are injured [9]. In liver transplantation, aRHA is either sacrificed or preserved and revascularized if it is large and replaces normal hepatic artery [10]. Such knowledge of variations in the origins of aRHA is essential to prevent intraoperative bleeding and complications during hepatobiliary surgeries [9]
According to a systemic review and meta-analysis of the origins of the RGA in 1,971 cases, the pooled prevalence of RGA originating from RHA is only 3.43% [16-19]. The variation of hepatic and gastric arteries is particularly important for gastrectomy. MDCT is highly suggested in revealing vascular anomalies in the preoperative stage [20].
Most HCC patients (>70%) are diagnosed with advanced unrespectable HCC [21]. Treatment options include systemic therapy, radiation therapy, transarterial chemoembolization (TACE), and hepatic arterial infusion chemotherapy (HAIC) [22]. TACE administers embolic and anti-cancer agents to HCCs via the hepatic artery. Understanding the variation in vascular patterns and pretreatment imaging such as MRI and CTA to identify arterial supply to HCC are imperative in preventing accidental embolization of the non-target artery [23]. In this case study, trifurcation of the common hepatic artery increases the risk of GDA injury when it is unintentionally embolized. Acute pancreatitis may occur when chemo-drugs flow through pancreaticoduodenal branches of GDA causing chemotherapyinduced ulceration [24]. RGA originating from RHA is also susceptible to damage if the catheter is positioned proximal to the opening of the RGA. Superselective TACE using microcatheter embolizes more distal hepatic artery. It can be used to prevent accidental delivery of chemodrugs into extrahepatic arteries such as GDA and RGA in our case [25,26]. HAIC continuously delivers high-concentration anticancer drugs to HCCs also depending on the vasculature distribution. Recent papers support that HAIC provides better survival [27], and tumour response rate [28]. It also lowers intrahepatic metastasis over TACE [29,30].
Patients with portal vein tumour thrombus (PVTT) are usually a contraindication to TACE since the process of treatment in TACE further reduces hepatic perfusion and worsens liver dysfunction [31]. According to a study documented by Chen et al in An Nan Hospital, China Medical University in Taiwan, the GDA, and RGA are embolized to prevent reflux of cytotoxic drugs into the stomach and the duodenum [27], which is particularly crucial attention to the treatment as the GDA and RGA can originate with aanatomical variants in our case findings.
Whitley et al documented in a systematic review and meta-analysis on 4,208 patients from 18 studies, that independent origins of RIPA and LIPA and the presence of CIPA have been observed in 75.8% and 24.2% of the patients respectively. The RIPA and LIPA arising from the CT have a prevalence of 35.7% and 46.1% respectively among all cases [32]. It should be noted that HCC occasionally receives parasitic blood supply from extrahepatic vessels, with RIPA(70-83%) and LIPA(12%) being the most common collateral arterial source [33]. Hence, although the branching pattern of RIPA and LIPA in our case (RIPA and LIPA arising from the CT separately) is relatively common, understanding the variations of the origins of RIPA and LIPA is important in the embolization of the IPAs during TACE. The knowledge could also be applied in handling IPAs injuries which can be caused by chest aspiration blunt trauma or cardiopulmonary resuscitation [34-34].
CONCLUSION
We report on anatomical multiple variants of the ventral roots branching from the upper abdominal aorta. The rare variants of (1) separating origins of inferior phrenic arteries from the celiac trunk, (2) trifurcation of the common hepatic artery, (3) an aberrant right gastric artery origin, and (4) accessory right hepatic artery originating from the celiac trunk in the donor body. Comprehending and understanding the numerous variations in the upper abdominal of the foregut is crucial for managing liver disease. The findings empower medical professionals to judge the critical arrangement and make decisions regarding treatment procedures, minimizing medication errors. Failure to recognize the patterns of vascular variations could lead to complications like misdiagnosis, intraoperative bleeding, and inadvertent damage to structures in the vicinity.
REFERENCES
- Panagouli E, Venieratos D, Lolis E, Skandalakis P. Variations in the anatomy of the celiac trunk: A systematic review and clinical implications. Ann Anat 2013;195(6):501-511.
- Sankar K, Bhanu P, Susan P. Variant anatomy of the celiac trunk and its branches. Int J Morphol 2011;29(2):581-584.
- Aslaner R. Variations in the origin of inferior phrenic arteries and their relationship to celiac axis variations on CT angiography. Korean journal of radiology 2017;18(2):336-344.
- Sutphin PD, Kalva SP. Hepatic Artery Origin and Course at Scale. Radiology: Cardiothoracic Imaging 2021;3(4):e210200.
- Opfell RW, Bowen J. Regional chemotherapy of liver cancer. Liver Cancer 1985;247-261.
- Gulnur O, Osman S, Esra K. Multiple variations of the abdominal vessels in a single cadaver. Int J Anat Var (IJAV) 2013; 6: 52–55.
- Prakashl, Rainil T, MokhasP V, GethanjalP BS, Sivacharanl PV, et al. Coeliac trunk and its branches anatomical variations and clinical implications. SingaporeMedJ 2012;53(5)329-331.
- Badagabettu SN, Padur AA, Kumar N, Reghunathan D. Absence of the celiac trunk and trifurcation of the common hepatic artery: a case report. J Vasc Bras 2016;15(3):259-262.
- Eleni P, Dionysios V, Evangelos L, Panagiotis S. Variations in the anatomy of the celiac trunk: A systematic review and clinical implications. Annals of Anatomy Anatomischer Anzeiger. 2013;195(6):501-511.
- Badagabettu SN, Padur AA, Kumar N, Reghunathan D. Absence of the celiac trunk and trifurcation of the common hepatic artery: a case report. J Vasc Bras. 2016;15(3):259-262.
- Huang Y, Mu GC, Qin XG, Chen ZB, Lin JL, et al. Study of celiac artery variations and related surgical techniques in gastric cancer. World J Gastroenterol 2015;14(22):6944-6951.
- Noussios G.The main anatomic variations of the hepatic artery and their importance in surgical practice review of the literature. Journal of clinical medicine research 2017;9(4):248.
- Sureka B, Mittal MK, Mittal A, Sinha M, Bhambri NK, et al. Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging 2013;23(3):223-33.
- Farghadani M, Momeni M, Hekmatnia A, Momeni F, Baradaranmahdavi M. Anatomical variation of celiac axis, superior mesenteric artery, and hepatic artery: Evaluation with multidetector computed tomography angiography. J Res Med Sci 2016;21:125.
- Mahesh KM, Aliza M, Mukul S, Narendra KB, Thukral BB, et al. Variations of celiac axis, common hepatic artery and its branches in 600 patients. July 2013. The Indian journal of radiology and imaging 2013; 23(3):223-233.
- Bastos-Neves D. Right Accessory Hepatic Artery Arising From Celiac Trunk-Case Report of a Variation that Must Be Looked for During Multiorgan Procurement. in Transplantation Proceedings 2016; 48(7):2387-2388.
- Malviya KK, Verma A. Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries A Review Emphasizing Origin and Branching. Diagnostics 2023;13(7):1233.
- Madhu Y, Harish K. Accessory right hepatic artery and its implications for a surgeon. Indian Journal of Surgery 2013;75:492-494.
- Abelleyra Lastoria DA, Smith R, Raison N. Variations in the origin of the right gastric artery: a systematic review and meta-analysis. Surg Radiol Anat 2023;45(6):709-720.
- Celik A, Celik AS, Altinli E, Beykal O, Caglayan K, Koksal N. Left gastric and right hepatic artery anomalies in a patient with gastric cancer: images for surgeons. Am J Surg. 2011 Aug;202(2):e13-e16.
- Wang EA, Stein JP, Bellavia RJ, Broadwell SR. Treatment options for unresectable HCC with a focus on SIRT with Yttrium-90 resin microspheres. Int J Clin Pract 2017;71(11).
- Quirk M, Kim YH, Saab S, Lee EW. Management of hepatocellular carcinoma with portal vein thrombosis. World J Gastroenterol 2015;28;(12):3462-3471.
- Cho Y, Choi JW, Kwon H, Kim KY, Lee BC, et al. Transarterial Chemoembolization for Hepatocellular Carcinoma 2023: Expert Consensus-Based Practical Recommendations of the Korean Liver Cancer Association. Korean J Radiol 2023;24(7):606-625.
- Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol 2006;23(2):119-125.
- de Baere, Ronot M, Chung JW. Initiative on Superselective Conventional Transarterial Chemoembolization Results (INSPIRE). Cardiovasc Intervent Radiol 2022;45:1430–1440.
- Gonsalves CF, Brown DB. Chemoembolization of hepatic malignancy. Abdom Imaging 2009; 34:557–565.
- Chen KT, Tsai KF, Leung HWC, Chan ALF, Wang SY, et al. Hepatic Arterial Infusion Chemotherapy Followed by Lipiodol Infusion for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: A Single-Center Experience. Medicina 2021; 57(8):779.
- Hu J, Bao Q, Cao G et al. Hepatic Arterial Infusion Chemotherapy Using Oxaliplatin Plus 5-Fluorouracil Versus Transarterial Chemoembolization/Embolization for the Treatment of Advanced Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis. Cardiovasc Intervent Radiol 2020;43:996–1005.
- Tsai WL, Sun WC, Chen WC, Chiang CL, Lin HS, et al. Hepatic arterial infusion chemotherapy vs transcatheter arterial embolization for patients with huge unresectable hepatocellular carcinoma. Medicine Baltimore 2020; 99(32):e21489.
- Kawabe N, Hashimoto S, Nakano T, Nakaoka K, Fukui A, et al. Transcatheter arterial infusion chemotherapy with cisplatin in combination with transcatheter arterial chemoembolization decreases intrahepatic distant recurrence of unresectable hepatocellular carcinoma. JGH Open 2021;18(6):705-711.
- Amr SM, Ahmed KAA, Yat Shun Mak, Nael S, Derek D, et al. Chemoembolization of Hepatocellular Carcinoma with Extrahepatic Collateral Blood Supply: Anatomic and Technical Considerations. RadioGraphics 2017;37(3):963-977.
- Adam W, Jan K, David K. The inferior phrenic arteries: A systematic review and meta-analysis. Annals of Anatomy - Anatomischer Anzeiger 2021; 235:151679.
- Aoki M, Shibuya K, Kaneko M, Koizumi A, Murata M, et al. Massive hemothorax due to inferior phrenic artery injury after blunt trauma. World J Emerg Surg 2015 24;10:58.
- Sanmoto Y, Hanari N, Kinuta S. A case of inferior phrenic artery injury after chest drainage treated laparoscopically. Trauma Case Rep 2023;9(43):100763.
- Mohamed EA, Sayed H, Mohamed A, Nady, AG, Hany Seif. Right inferior phrenic artery injury as a complication of needle aspiration: a case report. Cardiothorac Surg 2021;29:8.
- Rossi UG, Ierardi AM, Cariati M. Diaphragmatic rupture with inferior phrenic artery bleeding caused by cardiopulmonary resuscitation. Clin Exp Emerg Med 2020;7(3):238-240.
- Adel ME, Hassen S, Nady MA, et al. Right inferior phrenic artery injury as a complication of needle aspiration: a case report. Cardiothorac Surg 2021;29:8.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref