Nanotechnology in the development of small and large molecule tyrosine kinase inhibitors and immunotherapy for the treatment of HER2-positive breast cancer
Received: 04-Apr-2022, Manuscript No. PULCMR-22-4495; Editor assigned: 09-Apr-2022, Pre QC No. PULCMR-22-4495(PQ); Accepted Date: Apr 27, 2022; Reviewed: 20-Apr-2022 QC No. PULCMR-22-4495(Q); Revised: 24-Apr-2022, Manuscript No. PULCMR-22-4495(R); Published: 28-Apr-2022
Citation: Ejigah V, Mandala B, Akala E.O. Nanotechnology in the development of small and large molecule tyrosine kinase inhibitors and immunotherapy for the treatment of HER2-positive breast cancer. J Cancer Metastasis Res. 2022; 4(2):6-31.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The HER2 receptor tyrosine kinase is a member of the epidermal growth factor receptor family which includes EGFR, HER3 and HER4. They are known to play critical roles in both normal development and cancer. A subset of breast cancers is associated with the HER2 gene, which is amplified and/or overexpressed in 20-25% of invasive breast cancers and is correlated with tumor resistance to chemotherapy, Metastatic Breast Cancer (MBC) and poor patient survival. The advent of receptor tyrosine kinase inhibitors has improved the prognosis of HER2-postive breast cancers; however, HER2+MBC invariably progresses (acquired resistance or de novo resistance). The monoclonal antibody-based drugs (large molecule TKIs) target the extracellular binding domain of HER2; while the small molecule TKIs act intracellularly to inhibit proliferation and survival signals. We reviewed the modes of action of the TKIs with a view to showing which of the TKIs could be combined in nanoparticles to benefit from the power of nanotechnology (reduced toxicity, improved solubility of hydrophobic drugs, long circulation half-lives, circumventing efflux pumps and preventing capture by the reticuloendothelial system (mononuclear phagocyte system). Nanotherapeutics also mediate the synchronization of the pharmacokinetics and biodistribution of multiple drugs incorporated in the nanoparticles. Novel TKIs that are currently under investigation with or without nanoparticle delivery are mentioned, and nano-based strategies to improve their delivery are suggested. Immunotherapies currently in clinical practice, clinical trials or at the preclinical stage are discussed. However, immunotherapy only works well in relatively small subsets of patients. Combining nanomedicine with immunotherapy can boost therapeutic outcomes, by turning “cold” non-immunoresponsive tumors and metastases into “hot” immunoresponsive lesions.
Keywords
Breast cancer; Nanoparticles; Trastuzumab; Pertuzumab; Antibody drug conjugates; Antibody conjugated nanoparticles; Immunotherapy
Introduction
The Human Epidermal Growth Factor Receptor 2 (HER2) is a type I transmembrane glycoprotein that plays a pivotal role in activating the downstream signaling cascades that control cell proliferation, survival, and apoptosis in HER2-positive breast cancer [1]. The ErbB receptor family of Receptor Tyrosine Kinases (RTKs) comprises four distinct receptors: the EGFR (also known as ErbB1/HER1), ErbB2 (neu, HER2), ErbB3 (HER3) and ErbB4 (HER4) [2,3]. HER2 can dimerize with itself or with other EGFR family members, thus activating downstream signal transduction pathways of tyrosine phosphorylation, and eventually resulting in the regulation of various cellular functions [2,3]. The ErbB receptors are triggered by several peptide ligands, comprising TGF-α, EGF, amphiregulin (for EGFR), and neuregulin (for HER3 and HER4) [2]. Ligand binding triggers receptor dimerization, activation of the kinase domains, autophosphorylation, and initiation of down-stream signaling. However, HER3 contains no kinase domain (which can be activated) and HER2 has no known ligand (that binds its receptors). Therefore, HER2 and HER3 depend on mutual dimerization with each other and other ERBB receptors to initiate their cellular effects [2]. HER2 is moderately expressed in normal adult tissues; however, an average of 20%–30% of human breast cancers overexpress HER2 receptors by amplification of the HER2/neu gene, which is a marker of aggressive cancer with an unfavorable prognosis [4] that correlates with tumorigenesis and metastasis [5-7]. HER2 is also overexpressed in approximately 20% of gastroesophageal junction and gastric cancers with an equally poor prognosis [3,8].
Several bioactive agents that target HER2 positive breast cancer have been approved by the FDA and are currently being used in the clinic (Figure1). Lapatinib was the first small molecule TKI approved by the FDA in 2007 [9] followed by neratinib in 2017 [10], pyrotinib in 2018 (onl--ly in China) [11] and tucatinib in 2020 based primarily on the outcome of the HER2CLIMB clinical trial [12]. The first drug developed in the class of large molecule TKIs is the monoclonal antibody, Trastuzumab (TRZ), which was approved in 1998. The approval of Trastuzumab (TRZ) was followed by the development of Pertuzumab (PTZ) in 2012 and Phesgo ® (Fixed Dose Combination of TRZ and PTZ) in 2020 as an option to improve efficacy and patient compliance based on results from the landmark CLEOPATRA study [13]. A recent entrant is margetuximab that was approved in December 2020 for use in anti-HER2+ experienced patients with metastatic HER2-positive breast cancer [14]. In a bid to increase efficacy, patient compliance and reduce the adverse effects of the partner cytotoxic agents, anti-HER2 monoclonal antibodies have been conjugated with cytotoxic partners. A popular example is adotrastuzumab (T-DM1: TRZ conjugated to emtansine) which was approved in 2013 and is currently the second line in several guidelines [15].
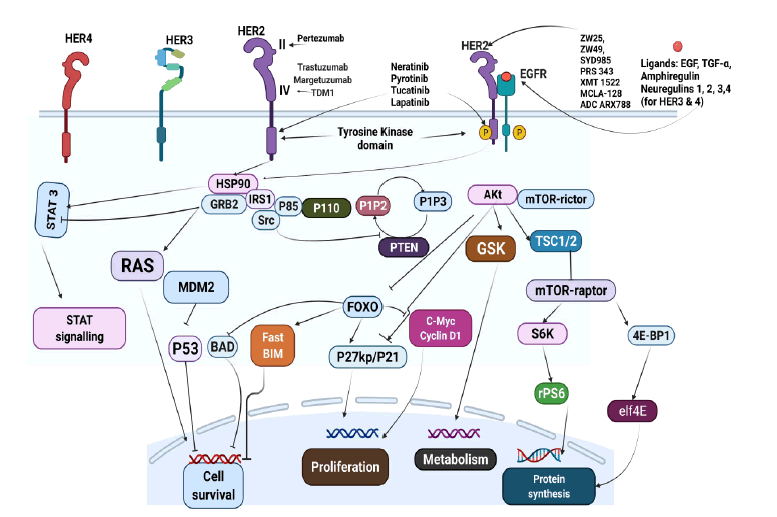
Figure 1: The ErbB family of receptors and site of action of anti-HER2 agents. (EGFR Epidermal Growth Factor Receptor; P13K, phosphatidylinositol-3-kinase; PTEN Phosphatase and Tensin Homolog; GRB2 Growth Factor Receptor Bound. PIP3 Phosphatidylinositol-3,4,5- Diphosphate; MDM2, Murine Double Minute. BAD Bcl- 2-Associated Death Promoter; FOXO, FORKHEAD homologin rhabdomyosarcoma; mTOR Mammalian Target of Rapamycin; STAT, Signal Transducer and Activator of Transcription; MAPK/ ERK Mitogen-Activated Protein Kinase/Extracellular Signal Regulated Kinase; HRG, Heregulin, Adapted from [44]).
Despite the clinical successes recorded with these bioactive agents due to their targeted design, resistance to monoclonal antibodies [16,17] remains a challenge in addition to the toxicity of cytotoxic partners which invariably affects their efficacy, safety, and compliance in the treatment of HER2-positive breast cancer. It is on the backdrop of these problems that the design of novel drug delivery systems has been proposed: encapsulation of cytotoxic drugs in nanoparticles which are then decorated by HER2 specific monoclonal antibodies for targeting two or more drugs simultaneously to neoplastic tissues.
Nanotherapeutics is an important and fast emerging field that is focused on the design of nanocarriers from biocompatible and biodegradable components (proteins, polymers, peptides, RNA/DNA aptamers, lipids, liposomes, and inorganic materials) for the delivery of bioactive agents in the treatment and diagnosis of HER2 positive breast cancer and other applications [18]. To achieve the goal of eliminating death and suffering from cancer, the USA National Cancer Institute has embraced the power of nanotechnology to radically change the procedure for diagnosis, imaging and treating of cancer. The integration of nanotechnology with advances in cancer research can be done using nanoparticles which are capable of specific delivery of large amounts of single or multiple therapeutic agents as well as imaging agents embedded in the core per targeting biorecognition event compared to simple immune-targeted drugs. Both targeting (spatial/ distribution control) and controlled release (temporal control) of therapeutic agents can be achieved [19-21]. Nanoparticles for biomedical applications have evolved from the first-generation, suitable for liver targeting, through the second generation of stealth nanoparticles for long blood circulation and passive targeting to the third generation with molecular recognition [22,23] and fourth generation (theranostics): that incorporates diagnostic/reporter agents in the same nanodevice [24].
Site specific nano-based drug delivery has the advantages of passive tumor targeting stemming from high permeability and retention effect (EPR), active targeting via the coupling of receptor specific ligand to carriers, lower toxicity [25,26] of encapsulated drug and intracellular endocytosis uptake which mediates evasion of mechanisms of multidrug efflux pump systems including p-glycoprotein [27]. In addition, nanotherapeutic carriers can be potentially functionalized to enhance drug-carrying capacity for delivery of multiple therapeutics to cancer cells simultaneously [18,28]. The controlled drug release attribute of nanoparticle systems can normalize and synchronize the pharmacokinetics, biodistribution, bioavailability and stability of multiple drugs that possess very different physicochemical properties that would have independently produced contrasting pharmacological behaviors if administered separately [28]. The use of nanocarriers enables the formulation of FDCs (Fixed Dose Combinations) with the capacity for ratiometric dosing, providing the ability to tailor the relative ratios of each agent based on their respective pharmacological disposition [29]. Further, therapeutic nanoparticles can improve the solubility of anticancer drugs when encapsulated in the hydrophobic core while surface stealth property of incorporated water-soluble polymers, like PEG, prevents opsonization and capture by the Reticuloendothelial System (RES). Dose reduction with better quality of life during chemotherapy would improve patient compliance [30].
The advantages of nanotherapeutics have led to the approval of several non-targeted nanodrugs including Doxil®, Thermodox®, Myocet® Genexol® and Opaxio™. These products have the advantages of lower toxicity (prevention of immediate release of cytotoxic components) and longer circulation time either by the impact of surface pegylation and/ or controlled release based on the ratio of specific nanomaterials used. Nevertheless, these products still lack the desired specificity required for targeted delivery at the site of neoplasia, stability in blood circulation as well as issues with the emergence of novel toxicities such as Palmar Plantar Erythrodysesthesia (PPE) and exanthema, oral mucositis, and hematological toxicities [31,32]. It is hoped that the conjugation of targeting ligands will overcome these challenges and lead to better therapeutic outcome.
It is important to differentiate between Antibody Drug Conjugates (ADCs) in which a partner cytotoxic agent is covalently linked to the antibody as seen in T-DM1 and Antibody-Conjugated Nanoparticles (ACNPs) which are designed to exploit the benefits of both antibody conjugation (such as site-specificity) and nanotechnology [33,34]. While ADCs can be targeted via the use of ligands that bind endogenous targets, they are unable to deliver drugs in a controlled manner; neither can they maintain the chemical structure of conjugated partners, avoid irregular metabolism, nor diminish toxicity when compared to ACNPs [35]. Moreover, ACNPs present the extra benefit of increasing endocytosis which translates to higher tumor cell permeability and accumulation, and stability against multi-resistant efflux pumps. Here we review the development of nanoparticle based small and large molecule Tyrosine Kinase Inhibitors (TKI) that are currently in clinical practice, in clinical trials or at the preclinical stage. Mention will be made of novel TKIs that are currently in development with or without nanoparticle delivery, and nano-based strategies to improve their delivery will be proposed.
LARGE MOLECULE TYROSINE KINASE INHIBITORS (TKI)
Trastuzumab
Trastuzumab is the premier humanized murine monoclonal antibody developed for the treatment of HER2 positive breast cancer. The efficacy of trastuzumab has been demonstrated in both neoadjuvant and adjuvant treatment of Metastatic Breast Cancer (MBC) as shown in several studies: CLEOPATRA [16,36]; TRYPHAEN [37]; KATHERINE [38]; EMILIA [39], THR3ESA [40] and MARIANNE [41]. Trastuzumab is recommended by both St. Gallen and National Comprehensive Cancer Network (NCCN) guidelines for use as monotherapy after adjuvant treatment [15]. Current guidelines recommend dual-blockade and the most preferred first line of treatment is a combination of trastuzumab + pertuzumab while T-DM1 and lapatinib are considered as second and third line options respectively [2,3,15].
Mechanism of action
Trastuzumab acts by binding to the Extracellular Domain (ECD) IV of the HER2 receptor with high specificity and avidity to block downstream signaling, attracting immune cells and inducing internalization of the antibodyreceptor complex leading to inhibition of cell growth and proliferations [17,18,42-44]. These effects are elicited in various ways and are manifested via intracellular and extracellular mechanisms [42,44-46]. First, by binding the domain IV on the ECD of the HER2 receptor, trastuzumab is recognized by the Fcγ receptors expressed on the surfaces of cells of the innate immune system, including APCs (Antigen-Presenting Cells), NK cells (Natural Killer), and effector immune cells that readily eliminate TRZ tagged tumor cells via Antibody-Dependent Cellular Cytotoxicity (ADCC) and Complement- Dependent Cytotoxicity (CDC) [44]. Second, TRZ inhibits HER2 shedding. In some breast cancer-derived cell lines, the ECD of HER2 is proteolytically discarded from the cell surface [47,48] and the residual truncated receptor (‘p95 HER2’ or ‘t95 HER2’) [49], relieved of autoinhibition by the receptor’s ECD), engages in constitutive tyrosine kinase activity [50,51] to promote cellular signal transduction. By binding to HER2 receptor, TRZ blocks this proteolytic shedding, either through a steric or allosteric mechanism, thus preventing or downregulating HER2 kinase activity [50,52]. Finally, TRZ stimulates endocytosis and degradation of HER2 domain hence preventing HER2 homodimerization [44,52] and HER2/HER3 heterodimerization that are required for downstream signal transduction. This is achieved through TRZ mediated recruitment of c-CBL, an E3 ubiquitin-protein ligase involved in cell signaling and protein ubiquitination [53,54]. The impact of these mechanisms leads to the inhibition of HER2 downstream pathways including MAPK and PI3K/AKT/mTOR thus downgrading cell growth and proliferation [17]. Other confirmed mechanisms associated with the therapeutic effect of TRZ include inhibition of angiogenesis, induction of cell-cycle arrest and apoptosis [55] and interference with DNA repair [56-57].
Trastuzumab (TRZ) Nanoparticle Formulations
There is currently no nanoparticle formulation of TRZ in the clinic [18,58] neither is any in ongoing clinical trials as revealed by ClinicalTrials.org. In the only trial involving nanoparticles (NCT01730833), TRZ was not conjugated to paclitaxel albumin-stabilized nano-formulation. That notwithstanding, TRZ conjugated nanoparticles have been studied extensively in numerous preclinical trials involving cell cultures and animal models to explore several novel strategies to increase the precision of tumor targeting, tissue accumulation and cellular uptake of various nano-encapsulated cytotoxic and theranostic agents.
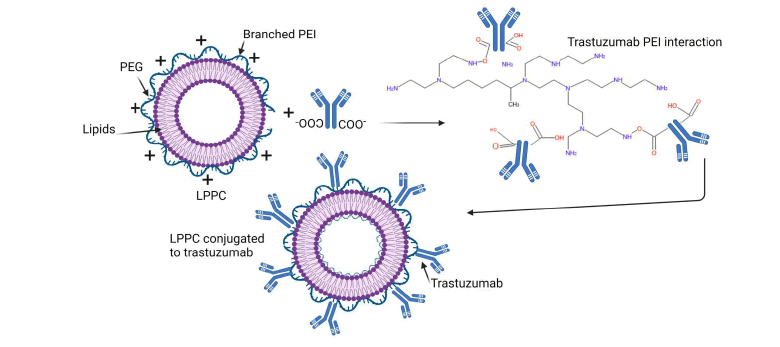
In one study [27], TRZ was electrostatically linked to a cationic lipoplex nanoparticle (Figure 2) formulated from PEG, PEI, and lipids (LPP), with a payload of curcumin and doxorubicin (LPPC) based on the ability of LPP component to strongly adsorb various biologically functional proteins on its surface (presence of NH2) which are difficult to displace by proteins in vivo [59]. In addition to its adsorptive effect, LPP demonstrated capacity to protect the structure of encapsulated drugs against oxidation as well as provide a strong cytotoxic effect against drug-resistant tumor cells [60,61]. The effect of this ACNP against HER2 positive SK-BR-3 (HER2 positive) breast cancer cell line in xenograft mouse model of HER2+ breast cancer showed that 50% of the mice treated with LPPC/doxorubicin/TRZ ACNPs were tumor free during 66 days of observation translating to 4x the lifespan of the group treated with PBS (Phosphate Buffered Saline). PEI and PLA (Poly Lactic Acid)-based NPs conjugated TRZ have been reported in a strategy [62] to deliver dasatinib for the purpose of rescuing TRZ resistance in HER2+ BT474 and resistant BT474-HR cell lines. Dasatinib is a multikinase inhibitor of SRC, a tyrosine kinase transducer whose alteration has been implicated in TRZ resistance [63,64]. The use of high molecular weight (MW 25,000) PEI may limit the translation of this strategy since it is well documented that HMW PEI is toxic in vivo due to its proton sponge effect, though this can be limited by using low MW PEI, PEGylation or by blocking the excess NH2 groups. The last option may increase the particle size of the LPPC. It should be noted that although covalent conjugation involves several steps and may be time consuming; however, it still provides a more stable conjugate compared to conjugates formed from electrostatic interaction (used in the cited work) which may be hampered by the heterogeneous nature (proteins, salts etc.) of the in vivo environment leading to desorption of the conjugated ligand thus diminishing its specificity.
Figure 2: Synthesis of LPPC Lipid-PEI-Cucurmin nanoparticle PEG- Polyethylene glycol, PEI-Polyethylene imine. Adapted from [27]
One of the challenges of targeted delivery using monoclonal antibodies is the need to strike the sweet spot between the total number of moieties conjugated to the surface of a nanocarrier and the minimization of immunological reactions due to recognition and rapid clearance by macrophages and other scavenger cells. An attempt to quantify the absolute number of conjugated mABs has been demonstrated in a liposome Nanocarrier (NC) system [65]. In this report, docetaxel was incorporated in a liposome system terminated with Mal-PEG and later mixed with an already thiolated trastuzumab for surface conjugation to terminal Mal-PEG in various ratios of (508:1, 1.127, and 1.16:1) to determine the ratio with the best affinity and minimal immunological challenge. Exposure of these immunoliposomes to a MDA-MB-453 spheroid showed a significant difference in cell viability between the liposomes, free docetaxel and control. However, there was no significant difference in cell viability among the immonoliposomes. This result bolsters the opinion that a high mAB surface density may not necessarily translate to site specific avidity probably due to certain factors including steric hinderance. Leakage of docetaxel and DiD fluorescent molecules from the hydrophilic phase affected proper quantification of the conjugated mABs. Nevertheless, this formulation has shown superiority when indexed against free docetaxel, free TRZ and TDM-1 [66].
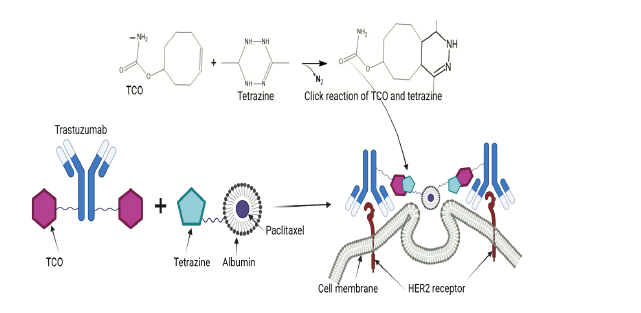
The concept of in situ click chemistry with trans-cyclooctene and Tetrazine (TCO/Tt cycloaddition) has been explored in a two-component, two-step, pre-targeting drug delivery system integrated with image guidance to overcome the challenge of resistance and non-specific toxicity associated with ADCs [5]. In this study, TRZ was conjugated to TCO and a fluorescent agent to form [Tz(TCO)6(CF-680)2] while paclitaxel was conjugated to albumin and another fluorescent agent to form ALB(Px)2.6(PEG4-Tt) (CF-750). The click treated, fluorescent labelled trastuzumab [Tz(TCO)2.6 (CF-680)2] was injected i.p into mice with orthotopically placed tumor xenografts and given enough time to bind to HER2+ expressing tumor cells. The click treated ALB(Px)2.6(PEG4-Tt) (CF-750) was injected 12 hours later to activate click reaction with the already bound click treated TRZ as shown in Figure 3. This strategy harnesses the close proximity of overexpressed HER2 receptors on cancer cells and multiply derivatized pre-targeting and delivery components, leading to multiple cross-linking reactions which induces self-assembly of cell membrane-bound nanoclusters in situ which are effectively internalized by clathrin-mediated endocytosis [5,67]. The result showed a superior cellular uptake of this formulation as monitored via imaging compared to control. The extent of tumor regression in the click treated group was substantially better than the untreated and mock treated controls.
Figure 3: In vivo click reaction between TCO and HER2 at the receptor site (TCO Trans-cyclooctene. Adapted from [5]).
The synchronized delivery of TRZ and neratinib with PAMAM (polyamidoamines) dendrimers has been described [68]. Neratinib was loaded into the core of a G4 PAMAM dendrimer before conjugating the NP surface with FITC (Fluorescence isothiocyanate) for imaging. Thereafter, a heterocross-linker, [maleimide-poly(ethylene) Glycol-N-hydroxysuccimide (NHS-PEG-MAL)] was covalently linked to the NPs followed by bioconjugation with an already thiolated TRZ. The resulting nanocarrier was shown to have superior cytotoxic effect on SK-BR-3 (HER2+) cells when compared to free neratinib most likely due to the targeting ability and anti- HER2 property of TRZ. The synthesis of the NPs had several steps which could interfere with the loading efficiency and drug release as acknowledged by the authors considering the 19.24% loading efficiency achieved and a 10% decrease in drug release. In terms of clinical translation, dendrimers present a challenging prospect because of their non-specific toxicity and tendency to accumulate in the liver due to their high cationic density. Besides, the LMW dendrimers are rapidly cleared in vivo and there is a poor control over the release profile of encapsulated drugs [69].
In another strategy [70], the combination of ultrasound with TRZ conjugated nanobubbles (PTX-NBs-HER) serving as a contrast agent and drug carrier has been explored for image-guided drug delivery and precise targeted therapy at the tumor site. The nanoparticles were prepared by the double emulsion method (W/O/W) from PLGA in which paclitaxel was encapsulated and made into nanobubbles by passage of perfluoropropane gas before linkage to TRZ. On administration, the nanobubbles were delivered to the tumor site through the tropism of TRZ and the encapsulated paclitaxel released by the application of exogenous ultrasound stimulus which triggered controlled release of the drug as well as cavitation of the cell membrane to promote enhanced permeability and retention (Figure 4). The mechanism relies on ADCC and enhanced endocytosis promoted by TRZ and the cytotoxic effects of paclitaxel which accelerate tubulin polymerization and inhibition of depolymerization, resulting in the formation of stable non-functional microtubule bundles, thus extinguishing mitosis and cell proliferation [70]. PLGA tagged TRZ has also been used to safely deliver epirubicin to MDA-MB-453 and BT-20 HER2+ cell lines [71] with success. The dose dependent side effect of epirubicin including cardiotoxicity [72] has limited its clinical application. Therefore, the translation of a PLGATRZ- epirubicin targeted nanocarrier will be a daunting proposition due to possible additive cardiotoxic effect when epirubicin is combined with TRZ.
Figure 4: Synthesis and controlled release of paclitaxel nanobubbles via external ultrasound activation. (NBs Nanobubbles; PLGA Polylactide-co-glycolide; NPs Nanoparticles; Adapted from [70]).
The role of other exogenous ligands in the efficient and targeted delivery of gene therapy to the nucleus of tumor cells has been reported [73]. In this publication, nanoparticles were formed from a 10:1 ratio of NHS-PEG3500-Mal and 25kDa PEI which was linked to either anti-HER2 nanobody (RR4) or TRZ in the presence of Traut’s reagent. Terminal PEI groups were subsequently linked to a genetic material- p15BID (tbid), a proapoptotic gene and its promoter pMUC1. The results showed that the highest expression of tBid gene and consequently the least amount of cell viability in HER2-positive cells (SK-BR-3 and BT-474) was observed in the group treated with TRZ modified nanoparticles. This result was largely due to the efficient delivery and expression of the killer gene in these cells. Low levels of specificity and targeting was observed with the low/non-HER2 expressed cell line (MCF-10A) thus signifying the safety potential of this design towards healthy cells. It is worth noting that this nanobody (RR4) led to good gene delivery and expression, hence presenting an opportunity for further exploration in targeted tumor delivery in preclinical and clinical studies.
Inorganic materials have been used for the delivery of multiple payloads to solid tumors. Examples include gold, SPIONs (Supra Paramagnetic Iron Oxide Nanoparticles), carbon nanotubes, quantum dots, gold, and silica, to mention a few [74]. They are particularly attractive because of the ease of synthesis, the large surface area to volume ratio, capacity for derivatization that allows the electrostatic or covalent linkage with several surface conjugates, and the ease of scale up. In a recent study [75], radiotoxic alphaemitter Ac radionuclide was incorporated into SPIONs produced via the coprecipitation method to form 225Ac@Fe3O4 nanoparticles. The NPs were linked to the surface of Fe3O4 nanoparticles via a 3-phosphonopropionic acid (CEPA) linker to form Ac@Fe3O4-CEPA nanoparticles that were conjugated to TRZ at pH 8.0 in a borate buffer in the presence of NHS/EDC. In vitro incubation of SKOV-3 cells to the plain 225Ac showed a surviving fraction of 67.7 ± 4.3%, 16.9 ± 6.6% for 225 Ac@Fe3O4-CEPA nanoparticles and 7.9 ± 1.5% for 225Ac@Fe3O4-CEPA-trastuzumab radio-bioconjugate. These outcomes clearly indicate that the TRZ conjugate is more toxic to SKOV-3 cells than other unvectorized NPs. However, in vivo biodistribution of these conjugates showed a considerable uptake by cells of the RES in the liver and spleen of SKOV-3 tumor-bearing SCID mice due to high hydrodynamic size of the NPs. The poor accumulation of these NPs at the tumor site due to sequestration by the RES [76,77], the propensity of radionuclides to decay into toxic metabolites that can cause genotoxicity, poor water solubility [78,79] and poor biodegradation [74,80] are some of the drawbacks of inorganic nanoparticles that make their translation to the clinic a daunting task. Nevertheless, inorganic materials in form of ultrasmall silica NPs linked to TRZ have been used for dual drug delivery and theranostic purposes [1].
PEG coating on NPs provides stealth property that aids evasion of the RES thus improving the circulation time of nano-constructs. However, it has been observed that when layers of targeting moieties are conjugated directly to PEG molecules on the surfaces of NPs, the stealth property is diminished [81]. In a strategy to improve the hydrophilicity of conjugated systems, a study [81] explored the concept of linking another PEG molecule to an already conjugated TRZ. Nanoparticles were formed by the nanoprecipitation method from di-block copolymers, PLGA-b-PEG-N3 and PLGA-b-Mpeg before clicking to TRZ via a 2kDa NHS-PEG-alkyne linker. Another short linker of NHS-PEG-methoxy with a MW of 333 Da was used to modify the already conjugated TRZ at a molar ratio of 200:1. Cellular uptake studies of single and double modified TRZ in SK-BR-3, MCF-7, and MDA-MB-231 showed that SK-BR-3 had the highest cellular uptake for all the conjugated NPs. This result showed that contrary to expectation, additional PEG did not alter the affinity of the modified TRZ for HER2 receptors. Exposure to RAW 264.7 cells revealed that the doubly modified PEG NPs with 10% and 50% azide heads showed a 70% and 82% reduction, respectively, in uptake and elimination by RAW 264.7 cells compared to their single modified counterparts.
PERTUZUMAB
Pertuzumab is a recombinant humanized monoclonal antibody that binds to a distinct epitope, precisely the subdomain II of the extracellular domain of HER2 [82]. Pertuzumab is composed of two heavy chains with 449 amino acid residues and two light chains with 214 residues in an immunoglobulin G1 kappa framework [83]. It is the second most popular monoclonal antibody used for HER2+breast cancer treatment.
Mechanism of Action
Pertuzumab inhibits ligand-dependent HER2 signaling thus preventing ligand-activated HER2/HER3 or HER2/HER1 heterodimerization, a potential escape mechanism from the inhibitory effect of trastuzumab [82,84]. Pertuzumab inactivates multiple downstream signaling networks [85] and triggers ADCC [86] like TRZ but differs in being effective in cases of normal HER2 and high HER1 (EGFR) levels, or breast cancers with characteristically low HER2 expression [43].
The efficacy of pertuzumab has been demonstrated in several studies. In the landmark CLEOPATRA study, pertuzumab in combination with docetaxel and trastuzumab led to a 40% overall response rate (ORR), significantly improving Progression-Free Survival (PFS) and Overall Survival (OS) with multiple complete and partial responses [13], hence approval of this combination as a first-line treatment in HER2-positive MBC [85]. In particular, the median overall survival was extended by more than a year and reached an excess of 4.5 years [2]. Furthermore, pertuzumab was the first drug to be approved with an endpoint of Pathological Complete Response (PCR) in the neoadjuvant setting [39]. In the NeoSphere study [87,88], neoadjuvant combination of pertuzumab + doxetacel + trastuzumab achieved superior pCR compared to pertuzumab + docetaxel, and trastuzumab + docetaxel. Pertuzumab monotherapy is well tolerated and shows no increase in cardiotoxic effect when added to trastuzumab or anthracycline free cytotoxic regimen [87,89]. The pharmacokinetic profile of pertuzumab has been shown to be stable in both monotherapy and combination therapy (trastuzumab/docetaxel) with no currently reported Drug-Drug Interaction (DDI), indicating that a Fixed Dose Combination (FDC) is feasible with pertuzumab [13,82,85,89].
Pertuzumab Nano-Formulations
There are no nano-formulations of pertuzumab in the clinic nor in ongoing clinical trials as assessed from Clinical trials.gov. In a recently published paper from our laboratory [90], a technique leading to nanoparticles fabrication by in situ dispersion polymerization (free of surfactants), involving the use of biodegradable macromonomer, a pH sensitive crosslinker, a redox initiator system and a hydrophilic macromonomer to provide stealth attributes was described. Specifically, PEG-monomethacrylate 2000 was conjugated to pertuzumab via carbodiimide (CDI) reaction followed by hydrazide derivatization. Assessment of cell viability in SKBR-3 cell line (HER2- positive) showed a dose dependent reduction in cell viability comparable to unmodified pertuzumab, though the free mAB had superior impact at lower concentration. However, at high concentration (30μm), PEG-pertuzumab had a drastic effect on cell viability, considerably decreasing cell population as it became more active.
Margetuximab
Margetuximab is a chimeric IgG1 monoclonal antibody that targets domain IV of the extracellular loop of HER2 receptor like trastuzumab [14,45]. The difference lies in the optimization of the Fcγ region via the substitution of five amino acids which increases the binding affinity for CD16A polymorphs and a decreased affinity for FcγRIIB (CD16B) that inhibits the activity of trastuzumab. Such reduced affinity frees the Fc region of margetuximab to bind more tightly to effector cells (macrophages and NK cells) leading to increased ADCC [91]. Margetuximab preserves binding to the high affinity activating FcγRI (CD64) [92] hence conserving the antiproliferative properties of trastuzumab.
Mechanism of Action
Margetuximab-cmkb binds to domain IV of the extracellular loop of HER2 receptor. It inhibits tumor proliferation, reduces shedding of HER2 extracellular domain (a resistant mechanism) and binds tightly to effector cells to mediate strong ADCC [14,93].
Margetuximab (Margenza) received approval by the FDA in December 2019 as part of a chemotherapy regimen for treatment of anti-HER2 experienced adult patients with metastatic HER2-positive breast cancer [94]. This approval was based on the successes recorded in clinical trials including the phase I study, MGAH22 [95] which revealed margetuximab as a promising single agent in the treatment of experienced patients with HER2-positive solid tumors. In this study, the objective response rate (ORR) was 17% and 3 out of 4 responders remained on treatment for 39 months-54 months. Unlike trastuzumab, no cardiotoxicity was observed. Interim analysis in the SOPHIA trial [96] showed that margetuximab improved primary PFS(RR=24%) and ORR (24% vs 22%) over trastuzumab at different time points. The frequency of infusion-related reactions in the first cycle was higher with margetuximab compared to trastuzumab (35 [13.3%] vs 9 [3.4%]); however, they were both comparable with respect to other side effects. Other planned trials of margetuximab include the MAHOGANY trial (NCT04082364) [97] and MARGOT study (NCT04425018). There are currently no nano-formulations of margetuximab either in preclinical or clinical trials.
ANTIBODY DRUG CONJUGATES (ADCS)
Trastuzumab emtansine (TDM1)
Ado-trastuzumab emtansine (KadcylaTM, Genentech/Roche) was the first developed HER2 ADC synthesized by the conjugation of TRZ to emtansine via a non-cleavable thioether linker-MCC (maleimidomethyl cyclohexane- 1-carboxylate) [17]. TDM1 was designed to harness the cytotoxic effect of emtansine on tubulin polymerization and the targeted delivery/antibody mediated cell cytotoxicity of TRZ [58,98]. Emtansine is an inhibitor of microtubules and is about a thousand times more potent than doxorubicin and paclitaxel [99,100]. When administered alone, emtansine, like other cytotoxic agents, harms both healthy and cancerous cells; however, conjugation to TRZ prevents this nonspecific effect since TRZ ensures active targeting of only HER2+ neoplastic tissues thus enhancing the overall clinical outcome for patients [101].
Mechanism of Action of TDM1
Upon administration, T-DM1 selectively binds exquisitely to subdomain IV of the HER2 receptor on the surface of antigen-positive cells via the natural tropism of TRZ [17]. The interaction of TRZ with HER2 receptor leads to endocytosis [102], degradation of endosomes on fusion with lysosomes [103] and liberation of MCC-DM1 which navigates through the nuclear membrane to interfere with the polymerization of tubulin [104]. Specifically, inhibition of tubulin assembly into a functional mitotic spindle prevents the important nexus between kinetochores on chromatids and the spindles, hence inducing cell-cycle arrest in metaphase culminating in ultimate mitotic disaster [105].
Trastuzumab related mechanism: TDM1 retains the mechanism of action of TRZ which include inhibition of the homo and heterodimerization of HER2, HER1, HER3, and HER4 receptors, activation of ADCC, inhibition of domain shedding [54], angiogenesis and DNA repair [27].
The efficacy of TDM1 has been demonstrated in several studies [39- 41,106]. In the EMILIA study, subjects with HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane showed improvements in median overall survival (OS) and PFS in the T-DM1-treated group compared to subjects treated with lapatinib and capecitabine in the control group [39]. This result was also replicated in the THR3SA study [40]. In the MARIANNE trial [41], TDM1 either alone or in combination with pertuzumab were not superior to TRZ/taxane; however, they were better tolerated than TRZ/taxane. Like EMILIA and THR3SA, TDM1 in the MARRIANE study equally had a prolonged PFS compared to TRZ/taxane. In the KRISTINE study [106], TDM1/pertuzumab was inferior to TRZ/ pertuzumab/docetaxel/ carboplatin; however, 44% of subjects achieved pCR without exposure to a retinue of potentially toxic systemic chemotherapy, clearly indicating that a subgroup of patients with particularly high and homogeneous HER2 expression, may not necessarily need neoadjuvant chemotherapy. This trend was equally observed in subjects in the Phase 2 PREDIX trial [107]. In the KATHERINE study, TDM1 administered to subjects with HER2-positive early breast cancer with residual invasive disease showed a lower risk of recurrence when compared to adjuvant trastuzumab, though with less tolerable outcome [38].
Despite the efficacy of TDM1 in HER2+breast cancer treatment, several resistance mechanisms have been reported. These include reduced expression of HER2 receptors [17], upregulation of alternative RTKs [108], increased expression of efflux transporters (MRP1,2&4, MDR1, P glycoprotein and BCRP) [109-112], impaired lysosmal release of lysine MCC-DM1(active DM1 metabolite) and enhanced recycling of HER2–T-DM1 complexes which is associated with reduced DM1 intracellular release [110,113]. Literature is replete with comprehensive reviews of TRZ and TDM1 resistance mechanisms. It should be recalled that unlike other members of the ErBb family, HER2 has no natural ligand, and this is thought to be instrumental to its impaired lysosomal trafficking [113,114]. As a result, HER2 is largely recycled back to the plasma membrane after spontaneous endocytosis [17]. Consequently, only a small fraction of T-DM1 is channeled to lysosomes for degradation and release within the cytoplasm [115] thus contributing to poor treatment outcome. Therefore, it is imperative to deploy other strategies including nano-delivery systems since they are not susceptible to extrusion by efflux pumps to overcome the dual challenge of resistance and poor delivery of TDM1 [116].
TDM1 Nano-Formulations
A search of the literature and clinical trials.gov revealed no nanoparticle formulation of TDM1 either in clinical practice or ongoing trials. At the preclinical stage, the most recent study by Rong et. al. [117] that conjugated TRZ to a three-arm star-shaped block copolymer of D-α-tocopheryl polyethylene glycol 1000 succinate-poly (D, L-lactide) (PLA-TPGS) in which TDM1 was incorporated, was withdrawn on 1/11/2021 due to an error in the representation of an image that could result in misleading interpretations.
Novel ADCs
In an attempt to overcome the HER2 receptor recycling partly responsible for T-DM1 resistance, newer ADCs are designed with a cleavable drug linker that mediates bystander killing effect. This is the passive extension of the cytotoxic effect of free toxins via diffusion from target HER2-positive cancer cells to neighboring cancer cells that cannot be targeted due to limited or complete absence of receptor expression. This effect is beneficial in the overall efficacy of ADCs that target HER2+ receptor, considering the inherent heterogeneity of HER2-positive breast neoplasia [45]. However, this design is also the Achille’s heel of most ADCs as evidence has shown that they are intrinsically toxic and can produce severe side effects due to their long circulatory half-life and toxicity in healthy tissues [118]. This concern has led to the withdrawal of a couple of ADCs in development as discussed in this section. There are no nano-based formulations of these products besides XMT1522 as most are still in early development.
Preclinical ADCs
A major impediment in the treatment of HER2+ breast cancer is the inherent intratumoral heterogeneity which has been observed in 16%-36% of patients with HER2+ tumors that is associated with aggressive tumor cell proliferation, high relapse rates, and poor survival [119-122]. An attempt to accelerate the treatment of highly heterogenous tumors with a homogenous ADC coupled to two distinct payloads has been published recently [123]. In this work, MMAE (monomethyl auristatin E) or MMAF (Monomethyl Auristatin F) were linked to Para-aminobenzyloxycarbonyl (PABC), glutamic acid-valine-citrulline cleavable linker, a PEG spacer and, terminal DBCO (Dibenzylcycolooctyne) and TCO (Transcyclooctyne), respectively. In a separate reaction, an anti-HER2 derivatized N297A trastuzumab was coupled to a “tri-arm linkers” with azide and methyl tetrazine heads via the transpeptidase action of Microbial Transglutamase (MTGase) at the glutamine side chain. The functionalized TRZ was then conjugated to the previously synthesized payloads via cycloaddition of azide/DBCO and Methyltetrazine/TCO pair to produce a branched double loaded ADC. There is inherent synergy in this ADC as MMAE has a pan-cytotoxic effect on a broad range of breast cancer cells but is susceptible to extrusion by efflux pumps; however, MMAF does not possess a bystander effect due to poor diffusivity but is resistant to the action of efflux pumps. Assessment of the cytotoxic effect of different ratios of MMAE and MMAF in cell lines with highly expressed HER2 (KPL-4, and SK-BR-3), refractory HER2+(JIMT-1), and HER2 negative (MDA-MB-231) cancer cell lines showed a considerable cytotoxic effect only in the HER2+ cell lines- with EC50 values of 0.017 nM- 0.029nM in KPL-4 cells, 0.024nM-0.045nM in JIMT-1 cells, and 0.18nM -0.34nM in SK-BR-3 cells. In vivo evaluation in orthotopically placed mice model of heterogeneity (MDA-MB 231+JIMT-1) demonstrated high efficacy in tumor regression while maintaining a comparable pharmacokinetic and safety profile. The safety concerns of ADCs are well known, and a good safety profile makes this dual construct a promising option in the treatment of HER2+ breast cancer. Further safety measures can be built in by encapsulating the payloads in nanoparticles before clicking to trastuzumab tri-arm linkers. This will ensure a gradual in vivo release as well as help MMAE overcome the problem of extrusion by efflux pumps.
Li and co-workers [115] designed a biparatopic antibody consisting of a Fab domain made by the fusion of a single chain variable fragment (scfv) derived from TRZ to the heavy chain of 39S Fv designed de novo via hybridoma technology. The biparatopic monoclonal antibody was then conjugated to a maleimidocaproyl functionalized tubulysin AZ13599185, an inhibitor of microtubule polymerization. The efficacy of this construct was tested in a panel of cell lines with varying (high, SKBR-3; low, RT-112; limited, T47D and resistant, JIMT-1) HER2 expression and the results showed extensive cytotoxicity in cells with limited HER2 expression as observed in TNBC (Triple Negative Breast Cancer). Some mechanisms advanced for this outstanding effect involves clustering of the ADC at the cell surface, efficient endocytosis, inhibition of receptor recycling to the cell surface and bystander effect. It has been documented that avidity at receptor site on the cell surface accelerates antibody internalization, inhibition of recycling, and lysosomal degradation [124-126]. This biparatopic ADC can be deployed differently by conjugating it to an appropriate nanoparticle that incorporates tubulysine and another small molecule that targets metastatic HER2 positive tumors through less permeable barriers like the BBB. The triple synergy will harness the existing advantages of tubulysine and the monoclonal antibody in addition to the impact of another cytotoxic agent, and the controlled delivery, efflux pump evasion and enhanced endocytic effect of nanoparticles.
Trastuzumab Deruxtecan (DS-8201/T-DXd) manufactured by Daiichi Sankyo, is a humanized monoclonal IgG1 anti-HER2 ADC of trastuzumab, a self-immolative maleimide linker, and a topoisomerase-I payload-deruxtecan [127,128]. T-DXd shares a common mechanism of tumor delivery with TDM- 1, however, preclinical studies demonstrated broader anti-tumor activity that extended to low HER2 expressing tumors [45] compared to T-DM1. The broader effect of T-DXd has been attributed to a better handling of TDM- 1 resistance mechanisms due to differences in linker and payload, stability against extrusion by efflux transporters and bystander effect [110,112,127]. T-DXd efficacy has been demonstrated in phase 1 trials [129,130]. In the landmark DESTINY Breast 03 trial [131], among the 261 patients in the T-DXd arm, the 12-month PFS rate was 75.8% while the 12-month OS rate was 94.1%. T-DXd is currently approved as a second line [132] for anti– HER2 experienced patients with unresectable, metastatic HER2-positive breast cancer [133]. Aside T-DXd, other emerging strategies for targeting HER2+ BC with ADCs are summarized in Table 1.
TABLE 1. Novel ADCs and bispecific anti-HER2 mABs in preclinical and clinical trials
| Name | Components | Target | Nano Formulation | Mechanism | Trials | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Novel ADCs | |||||||
| SYD985 | Trastuzumab duocarmazine | Domain IV of HER2 | No | ADCC, alkylation | Phase I | [135,136] | |
| Phase III | ORR 33%, PFS 9.4 months in HER2 overexpressed tumors and 27%/40% in HR+ low | ||||||
| HER2 expression and HR- low | |||||||
| HER2 expression | |||||||
| Phase III TULIP NCT03262935 | Expected | clinical trials.gov | |||||
| MEDI4276 | scFv trastuzumab/ mAB 39S and AZ13599185 (tubulysin) | Domains IV and II of HER2 | No | ADCC, inhibition of tubulin polymerization | Phase I | Substantial toxicity led to suspension for treatment of breast and lung cancers | [137] |
| ADCT-502 | Trastuzumab, pyrolobenzodiazepine | HER2 | No | Phase I | Trials terminated as efficacy fell below expectation (NCT03125200) | [42] | |
| XMT 1522 | Trastuzumab, auristatin F hydroxypropylamide | Yes | ADCC, alkylation | Trials halted due to perceived fatal outcome but lifted subsequently | [138] | ||
| ADC ARX788 | Dolostatin, HER2IgG | HER2 | No | ADCC, alkylation | Phase I NCT03255070 | Ongoing | [133], clinical trials.gov |
| NCT02512237 | clinical trials.gov | ||||||
| Phase II NCT04829604 | clinical trials.gov | ||||||
| NCT04983121, | clinical trials.gov | ||||||
| NCT05018702 | clinical trials.gov | ||||||
| 5G9 | trastuzumaboptimal-synergistic epitope (TOSE) | No | Pre-clinical | Better uptake compared to pertuzumab. Superior synergistic outcome when combined with pertuzumab | [139] | ||
| Bispecific Antibodies | |||||||
| MCLA-128 | IgG1 BsAb | HER2, HER3 | No | ADCC | Phase I/II NCT 02912949 phase II NCT03321981 | 70% clinical benefit | [45,140] |
| Phase II NCT03321981 | Expected | clinical trials.gov | |||||
| ZW25 | Azymetric BsAb | Domains II/1V HER2 | No | ADCC | Phase I | Promising outcome with no dose limiting toxicity | [45,141] |
| NCT05035836 | Ongoing | clinical trials.gov | |||||
| NCT04224272 | - | clinical trials.gov | |||||
| NCT04276493 | - | clinical trials.gov | |||||
| ZW49 | N-acyl sulfonamide auristatin, ZW25 | HER2 | No | ADCC, cytotoxic agent | - | - | [141] |
| GBR1302 | 2* HER2 +CD3 BsAb | HER2, CD3 | No | ADCC | NCT03983395 | Terminated due to business decision | [45] |
| PRS-343 | HER2, CD137 | No | ADCC | Phase 1 NCT03330561 | Expected | [45,142] | |
| NCT03650348 | clinical trials.gov | ||||||
| Tribody design | 2*HER2+CD16 BsAb | HER2, CD16 | No | ADCC via degranulation of immune cells | Preclinical | Superior effect in pancreatic ductal adenocarcinoma, breast cancer and autologous ovarian tumor cell lines | [143] |
Novel ADCs in clinical trials
Bispecific Antibodies: Bispecific antibodies (BsAbs) are designed to harness the benefits of dual inhibition of different targets or epitopes, either in the same or different receptors. For instance, BsAbs can inhibit signal transduction in two or more RTK signaling pathways by deactivating either the RTKs or their ligand. Several BsAbs are currently being studied in patients with advanced HER2-positive disease [45].
Bispecific Nanodelivery Approach
It was thought that the inhibition of more than one epitope on the HER2+ receptor will improve inhibition of signal transduction compared to monoinhibition. This is the basis for the development of Phesgo®, a fixed dose combination of pertuzumab and TRZ that inhibits domain II and IV of the HER2+ receptor, respectively. To harness this strategy while incorporating a cytotoxic agent in a nano-delivery system, a study [134] designed a bispecific anti- mPEG/anti-HER2 antibody (BsAbs) conjugated to a docetaxel (DTX)- loaded methoxy-Pegylated lecithin-stabilized micellar drug delivery system (LsbMDDs) for targeting HER2+ cells (Figure 5). DTX was encapsulated in an amphiphilic polymer DSPE-PEG2K (1,2-Distearoyl-sn-glycero-3- phosphoethanol-amine-N[methoxy(polyethyle- neglycol), in a 1:5 ratio to form lecithin-stabilized NCs (LsbMDDs).
The anti-mPEG/anti-HER2 BsAbs delivery system comprised a Fab fragment linked to the methoxy ends of the mPEG on LsbMDDs surface, and a free scFv moiety that targets HER2+ receptors on tumor cells. On exposure to HER2+ SKBR-3 and MDC-7 cell lines, BsAbs-LsbMDDs demonstrated better release and biodistribution that ensured sustained delivery over a long period compared to controls. BsAbs-LsbMDDs also demonstrated a higher cellular uptake by SK-BR-3 and HER2+/MCF-7 cell lines compared to HER2-/MCF-7 cell lines (due to poor targeting) while there was 1.3-2.3 folds higher biodistribution and a 5 folds-10 folds higher retention of DTX at the site of action when indexed against comparators in in vivo evaluation. Other bispecific mABs are summarized in Table 1.
SMALL MOLECULE TYROSINE KINASE INHIBITORS (SMTKIS)
In general, SMTKIs are oral non-peptide anilinoquinazolone compounds [144] that are homologous to Adenosine Triphosphate (ATP) [145] and compete reversibly or irreversibly at the cytoplasmic catalytic kinase domain, preventing tyrosine phosphorylation, and consequently, inhibiting the activation and transduction of downstream signal pathways [146,147]. Since cancer cell survival and proliferation are aided by HER2 and its homodimerization or heterodimerization with HER1, HER3, or HER4 [148], inhibition of EGFR function disrupts EGFR-HER2 crosstalk resulting in a variety of therapeutic effects [145]. The smaller size of SMTKIs compared to mABs makes them useful in treating Metastatic Brain Cancer (MBC) because of the inherent ability to cross the BBB more effectively [45].
Lapatinib
Lapatinib is a dual inhibitor of EGFR, HER2 and p-glycoprotein [149] that was approved in 2013 in combination with TRZ as a chemotherapy-free regimen for patients with HR-negative and HER2-positive advanced BC [150]. The efficacy of lapatinib alone or in combination with other therapy in MBC (including brain metastases) [151] has been proven in many studies [152,153].
Mechanism of action
Lapatinib reversibly binds to the cytosolic ATP domain of EGFR and HER2 receptors to inhibit tumor cell proliferation primarily through the PI3K/Akt and MAPK pathways [154]. Inhibition of tyrosine kinase activity results in reduced phosphorylation of RAF, ERK, Akt, and PLC1 proteins[155-157]. Lapatinib also inhibits Insulin-Like Growth Factor I (IGF-I) signaling in trastuzumab-sensitive and trastuzumab-resistant HER2 overexpressing cells [158]. This is important because crosstalk between the IGF-I receptor and HER2 in trastuzumab-resistant cells increases HER2 receptor phosphorylation [151,159] leading to tumor cell proliferation. Further, lapatinib increases fragmentation of poly ADP-ribose polymerase (PARP), a protein implicated in programmed cell death, and downregulated survivin expression [160,161]. In HER2-positive breast cancer cells, lapatinib regulates numerous microRNAs (miRNAs), which play crucial roles in anti-tumor activity [162-164]. In this context, lapatinib treatment increases the expression of miR575 and miR-1225-5 resulting in the downregulation of the oncogenic enzyme phospholipase C PLCXD1 (phosphatidylinositol-specific phospholipase-C-X-domain-containing-1), an miR-575 and miR1225-5p target gene [164].
Lapatinib nanoparticle formulations
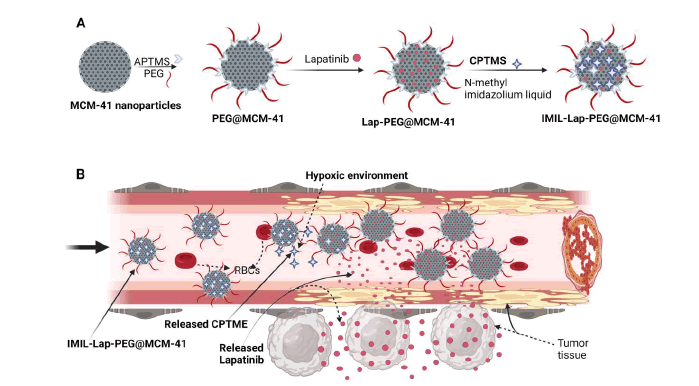
According to ClinicalTrials.org, there is presently no nanoparticle formulation of lapatinib in clinical use or ongoing trials. Regardless, lapatinib conjugated nanoparticles have been extensively examined in several preclinical experiments involving cell cultures and animal models. The concept of pH-responsive nano-delivery has been explored in a study [165] with lapatinib. In this evaluation, lapatinib was encapsulated in NPs formulated from mesoporous silica materials, decorated with PEG (ImIL-PEGylated@MCM-41) before sealing of the porous surface with covalently linked imidazolium-based ionic seal to achieve controlled release (Figure 6). The presence of N-methyl imidazole ionic liquid seal on the porous surface of the NPS in a neutral medium prevents premature release [166]; however, in an acidic/hypoxic environment, the seal breaks down to release the loaded drug due to hydrolysis of imidazolium ionic liquid [167]. The result of this study showed that when Lap@ImIL-PEG@MCM-41 was exposed to SK-BR-3 cell lines and control (HEK-293), there was less cell viability in SK-BR-3 cells compared to HEK. This was primarily attributed to lapatinib’s affinity for HER2 receptors, excellent lapatinib entrapment efficiency and extended controlled release property of the nanocarrier. In general, the surface density of silanol groups interacts readily with phospholipids on the red blood cell membranes, leading to hemolysis-a drawback for mesoporous silica nanoparticles [168]. However, in this study, hemolysis was mitigated via the incorporation of PEG in the nanocarrier to reduce interaction between RBCs and the mesoporous silica layer.
Figure 6: Illustration of the A) Synthesis and drug loading of modified mesoporous nanoparticles B) Expected drug release model in vivo APTMS 3.(aminopropyl) trimethoxysilane CPTMS: 3- Chlororpropyltriethoxysilane; PEG: Polyethylene Glycol (PEG3000); MCM-41: Mesoposorous silicon nanoparticles. Adapted from [165]
Other novel nanodelivery strategies with lapatinib have been used for the treatment of other types of Breast cancer; they could be applied to treating HER2+ BC. In one study [169], an attempt was made to design pH-responsive nano-delivery systems without conjugation to ligands that are prone to opsonization and clearance by the RES [170]. In this study, lapatinib was loaded into pH-responsive, charge switchable, polycarbonate-doxorubicin conjugate micelles for synergistic breast cancer treatment. This design takes advantage of the knowledge that anionic NPs have long circulation time in blood; however, they can be easily repelled by the negatively charged cell membrane. One way to circumvent this challenge is switching of polarity to cationic charge at the target site to induce local fluidization causing cell membranes to lose their rigidity thus allowing in rapid internalization and penetration across multiple cell layers [152,153,171].
The rich glutathione (GSH) level in tumor cells relative to normal cells has been explored in intracellular drug release triggered by disulfide redox sensitivity [172-174]. In a study that exploited this strategy nanoparticles encapsulating several hydrophobic anticancer drugs including Gefitinib (GEF), Lapatinib (LAP), Olaparib (OLA) were synthesized via the nanoprecipitation method with polydisulfide amide (L-Cystine Dimethyl Ester Dihydrochloride (Cyst-8E)) and 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine polyethylene glycol 3000 (DSPE-PEG3000) to form SMA-NPs (Small Molecule Anticancer-Nanoparticles) [175]. Evaluation of the in vivo effect of LAP-NPs showed a strong and efficient suppression of tumor volume compared to free LAP and control NPs. Particularly, the tumor inhibition rate for LAP-NP was 62.5% compared to 42.29% for free LAP which reflects the extent of tumor volume regression. This study relies solely on EPR and would benefit from active targeting if conjugated to HER2 specific ligands.
Neratinib
Neratinib is an oral pan-HER TKI that has demonstrated promising anti-tumor results in trastuzumab pretreated individuals based on the phase III ExteNET trial [176]. In combination with capecitabine, neratinib decreased CNS lesions by 49% in the TBCRC 022 study [177]. The incidence of CNS tumor recurrence and time to CNS metastases has been shown to be significantly lower (p=0.002), (HR 0.45 95% CI: 0.26-0.78, P=0.004) [177], respectively, with neratinib. It has demonstrated clinical activity with a response rate of 69% in patients with prior taxane, trastuzumab, and lapatinib therapy [16].
Mechanism of action
Neratinib binds covalently and irreversibly to a unique and shared cysteine residue in the ATP-binding pocket of the EGFR family of receptors [178]. In addition, neratinib binds to the cysteine residues Cys-773 and Cys-805 in the HER1 and HER2 proteins, respectively [179] thus inhibiting PI3K/Akt and MAPK downstream signaling induced by HER2 receptor activation [45,180,181] resulting in the stagnation of the G1-S phase transition, increase in p27 (cyclin dependent Kinase inhibitor), and decrease in Phosphorylated Retinoblastoma Protein (PRB) and cyclin D1 [148,182] that are involved in cell proliferation. Neratinib has been shown to reverse membrane-bound ATP transporter-mediated multidrug resistance in cancer cell lines [183] thus providing an alternative method for improving chemotherapy response in HER2-positive breast cancer. However, concurrent suppression of wild-type EGFR leads to common toxicity of diarrhea associated with neratinib. This has limited neratinib's clinical application [184].
Following a search of the literature and clinical trials.gov, there is no nanoparticle formulation of neratinib in clinical practice or current trials. A study that synchronized delivery of TRZ and neratinib has been discussed earlier in this review [68].
Tucatinib is an orally active reversible inhibitor of the MAPK and P13K/AKT signaling pathway via inhibition of the cytosolic tyrosine kinase domain of HER2 and HER3 receptors [185]. Tucatinib is highly selective for HER2 [186] has no effect on EGFR, and thus fewer side effects (rashes and diarrhea) compared to other small molecule TKIs [148].
Mechanism of action
In vitro and in vivo studies revealed tucatinib as a competitive and reversible ligand that binds the ATP pocket of the cytosolic domain of the HER2 receptor, blocking HER2 phosphorylation and thereby suppressing signal transduction downstream the MAPK and PI3K/AKT pathways [185]. Its selectivity confers a safety and tolerability profile that favors combination therapy with various therapeutic agents, including monoclonal antibodies and ADCs (Antibody-Drug Conjugates) [187]. Besides, tucatinib demonstrates a more significant potential to cross the Blood-Brain Barrier (BBB) when compared to monoclonal antibodies; hence its application in therapies aimed at treating and preventing brain metastases, which occurs in 30%-50% of patients with advanced HER2-positive breast cancer [188].
A search of the literature and clinical trials.gov did not turn up any published article on nano-formulations of tucatinib either in clinical practice or clinical trials. In addition, a search of the literature for tucatinib conjugated nano-formulations revealed no article in the preclinical setting.
Pyrotinib
Pyrotinib is a new oral small molecule TKI that inhibits cell proliferation via the PI3K/AKT/mTOR and MEK/MAPK pathways and acts against numerous HER family members (HER1, HER2, and HER4) [189,190]. It specifically binds to ATP binding sites in the intracellular kinase sections of HER1, HER2, and HER4, effectively blocking the downstream signaling pathways initiated on the tumor cell membrane by homodimers or heterodimers of the HER family [191]. In two clinical trials [192,193] pyrotinib in combination with other HER2 therapies, was found to have a greater progression-free survival rate than placebo. However, based on our literature search, nanoparticle formulations of pyrotinib are yet to be reported.
Afatinib is an orally active, second-generation anilinoquinazoline derivative that inhibit members of the ErbB receptor family by binding irreversibly to an intracellular tyrosine kinase domain [194,195]. The irreversible binding properties of afatinib may help impede mutant cell lines, such as EGFR L858R/T790M mutations, which are typically resistant to erlotinib and gefitinib [196]. The FDA approved afatinib only for treatment of metastatic Non-Small Cell Lung Cancer despite its effect on HER2 receptor. This is largely due to toxicities observed during Phase III [197,198] trials in spite of apparent efficacy in Phase I and II trials. Nano-formulation strategies are currently underway to reduce toxicity and investigations are focused on evaluating efficacy and safety in lung cancer [199,200] with no mention of such investigation in HER2+BC.
Most studies reported on nanotechnology formulations are largely reliant on the EPR mechanism, especially among studies involving small molecule TKIs [165,201,202]. It is our strong believe that TKIs can benefit from strategies that incorporate active targeting of tumor cells. Hence there is ample opportunity for various research groups to exploit the numerous targeted delivery techniques discussed in this review for enhancing the efficacy of TKIs in combination with other antineoplastic agents.
THE ROLE OF NANOTECHNOLOGY IN IMMUNOTHERAPY IN HER2-POSITIVE BREAST CANCER
A new generation of treatment modality that stimulates the host immune system to selectively kill cancer cells with relatively fewer side effects on healthy tissues has been dubbed cancer immunotherapy; it also promotes systemic immune surveillance that can eliminate both primary and metastatic tumors. Compared to chemotherapy, surgical resection and radiotherapy, the anti-tumor immune cells activated by immunotherapeutic strategies can even kill Circulating Tumor Cells (CTCs) and thus inhibit the formation of metastatic foci. Further, immunotherapy can establish a long-term immune memory to prevent tumor recurrence [203]. These immunotherapeutic agents are exemplified by cytokines, checkpoint blockers, anti-cancer vaccines and Chimeric Antigen Receptor T-Cells (CAR-T).
Checkpoint Blockers for Cytotoxic T-lymphocyte antigen 4(CTLA4)
Advances in cancer therapy in the past few years have included the development of medications that modulate immune checkpoint proteins. Cytotoxic T-lymphocyte antigen 4 (CTLA4) and programmed death 1 (PD1) are two coinhibitory receptors that are expressed on activated T cells to which therapeutic blocking antibodies have reached routine clinical use [204]. To prevent the immune system from causing harm to the host when reacting to a foreign antigen, humans have evolved immune checkpoint proteins and mechanisms to quickly halt an immune response. However, in the case of cancer, malignant cells have developed many mechanisms to evade the human immune system, including the ability to limit immune responses through such immune checkpoints. Monoclonal antibodies that target CTLA4, such as ipilimumab (approved by the FDA for treatment of unrespectable or metastatic melanoma), have demonstrated efficacy in cancer treatment. With CTLA4 blocked, activated T cells proliferate and achieve a persistent state of activation, which enables the targeting of otherwise poorly immunogenic tumor antigens on cancer cells [205]. Tremelimumab works through a similar mechanism as ipilimumab to block CTLA4.
Checkpoint Blockers for Programmed death-1 (PD-1) and PD-L1
Programmed death 1 (PD1) is a protein on the surface of T cells of the immune system and programmed death ligand 1 (PD-L1) and ligand 2 (PD-L2) are on the tumor cells and antigen presenting cells. The interaction of PD-1 and its ligand (PD-L1) in the Tumor Microenvironment (TME) provides an immune escape for tumor cells by turning off cytotoxic T cells (when bound to a ligand, PD1 lowers the threshold for apoptosis, induces energy via blunted T-cell receptor signaling, and generally leads to T-cell depletion). Thus, blocking this interaction subjects the tumor cells to attack from cytotoxic T cells. In certain tumor cells, upregulation of PD-L1 expression has been seen, which leads to increased inhibition of T-cell activity in favor of tumor cell survival. A monoclonal antibody against PD1 can block this pathway (that is a PD1–PDL1 interaction) and can result in the upregulation of immune response and inhibition of tumor growth. Suppressing these immune checkpoints results in immune-mediated antitumor activity in mouse models and clinical trials. Thus recent advances in the understanding of tumor biology and immune checkpoint molecules (PD-1/PD-L1) have provided novel therapeutic strategies using Immune Checkpoint Blockade (ICB). Pembrolizumab and nivolumab were approved by the FDA for the treatment of patients already treated with ipilimumab for unresectable or metastatic melanoma and disease progression [207]. Both antibodies inhibit the interaction between PD1 and its ligands and increase the immune response against cancer cells and are efficacious against non-small cell lung cancer, renal cell cancer, bladder cancer and Hodgkin lymphoma [204]. The advent of anti–programmed death ligand 1 (PD-L1) monoclonal antibodies have transformed the paradigm of treatment in a variety of solid tumors, including the treatment of Non-Small Cell Lung Cancer (NSCLC). The U.S. Food and Drug Administration (FDA) granted approval to atezolizumab and durvalumab in March of 2019 and 2020, respectively, for use in combination with chemotherapy for first-line treatment of patients with small cell lung cancer. Further, combined CTLA4 and PD1 blockade has been explored in preclinical models and clinical trials in metastatic melanoma [204].
Chimeric antigen receptor T cell (CAR-T)
Chimeric antigen receptor T cell (CAR-T) therapy is an innovative form of immunotherapy in which autogenous T cells are genetically modified to express chimeric receptors encoding antigen-specific fragments and various co-stimulating molecules. Unlike physiological T cells with common T cell receptors, these modified CAR-T cells are transported to and recognized by cancer cells in ways unrelated to Human Leukocyte Antigens (HLA). At present, CAR-T therapy has performed well in the treatment of hematologic malignancies, but challenges remain in solid tumors such as breast cancer. Many targets are under study for CAR-T cell therapy for BCs such as anti-HER2 CAR-T cells (NCT02547961) [207]. T cells against HER2 can be successfully expanded ex vivo in mice models and have shown evidence of antitumor activity [208]. One patient with HER2+MBC was treated with autologous HER2369-377 T cells that were cocultured with HLA2-peptide-loaded DCs in a pilot trial [209]. There was evidence of tumor cell disappearance in the bone marrow but no penetration of T cells into the tumor. HER2 CAR T cells containing CD28 costimulatory domain were administered to the Central Nervous System (CNS) of mice and showed regression of HER2+ MBC in the CNS [210].
Breast Cancer
PD-Ll is a therapeutic target in breast cancer, and inhibition of PD1/ PD-Ll signaling has shown extremely promising signs of activity in breast cancer [211,212]. Triple-negative Breast Cancer (TNBC) has been found to have high immunogenicity among BC subtypes. Renewed attention and research directions have been attracted toward the immunotherapy for breast cancer, especially on TNBC [213]. The FDA granted an accelerated approval for the immunotherapy drug (atezolizumab) in combination with chemotherapy (nab-paclitaxel: Abraxane) for the treatment of women with advanced TNBC (for tumors that are positive for a protein called PD-L1). It is known that HER2-expressing breast cancer cells use the PD-1/PD-L1 cells checkpoint axis to evade cytotoxicity by immune system. Further, PD-L1, a ligand for PD-1, is constitutively expressed in a subset of HER2+ breast cancer patients. Higher PD-L1 expression on HER2+ breast cancer has been shown to have a significant positive correlation with a higher tumor grade and tumor-infiltrating lymphocytes [42]. Preclinical studies in immune-competent mice showed that PD-1 and CTLA-4 inhibition improves the immune-mediated effects of HER2-targeted therapies through synergistic activation of CD8+ T cells. These data provide a rationale for the clinical development of immune checkpoint inhibitors for the treatment of HER2+ breast cancer patients and the combination of these inhibitors with HER2-targeted therapies [212]. However, early phase clinical trials of new immune agents for the treatment of patients with HER2+breast cancer have shown modest results.
Nanotechnology
One limitation that accounts for the compromised efficacy of ICB immunotherapy is off target binding of ICB antibodies to normal tissues upon systemic administration. Ideally, ICBs should be delivered selectively to the tumor site to avoid systemic non-specific activation of the Immune System resulting in irAEs (Immune-Related Adverse Effects). Immune checkpoint blockades have been reported to induce Inflammatory Side Effects (IRAEs), which resemble autoimmune disease [204]. Recent years have witnessed novel developments in pharmaceutical nanotechnology, or nanomedicine, as a possible strategy to ameliorate immunotherapy technical weaknesses. To improve the long-lasting response rate of checkpoint blockade therapy, nanotechnology has been employed at first for the delivery of single checkpoint inhibitors; while therapy via single immune checkpoint blockade results in resistance and a short period of response, strong interest has been raised to efficiently deliver immunomodulators targeting different inhibitory pathways or both inhibitory and costimulatory pathways. Promising results of immune checkpoint blockade therapy in combination with nanotechnology delivery systems have been reported [214]. nanotechnology, an interdisciplinary science, represents an effective tool to design highly effective combinational therapies boosting the effectiveness of immunotherapy and overpowering its limits (useful in overcoming critical points of Immune Checkpoint Inhibitors (ICIs) therapy such as the localized/targeted and controlled ICI release (availability) and ICI stability after infusion; further, they may allow for a reduction of ICI dose and control over Adverse Immune-Related Events (AIEs).
Reports have been given that although anti-CTLA4 treatment allowed for the overall survival of melanoma to be reached, with even cure of metastatic disease, for about 20% of the patients; nevertheless, clinical application is limited because of its AIEs [215,216]. It has been suggested that CTLA-4 blockade can promote recruitment of peripheral T cells, thus enhancing the probability of autoimmune reaction [217]. Thus, the need for nanotechnological delivery of ICIs has presented itself which will enhance antibody bioavailability inside the tumor in order to improve the efficacy of the treatment, and at the same time limit the toxic effects due to the systemic exposure. Immunotherapy of HER2-positive breast cancer can benefit from the nanotechnology approach as it progresses to the clinic. NP-mediated ICI delivery seems an efficient and feasible approach to overcome the unsuccessful systemic administration of mAb against ICB in brain glioma treatment, due to drug failure in crossing the BBB. For this reason, combined nanotechnology and immunotherapy [218]. These authors covalently attached ICI mAb, such as anti-CTLA-4 (and/or anti-PD-1) to a biological polymeric scaffold made of Poly (-L-Malic Acid) (PMLA). These nanoscale immunoconjugates allowed the ICI mAb to go across the BBB and get to the cancer environment, where they modulated the immune response Mice bearing GBM showed an increase in survival when treated with nanocarriers loaded with anti-CTLA-4 (or anti-PD-1) with respect to the free mAb, and mice survival became even longer when treated with nanocarriers loaded with both ICI mAbs [218]. Moreover, mice bearing GBM showed a significantly longer survival when treated with double checkpoint inhibitor-bearing nanocarriers compared to those treated with single checkpoint inhibitors.
Nanotechnology is also useful in reducing drug dose as proposed by Schmid and colleagues [219]. They used Poly (Lactide-O-Glycolic) Acid (PLGA) and PEG NPs conjugated with anti-PD-1 mAb (PD-1 targeting NPs) and loaded with the Transforming Growth Factor- Receptor 1 (TGF- R1) inhibitor, SD-208, to target PD-1 cells and to block the immunosupressive activity of TGF-β in an MC38 colon cancer model. They showed that PD-1 targeting NPs-SD-208 acted on CD8+T cells, reduced tumor growth and ameliorated animal survival. The therapeutic effect was obtained at low doses of ICI (20 µg of anti-PD-1 and 40 µg of SD-208); whereas anti-PD-1 mAb and SD-208 in free administration did not have any effects. In a 4T1 tumor-bearing mice model used Iron-Oxide Nanoparticles (IONPs), which, after systemic administration, were strongly stable, and the small size favored their accumulation into the tumor site where they mediated hyperthermia after NIR irradiation. These authors suggested that local IONP-mediated photothermal therapy combined with ICI, such as anti-CTLA-4, can allow immunosuppression mediated by Treg to be overcome, thus boosting cancer immunotherapy [220].
Combining immunotherapy and multifunctional nanoprobes can achieve early cancer diagnosis and treatment [201]. Studies have shown that nanoparticle-assisted radiotherapy, chemotherapy, Photothermal Therapy (PTT) and Photodynamic Therapy (PDT) can stimulate the immune system by Inducing Immunogenic Cell Death (ICD) [221,222]. When combined with Immune Checkpoint Blockade (ICB), the localized therapies can activate tumor-specific immune response to target metastatic cancer cells, and induce immune memory to inhibit tumor recurrence [223,224]. Therefore, various therapeutic modalities can significantly augment the effects of immunotherapy against residual tumor cells. High-resolution imaging by multifunctional nanoparticles can also elucidate the mechanisms of immunotherapy [225,226] via real-time monitoring of immune cells in the Tumor Micro Environment (TME) and the biodistribution of immunomodulatory drugs at the target site [227-230].
Conclusion
Nanotechnology platforms for the delivery of small molecule TKIs relied on EPR mechanism for delivery to tumors. It is our strong believe that small molecule TKIs can benefit from strategies that incorporate active targeting of tumor cells using large molecule TKIs (monoclonal antibody) as targeting moiety and which can exert therapeutic effects. Hence there is ample opportunity for various research groups to exploit the numerous targeted delivery techniques discussed in this review for enhancing the efficacy of TKIs in combination with other antineoplastic agents. Further, nanotechnology platforms can augment the delivery and enhance the efficacy of the new generation of treatment modality (immunotherapy) that stimulates the host immune system to selectively kill cancer cells with relatively fewer side effects on healthy tissues. The clinical progress made with immunotherapy has been spectacular, achieving complete cures and inducing long-term survival in advanced-stage patients. Unfortunately, however, immunotherapy only works well in relatively small subsets of patients. Increasing amounts of preclinical and clinical data demonstrate that combining nanomedicine with immunotherapy can boost therapeutic outcomes, by turning “cold” nonimmunoresponsive tumors and metastases into “hot” immunoresponsive lesions. In fact, reports have shown that Nano-immunotherapy can be realized via three different approaches, in which nanomedicines are used:
- to target cancer cells,
- to target the tumor immune microenvironment, and
- to target the peripheral immune system
Acknowledgement
This publication was supported by NCI/NIH Grant #: 1SC1CA199810-01 awarded to Emmanuel O. Akala and Pharmaceutical Research and Manufacturers of America Foundation (PhRMA Foundation) 2022 Predoctoral Fellowship in Drug Delivery awarded to Victor Ejigah. This work was carried out in facilities supported by NCRR/NIH Grants #1C06 RR 020608-01 and #1 C06 RR 14469-01.
REFERENCES
- Chen F, Ma K, Madajewski B, et al. Ultrasmall targeted nanoparticles with engineered antibody fragments for imaging detection of HER2-overexpressing breast cancer. Nat Communication. 2018;9(1):1. [GoogleScholar] [CrossRef]
- Larionov AA. Current Therapies for Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Patients. Front Oncol. 2018;8:89. [GoogleScholar] [CrossRef]
- Shu M, Yan H, Xu C, et al. A novel anti-HER2 antibody GB235 reverses Trastuzumab resistance in HER2-expressing tumor cells in vitro and in vivo. Sci Rep. 2020;10(1):1-2.[GoogleScholar] [CrossRef]
- Harris AL, Nicholson S, Sainsbury R, et al. Epidermal growth factor receptor and other oncogenes as prognostic markers. J Natl Cancer Inst Monogr. 1992;(11):181-7.[GoogleScholar]
- Hapuarachchige S, Kato Y, Artemov D. Bioorthogonal two-component drug delivery in HER2 (+) breast cancer mouse models. Sci Rep. 2016;6(1):1. [GoogleScholar] [CrossRef]
- Sainsbury JR, Farndon JR, Harris AL, et al. Epidermal growth factor receptors on human breast cancers. Br J Surg. 1985;72(3):186-8. [GoogleScholar][CrossRef]
- Ferrero JM, Ramaioli A, Largillier R, et al. Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol. 2001;12(6):841-6. [GoogleScholar] [CrossRef]
- Mondaca S, Margolis M, Sanchez Vega F, et al. Phase II study of trastuzumab with modified docetaxel, cisplatin, and 5 fluorouracil in metastatic HER2-positive gastric cancer. Gastric Cancer. 2019;22(2):355-62. [GoogleScholar] [CrossRef]
- Ryan Q, Ibrahim A, Cohen MH, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008;13(10):1114-9. [GoogleScholar] [CrossRef]
- Singh H, Walker AJ, Amiri-Kordestani L, et al. U.S. Food and Drug Administration Approval: Neratinib for the Extended Adjuvant Treatment of Early-Stage HER2-Positive Breast Cancer. Clin Cancer Res. 2018;24(15):3486-91. [GoogleScholar] [CrossRef]
- Blair HA. Pyrotinib: First global approval. Drugs. 2018;78(16):1751-5. [GoogleScholar] [CrossRef]
- Shah M, Wedam S, Cheng J, et al. FDA approval summary: tucatinib for the treatment of patients with advanced or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27(5):1220. [GoogleScholar] [CrossRef]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015; 372(8):724-34.
[GoogleScholar] [CrossRef] - Tarantino P, Morganti S, Uliano J, et al. Margetuximab for the treatment of HER2positive metastatic breast cancer. Exp Opi Bio Ther. 2021;21(2):127-33.
[GoogleScholar] [CrossRef] - NCCN Guidelines for Patients Metastatic Breast Cancer. Published online 2020:78. [GoogleScholar]
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Exp Rev Antic Ther. 2011;11(2):263-75. [GoogleScholar] [CrossRef]
- Hunter FW, Barker HR, Lipert B, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2positive breast cancer. Br J Cancer. 2020;122(5):603-12. [GoogleScholar] [CrossRef]
- Kumar G, Nandakumar K, Mutalik S, et al. Biologicals to direct nanotherapeutics towards HER2-positive breast cancers. Nanomed: Nanotech, Bio Med. 2020;27:102197. [GoogleScholar] [CrossRef]
- Akala EO, Adesina S, Ogunwuyi O. Computer optimization of biodegradable nanoparticles fabricated by dispersion polymerization. Int J Environ Res Public Healt. 2016;13(1):47.[GoogleScholar] [CrossRef]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nature Rev Cancer. 2005;5(3):161-71. [GoogleScholar] [CrossRef]
- Haque S, Md S, Alam MI, et al. Nanostructure-based drug delivery systems for brain targeting. Drug Dev Ind Pharm. 2012;38(4):387-411. [GoogleScholar] [CrossRef]
- Hillaireau H, Couvreur P. Polymeric nanoparticles as drug carriers. Polym Drug Deliv. 2006;101-10. [GoogleScholar]
- Ogunwuyi O, Adesina S, Akala EO. d-Optimal mixture experimental design for stealth biodegradable crosslinked docetaxel-loaded poly-ε-caprolactone nanoparticles manufactured by dispersion polymerization. Pharm-Int J Pharm Sci. 2015;70(3):165-76. [GoogleScholar] [CrossRef]
- Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjugate Chem. 2011;22(10):1879-903. [GoogleScholar] [CrossRef]
- Rodallec A, Sicard G, Giacometti S, et al. Tumor uptake and associated greater efficacy of anti-Her2 immunoliposome does not rely on Her2 expression status: study of a docetaxel-trastuzumab immunoliposome on Her2+ breast cancer model (SKBR3). Anti-Cancer Drugs. 2020;31(5):463-72. [GoogleScholar] [CrossRef]
- Wang H, Zhao Y, Wu Y, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32(32):8281-90. [GoogleScholar] [CrossRef]
- Lin YL, Tsai NM, Chen CH, et al. Specific drug delivery efficiently induced human breast tumor regression using a lipoplex by non-covalent association with anti-tumor antibodies. J Nanobiotech. 2019;17(1):1-1. [GoogleScholar] [CrossRef]
- Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharm Nanotechnol. 2019;7(1):3-23. [GoogleScholar] [CrossRef]
- Lee JH, Nan A. Combination drug delivery approaches in metastatic breast cancer. J Drug Deliv. 2012;2012. [GoogleScholar] [CrossRef]
- Berko YA, Funmilola AF, Akala EO. Fabrication of Paclitaxel and 17AAG-loaded Poly-ε-Caprolactone Nanoparticles for Breast Cancer Treatment. J Pharm Drug Deliv Res. 2021;10(1).[GoogleScholar]
- Rafiyath SM, Rasul M, Lee B, et al. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp hematol oncol. 2012;1(1):1-9. [GoogleScholar] [CrossRef]
- Yamada Y, Kawaguchi R, Ito F, et al. Skin–mucous membrane disorder and therapeutic effect of pegylated liposomal doxorubicin in recurrent ovarian cancer. J Obstet Gynaecol Res. 2017;43(7):1194-99.GoogleScholar] [CrossRef]
- Fay F, Scott CJ. Antibody-targeted nanoparticles for cancer therapy. Immunotherapy. 2011; 3(3): 381-94. [GoogleScholar] [CrossRef]
- Johnston MC, Scott CJ. Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy. Drug Discov Today: Technol. 2018;30:63-69. [GoogleScholar] [CrossRef]
- Parakh S, Gan HK, Parslow AC, et al. Evolution of anti-HER2 therapies for cancer treatment. Cancer Treat. Rev. 2017;59:1-21. [GoogleScholar] [CrossRef]
- Pinto AC, Ades F, de Azambuja E, et al. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22:152-55. [GoogleScholar] [CrossRef]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann oncol. 2013;24(9):2278-84. [GoogleScholar] [CrossRef]
- Von Minckwitz G, Huang CS, Mano MS, et al. Investigators KN Engl. J Med. 2019;380:617-628.
[GoogleScholar] [CrossRef] - Cortés J, Diéras V, Lorenzen S, et al. Efficacy and safety of Trastuzumab Emtansine plus Capecitabine vs Trastuzumab Emtansine alone in patients with previously treated ERBB2 (HER2)-positive metastatic breast Cancer: a phase 1 and randomized phase 2 trial. JAMA oncol. 2020;6(8):1203-09. [GoogleScholar] [CrossRef]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689-99. [GoogleScholar] [CrossRef]
- Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2–positive advanced breast cancer: final results from MARIANNE. Cancer. 2019;125(22):3974-84. [GoogleScholar] [CrossRef]
- Costa RL, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer. 2020;6(1):1-1. [GoogleScholar] [CrossRef]
- Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther. 2019;4(1):1-22. [GoogleScholar] [CrossRef]
- Vernieri C, Milano M, Brambilla M, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol/Hematol. 2019;139:53-66. [GoogleScholar] [CrossRef]
- Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019;11:1758835919833519. [GoogleScholar] [CrossRef]
- Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer. 2021;7(1):1-2. [GoogleScholar][CrossRef]
- Pupa SM, Menard S, Morelli D, et al. The extracellular domain of the c-erbB-2 oncoprotein is released from tumor cells by proteolytic cleavage. Oncogene. 1993;8(11):2917-23. [CrossRef]
- Codony-Servat J, Albanell J, Lopez-Talavera JC, et al. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer research. 1999;59(6):1196-01. [GoogleScholar]
- Lafky JM, Wilken JA, Baron AT, et al. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochem Biophys Acta. 2008;1785(2):232-65. [GoogleScholar] [CrossRef]
- Molina MA, Codony-Servat J, Albanell J, et al. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer research. 2001;61(12):4744-79. [GoogleScholar]
- Christianson TA, Doherty JK, Lin YJ, et al. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer research. 1998;58(22):5123-29. [GoogleScholar]
- Wilken JA, Maihle NJ. Primary trastuzumab resistance: new tricks for an old drug. Annals of the New York Academy of Sciences. 2010 Oct;1210(1):53-65. [GoogleScholar] [CrossRef]
- Hurwitz E, Stancovski I, Sela M, Yarden Y. Suppression and promotion of tumor growth by monoclonal antibodies to ErbB-2 differentially correlate with cellular uptake. Proc Natl Acad Sci. 1995;92(8):3353-57. [GoogleScholar] [CrossRef]
- Klapper LN, Waterman H, Sela M, Yarden Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000;60(13):3384-88. [GoogleScholar]
- Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27(34):5838-47. [GoogleScholar] [CrossRef]
- Baselga J, Albanell J, Molina MA, et al. Mechanism of action of trastuzumab and scientific update. InSeminars oncol. 2001;28(1); 4-11. [GoogleScholar] [CrossRef]
- Albanell J, Codony J, Rovira A, et al. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4. New Trends in Cancer for the 21st Century. 2003;253-68. [GoogleScholar] [CrossRef]
- Juan A, Cimas FJ, Bravo I, et al. Antibody conjugation of nanoparticles as therapeutics for breast cancer treatment. Int J Mole Sci. 2020;21(17):6018. [GoogleScholar] [CrossRef]
- Liu YK, Lin YL, Chen CH, et al. A unique and potent protein binding nature of liposome containing polyethylenimine and polyethylene glycol: a nondisplaceable property. Biotechnol Bioeng. 2011;108(6):1318-27. [GoogleScholar] [CrossRef]
- Lin YL, Chang KF, Huang XF, et al. Liposomal n-butylidenephthalide protects the drug from oxidation and enhances its antitumor effects in glioblastoma multiforme. Int J Nanomed. 2015;10:6009-20. [GoogleScholar] [CrossRef]
- Lin YL, Liu YK, Tsai NM, et al. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomed. 2012;8(3):318-27. [GoogleScholar] [CrossRef]
- Niza E, Noblejas-López MD, Bravo I, et al. Trastuzumab-targeted biodegradable nanoparticles for enhanced delivery of Dasatinib in HER2+ Metastasic breast Cancer. Nanomat. 2019;9(12):1793.
[GoogleScholar] [CrossRef] - Qian XL, Zhang J, Li PZ, et al. Dasatinib inhibits c-src phosphorylation and prevents the proliferation of Triple-Negative Breast Cancer (TNBC) cells which overexpress Syndecan-Binding Protein (SDCBP). PLoS One. 2017;12(1):e0171169. [GoogleScholar] [CrossRef]
- Ocana A, Gil-Martin M, Antolín S, et al. Efficacy and safety of dasatinib with trastuzumab and paclitaxel in first line HER2-positive metastatic breast cancer: results from the phase II GEICAM/2010-04 study. Breast Cancer Res Treat. 2019;174(3):693-701. [GoogleScholar] [CrossRef]
- Rodallec A, Franco C, Robert S, et al. Prototyping trastuzumab docetaxel immunoliposomes with a new FCM-based method to quantify optimal antibody density on nanoparticles. Sci Reports. 2020;10(1):1-1. [GoogleScholar] [CrossRef]
- Rodallec A, Sicard G, Giacometti S, et al. From 3D spheroids to tumor bearing mice: efficacy and distribution studies of trastuzumab-docetaxel immunoliposome in breast cancer. Int J Nanomed. 2018;13:6677. [GoogleScholar] [CrossRef]
- Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6(1):12-21. [GoogleScholar] [CrossRef]
- Aleanizy FS, Alqahtani FY, Setó S, et al. Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. IJN. 2020;15:5433-43. [GoogleScholar] [CrossRef]
- Adesina SK, Akala EO. Nanotechnology approaches for the delivery of exogenous siRNA for HIV therapy. Mole Pharma. 2015;12(12):4175-87. [GoogleScholar] [CrossRef]
- Zhong S, Ling Z, Zhou Z, et al. Herceptin-decorated paclitaxel-loaded poly (lactide-co-glycolide) nanobubbles: ultrasound-facilitated release and targeted accumulation in breast cancers. Pharma Devel Tech. 2020;25(4):454-63. [GoogleScholar] [CrossRef]
- Fathian kolahkaj F, Derakhshandeh K, Khaleseh F, et al. Active targeting carrier for breast cancer treatment: Monoclonal antibody conjugated epirubicin loaded nanoparticle. J Drug Deliv Sci Technol. 2019;53:101136. [GoogleScholar] [CrossRef]
- Gu X, Jia S, Wei W, et al. Neoadjuvant chemotherapy of breast cancer with pirarubicin versus epirubicin in combination with cyclophosphamide and docetaxel. Tumour Biol. 2015;36(7):5529-35. [GoogleScholar] [CrossRef]
- Saqafi B, Rahbarizadeh F. Polyethyleneimine-polyethylene glycol copolymer targeted by anti-HER2 nanobody for specific delivery of transcriptionally targeted tBid containing construct. Artif Cells Nanomed Biotech. 2019;47(1):501-11. [GoogleScholar] [CrossRef]
- Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed Engl. 2008;47(8):1382-95. [GoogleScholar] [CrossRef]
- Cędrowska E, Pruszyński M, Gawęda W, et al. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules. 2020;25(5):e1025. [GoogleScholar] [CrossRef]
- Guo S, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol Adv. 2014;32(4):778-88. [GoogleScholar] [CrossRef]
- Fang M, Peng C wei, Pang DW, et al. Quantum Dots for Cancer Research: Current Status, Remaining Issues, and Future Perspectives. Cancer Biol Med. 2012;9(3):151-63. [GoogleScholar] [CrossRef]
- Draz MS, Fang BA, Zhang P, et al. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4(9):872-92.[GoogleScholar] [CrossRef]
- Song WJ, Du JZ, Sun TM, et al. Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery. Small. 2010;6(2):239-41. [GoogleScholar]
- Varkouhi AK, Foillard S, Lammers T, et al. SiRNA delivery with functionalized carbon nanotubes. Int J Pharm. 2011;416(2):419-25.[GoogleScholar] [CrossRef]
- Badkas A, Frank E, Zhou Z, et al. Modulation of in vitro phagocytic uptake and immunogenicity potential of modified Herceptin®-conjugated PLGA-PEG nanoparticles for drug delivery. Colloids and Surfaces B: Biointerfaces. 2018; 162:271-78.[GoogleScholar] [CrossRef]
- Quartino AL, Li H, Jin JY, et al. Pharmacokinetic and exposure–response analyses of pertuzumab in combination with trastuzumab and docetaxel during neoadjuvant treatment of HER2+ early breast cancer. Cancer Chemother Pharmacol. 2017;79(2):353-61.[GoogleScholar] [CrossRef]
- Robert M, Frenel JS, Bourbouloux E, et al. Pertuzumab for the treatment of breast cancer. Expert Rev Antican Ther. 2020;20(2):85-95. [GoogleScholar][CrossRef]
- Marquez BV, Ikotun OF, Zheleznyak A, et al. Evaluation of 89 Zr-pertuzumab in Breast Cancer Xenografts. Mol Pharmace. 2014;11(11):3988-95. [GoogleScholar] [CrossRef]
- Capelan M, Pugliano L, De Azambuja E, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Annals Onco. 2013;24(2):273-82. [GoogleScholar] [CrossRef]
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330-6. [GoogleScholar] [CrossRef]
- Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA Approval of Neoadjuvant Therapy for Breast Cancer: Pertuzumab for the Treatment of Patients with HER2-Positive Breast Cancer. Clin Cancer Res. 2014;20(21):5359-64. [GoogleScholar] [CrossRef]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Onco. 2012;13(1):25-32. [GoogleScholar] [CrossRef]
- Harbeck N, Beckmann MW, Rody A, et al. HER2 Dimerization Inhibitor Pertuzumab - Mode of Action and Clinical Data in Breast Cancer. Breast Care. 2013;8(1):49-55. [GoogleScholar] [CrossRef]
- Fisusi F, Brandy N, Wu J, Akala EO. Studies on polyethylene glycol-monoclonal antibody conjugates for fabrication of nanoparticles for biomedical applications. J Nanosci Nanomed. 2020;4(2):1-9. [GoogleScholar]
- Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67(18):8882-90. [GoogleScholar] [CrossRef]
- Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an antiHER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13(6):R123. [GoogleScholar] [CrossRef]
- Liu L, Yang Y, Burns R, et al. Margetuximab mediates greater Fc-dependent anti-tumor activities than trastuzumab or pertuzumab in vitro. Cancer Res. 2019;79(13). [GoogleScholar] [CrossRef]
- Research C for DE and. FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). FDA. 2020,2021.
- Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855861. [GoogleScholar] [CrossRef]
- Rugo HS, Im SA, Cardoso F, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Onco. 2021;7(4):573-84. [GoogleScholar] [CrossRef]
- Catenacci DVT, Kang YK, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b–2 trial. Lancet Onco. 2020;21(8):1066-76. [GoogleScholar] [CrossRef]
- Ultrasmall targeted nanoparticles with engineered antibody fragments for imaging detection of HER2-overexpressing breast cancer.
- Uppal H, Doudement E, Mahapatra K, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res. 2015;21(1):123-33. [GoogleScholar] [CrossRef]
- Piwko C, Prady C, Yunger S, et al. Safety profile and costs of related adverse events of trastuzumab emtansine for the treatment of HER2-positive locally advanced or metastatic breast cancer compared to capecitabine plus lapatinib from the perspective of the Canadian health-care system. Clinical drug investig. 2015;35(8):487-93. [GoogleScholar] [CrossRef]
- Erickson HK, Lambert JM. ADME of antibody-maytansinoid conjugates. AAPS J. 2012; 14(4):799-805. [GoogleScholar] [CrossRef]
- White BE, White MK, Adhvaryu H, et al. Nanotechnology approaches to addressing HER2-positive breast cancer. Cancer Nano. 2020;11(1):12. [GoogleScholar] [CrossRef]
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209. [GoogleScholar] [CrossRef]
- Phillips GL, Li G, Dugger DL, et al,“Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate,”. Cancer Res. 2008;68(22):9280-90. [GoogleScholar] [CrossRef]
- Barok M, Tanner M, Köninki K, et al. Trastuzumab-DM1 is highly effective in preclinical models of HER2positive gastric cancer. Cancer Lett. 2011;306(2):171-9. [GoogleScholar] [CrossRef]
- Okines AF. T-DM1 in the Neo-Adjuvant Treatment of HER2-Positive Breast Cancer: Impact of the KRISTINE (TRIO-021) Trial. Rev Recent Clin Trials. 2017;12(3):216-22. [GoogleScholar] [CrossRef]
- Bergh JCS, Andersson A, Bjohle J, et al. Docetaxel, trastuzumab, pertuzumab versus trastuzumab emtansine as neoadjuvant treatment of HER2-positive breast cancer: Results from the Swedish PREDIX HER2 trial identifying a new potential de-escalation standard? JCO. 2019; 3715:501. [GoogleScholar] [CrossRef]
- Ghosh S, Chandran A, Jansen JP. Epidemiology of HIV-related neuropathy: a systematic literature review. AIDS Res Hum Retroviruses. 2012;28(1):36-48. [GoogleScholar] [CrossRef]
- Li G, Guo J, Shen BQ, et al. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells. Mol Cancer Ther. 2018; 17(7):1441-53.
[GoogleScholar] [CrossRef] - Loganzo F, Tan X, Sung M, et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Ther. 2015; 14(4):952-63. [GoogleScholar] [CrossRef]
- Kovtun YV, Audette CA, Mayo MF, et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70(6):2528-37. [GoogleScholar] [CrossRef]
- Takegawa N, Nonagase Y, Yonesaka K, et al. DS-8201a, a new HER2-targeting antibody–drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141(8):1682-9. [GoogleScholar] [CrossRef]
- Austin CD, De Mazière AM, Pisacane PI, et al. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15(12):5268-82.[GoogleScholar] [CrossRef]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314(17):3093-106. [GoogleScholar] [CrossRef]
- Li JY, Perry SR, Muniz-Medina V, et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell. 2016;29(1):117-29. [GoogleScholar] [CrossRef]
- Welslau M, Diéras V, Sohn JH, et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2positive locally advanced or metastatic breast cancer. Cancer. 2014;120(5):642-51. [GoogleScholar] [CrossRef]
- Rong L, Zhou S, Liu X, et al. Trastuzumab-modified DM1-loaded nanoparticles for HER2 + breast cancer treatment: an in vitro and in vivo study. Artificial Cells, Nanomedicine, and Biotechnology. 2017;46(1):1-11. [GoogleScholar] [CrossRef]
- Lyass O, Uziely B, Ben-Yosef R, et al. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89(5):1037-47. [GoogleScholar] [CrossRef]
- Seol H, Lee HJ, Choi Y, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25(7):938-48. [GoogleScholar] [CrossRef]
- Glöckner S, Buurman H, Kleeberger W, et al. Marked Intratumoral Heterogeneity of c-myc and CyclinD1 But Not of c-erbB2 Amplification in Breast Cancer. Lab Invest. 2002;82(10):1419-26. [GoogleScholar] [CrossRef]
- Lee HJ, Seo AN, Kim EJ, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014;142(6):755-66. [GoogleScholar] [CrossRef]
- Hosonaga M, Arima Y, Sampetrean O, et al. HER2 Heterogeneity Is Associated with Poor Survival in HER2-Positive Breast Cancer. Int J Mol Sci. 2018;19(8):2158-63. [GoogleScholar] [CrossRef]
- Yamazaki CM, Yamaguchi A, Anami Y, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12(1):3528. [GoogleScholar] [CrossRef]
- Friedman LM, Rinon A, Schechter B, et al. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci USA. 2005;102(6):1915-20. [GoogleScholar] [CrossRef]
- Spangler JB, Neil JR, Abramovitch S, et al. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci USA. 2010;107(30):13252-7. [GoogleScholar] [CrossRef]
- Spangler JB, Manzari MT, Rosalia EK, et al. Triepitopic antibody fusions inhibit cetuximabresistant BRAF- and KRAS-mutant tumors via EGFR signal repression. J Mol Biol. 2012;422(4):532-44. [GoogleScholar] [CrossRef]
- Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097-108. [GoogleScholar] [CrossRef]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. New Engljmed. 2012;367(19):1783-91. [GoogleScholar] [CrossRef]
- Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512-22. [GoogleScholar] [CrossRef]
- Iwata H, Tamura K, Doi T, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: Long-term results of a large phase 1 study with multiple expansion cohorts. J Clin Oncol. 2018;36(15):25012501. [GoogleScholar] [CrossRef]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. New England Journal of Medicine. 2020;382(7):610-21. [GoogleScholar] [CrossRef]
- ESMO 2021. Top oncology breakthroughs shared at ESMO 2021. Clarivate. Accessed, 2021. [GoogleScholar] [CrossRef]
- ENHERTU for HER2 Positive Metastatic Breast Cancer. Accessed, 2021.
- Su CY, Chen M, Chen LC, et al. Bispecific antibodies (anti-mPEG/anti-HER2) for active tumor targeting of docetaxel (DTX)-loaded mPEGylated nanocarriers to enhance the chemotherapeutic efficacy of HER2overexpressing tumors. Drug Deliv. 2018;25(1):1066-79. [GoogleScholar] [CrossRef]
- Elgersma RC, Coumans RG, Huijbregts T, et al. Design, synthesis, and evaluation of linker-duocarmycin payloads: toward selection of HER2-targeting antibody-drug conjugate SYD985. Mol Pharm. 2015;12(6):1813-35. [GoogleScholar] [CrossRef]
- Banerji U, van Herpen CM, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124-35.[GoogleScholar] [CrossRef]
- Li JY, Perry SR, Wang X, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer cell. 2016;29(1):117-29. [GoogleScholar] [CrossRef]
- Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17(4):461-69. [GoogleScholar] [CrossRef]
- Ding H, Gangalum PR, Galstyan A, et al. HER2-positive breast cancer targeting and treatment by a peptideconjugated mini nanodrug. Nanomed. 2017;13(2):631-9. [GoogleScholar] [CrossRef]
- Alsina M, Boni V, Schellens JH, et al. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC). J Clin Oncol. 2017;35:2522. [GoogleScholar]
- Meric-Bernstam F, Beeram M, Mayordomo JI, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Oncol. 2018;36(15). [GoogleScholar] [CrossRef]
- Hinner MJ, Aiba RS, Wiedenmann A, et al. Costimulatory T cell engagement via a novel bispecific anti-CD137/anti-HER2 protein. J Immuno Cancer 2015;3(2):1-9. [GoogleScholar] [CrossRef]
- Oberg HH, Kellner C, Gonnermann D, et al. Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing γδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Front Immunol. 2018;9:814. [GoogleScholar] [CrossRef]
- Ismail RSM, Ismail NSM, Abuserii S, et al. Recent advances in 4-aminoquinazoline based scaffold derivatives targeting EGFR kinases as anticancer agents. Future J Pharm Sci. 2016;2(1):9-19. [GoogleScholar] [CrossRef]
- Segovia-Mendoza M, González-González ME, Barrera D, et al. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence. Am J Cancer Res. 2015;5(9):2531-61. [GoogleScholar]
- Traxler P. Tyrosine kinases as targets in cancer therapy - successes and failures. Expert Opin Ther Targets. 2003;7(2):215-234. [GoogleScholar] [CrossRef]
- Denny WA, Rewcastle GW, Bridges AJ, et al. Structure-activity relationships for 4anilinoquinazolines as potent inhibitors at the ATP binding site of the epidermal growth factor receptor in vitro. Clin Exp Pharmacol Physiol. 1996;23(5):424-27. [GoogleScholar] [CrossRef]
- Yang X, Wu D, Yuan S. Tyrosine Kinase Inhibitors in the Combination Therapy of HER2 Positive Breast Cancer. Technol Cancer Res Treat. 2020;19:1533033820962140. [GoogleScholar] [CrossRef]
- Wang J, Lv FM, Wang DL, et al. Synergistic Antitumor Effects on Drug-Resistant Breast Cancer of Paclitaxel/Lapatinib Composite Nanocrystals. Molecules. 2020;25(3):604. [GoogleScholar] [CrossRef]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633-40. [GoogleScholar] [CrossRef]
- Cetin B, Benekli M, Oksuzoglu B, et al. Lapatinib plus capecitabine for brain metastases in patients with human epidermal growth factor receptor 2-positive advanced breast cancer: a review of the Anatolian Society of Medical Oncology (ASMO) experience. Onkologie. 2012;35(12):740-45. [GoogleScholar] [CrossRef]
- Cho EC, Xie J, Wurm PA, et al. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano letters. 2009;119(3):1080-84. [GoogleScholar] [CrossRef]
- Zhang M, Chen X, Li C, et al. Charge-reversal nanocarriers: An emerging paradigm for smart cancer nanomedicine. J Cont Release. 2020;10;319:46-62. [GoogleScholar] [CrossRef]
- Medina PJ, Goodin S. Lapatinib: A dual inhibitor of human epidermal growth factor receptor tyro kinases. Clin Therap. 2008;30(8):1426-1447. [GoogleScholar] [CrossRef]
- Zhang D, Pal A, Bornmann WG, et al. Activity of lapatinib is independent of EGFR expression level in HER2-overexpressing breast cancer cells. Mol cancer thera. 2008 1;7(7):1846-50. [GoogleScholar] [CrossRef]
- Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J National Cancer Inst. 2008;100(15):1092-103. [GoogleScholar] [CrossRef]
- Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630-39. [GoogleScholar] [CrossRef]
- Nahta R. Deciphering the role of insulin-like growth factor-I receptor in trastuzumab resistance. Chem res practice. 2012. [GoogleScholar] [CrossRef]
- Rubin I, Yarden Y. The basic biology of HER2. Annals Oncol. 2001;12. [GoogleScholar] [CrossRef]
- Zimmer AS, Swearingen AEDV, Anders CK. HER2-positive breast cancer brain metastasis: A new and exciting landscape. Cancer Reports. 2021. [GoogleScholar] [CrossRef]
- Tanizaki J, Okamoto I, Fumita S, et al. Roles of BIM induction and survivin downregulation in lapatinib-induced apoptosis in breast cancer cells with HER2 amplification. Oncogene. 2011;30(39):4097-106. [GoogleScholar] [CrossRef]
- Van Schooneveld E, Wildiers H, Vergote I, et al. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast cancer res. 2015;17(1):1-5. [GoogleScholar] [CrossRef]
- Normann LS, Aure MR, Leivonen SK, et al. MicroRNA in combination with HER2-targeting drugs reduces breast cancer cell viability in vitro. Scientific reports. 2021;11(1):1[GoogleScholar] [CrossRef]
- Fisher JN, Terao M, Fratelli M, et al. MicroRNA networks regulated by all-trans retinoic acid and Lapatinib control the growth, survival and motility of breast cancer cells. Oncotarget. 2015;6(15):13176. [GoogleScholar] [CrossRef]
- Peyvand P, Vaezi Z, Sedghi M, et al. Imidazolium-based ionic liquid functionalized mesoporous silica nanoparticles as a promising nano-carrier: response surface strategy to investigate and optimize loading and release process for Lapatinib delivery. Pharm Dev Tech. 2020;25(9):1150-61. [GoogleScholar] [CrossRef]
- Wen J, Yang K, Liu F, et al. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem Soc Rev. 2017;46(19):6024-6045. [GoogleScholar] [CrossRef]
- Chen X, Yao X, Wang C, et al. Mesoporous silica nanoparticles capped with fluorescence-conjugated cyclodextrin for pH-activated controlled drug delivery and imaging. Microporous and Mesoporous Materials. 2015;6(217):46-53. [GoogleScholar] [CrossRef]
- Bharti C, Nagaich U, Pal AK, et al. Mesoporous silica nanoparticles in target drug delivery system: A review. Int J Pharm Investigation. 2015 Jul;5(3):124. [GoogleScholar] [CrossRef]
- Guo Z, Liang E, Sui J, et al. Lapatinib-loaded acidity-triggered charge switchable polycarbonate-doxorubicin conjugate micelles for synergistic breast cancer chemotherapy. Acta Biomater. 2020;118:182-195. [GoogleScholar] [CrossRef]
- Zhang Y, Cao J, Yuan Z. Strategies and challenges to improve the performance of tumor-associated active targeting. J Mat Chem B. 2020;8(18):3959-71. [GoogleScholar] [CrossRef]
- Wang B, Zhang L, Bae SC, et al. Nanoparticle-induced surface reconstruction of phospholipid membranes. Pro Nat Acade Sci.2008;105(47):18171-18175. [GoogleScholar] [CrossRef]
- Zhao Y, Chen H, Chen X, et al. Targeted nanoparticles for head and neck cancers: overview and perspectives. Wiley Int Rev: Nanomed Nanobiot. 2017;9(6):1469. [GoogleScholar] [CrossRef]
- Sun B, Luo C, Yu H, et al. Disulfide bond-driven oxidation-and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano letters. 2018;18(6):3643-50. [GoogleScholar] [CrossRef]
- Kaur S, Prasad C, Balakrishnan B, et al Trigger responsive polymeric nanocarriers for cancer therapy. Biomater Sci. 2015;3(7):955-987. [GoogleScholar][CrossRef]
- Wang L, You X, Lou Q, et al. Cysteine-based redox-responsive nanoparticles for small-molecule agent delivery. Bio sci. 2019;7(10):4218-29. [GoogleScholar] [CrossRef]
- Deeks ED. Neratinib: first global approval. Drugs. 2017;77(15):1695-704. [GoogleScholar] [CrossRef]
- Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clini Onco. 2019;37(13):1081. [GoogleScholar] [CrossRef]
- Wissner A, Overbeek E, Reich MF, et al. Synthesis and structure− activity relationships of 6, 7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2). J Med Chem. 2003;46(1):49-63. [GoogleScholar] [CrossRef]
- Wissner A, Mansour TS. The development of HKI‐272 and related compounds for the treatment of cancer. Archiv der Pharmazie: Int J Pharm Med Chem. 2008;341(8):465-77. [GoogleScholar] [CrossRef]
- Canonici A, Gijsen M, Mullooly M, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4(10):1592. [GoogleScholar] [CrossRef]
- Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Trans Targe Therapy. 2019;13;4(1):1. [GoogleScholar] [CrossRef]
- Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64(11):3958-65. [GoogleScholar][CrossRef]
- Zhao XQ, Xie JD, Chen XG, et al. Neratinib reverses ATP-binding cassette B1-mediated chemotherapeutic drug resistance in vitro, in vivo, and ex vivo. Mol pharmaco. 2012;82(1):47-58.[GoogleScholar] [CrossRef]
- Yin Y, Yuan X, Gao H, Yang Q. Nanoformulations of small molecule protein tyrosine kinases inhibitors potentiate targeted cancer therapy. Int J Pharmaceutics. 2020;573:118785.[GoogleScholar] [CrossRef]
- Kulukian A, Lee P, Taylor J, et al. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol Cancer Ther. 2020;19(4):976-87. [GoogleScholar] [CrosssRef]
- Borges VF, Ferrario C, Aucoin N, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA oncology. 2018;4(9):1214-20. [GoogleScholar] [CrossRef]
- Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126(19):4278-88. [GoogleScholar] [CrossRef]
- Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer treatment reviews. 2018;67:71-77. [GoogleScholar] [CrossRef]
- Dai J, Chen Y, Tang C, et al. Pyrotinib in the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer: A case report. Medicine (Baltimore). 2020;99(25):20809.[GoogleScholar] [CrossRef]
- Mezni E, Vicier C, Guerin M, et al. New therapeutics in HER2-positive advanced breast cancer: towards a change in clinical practices?.Cancers. 2020;12(6):1573. [GoogleScholar] [CrossRef]
- Xuhong JC, Qi XW, Zhang Y, Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Amer J Cancer Res. 2019;9(10):2103. [GoogleScholar]
- Ma F, Ouyang Q, Li W, et al. Pyrotinib or lapatinib combined with capecitabine in HER2–positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Onco. 2019; 37(29):2610-9.[GoogleScholar][CrossRef]
- Jiang Z, Yan M, Hu X, et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: A randomized phase III study. 2019;37:15:1001. [GoogleScholar][CrossRef]
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702-11.[GoogleScholar] [CrossRef]
- Zhang X, Munster PN. New protein kinase inhibitors in breast cancer: afatinib and neratinib. Expert opinion on pharmacotherapy. 2014;15(9):1277-88. [GoogleScholar] [CrossRef]
- Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102(21):7665-70. [GoogleScholar][CrossRef]
- Harbeck N, Huang, CS, Hurvitz, S, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Onco. 2016; 17(3):357-66. [GoogleScholar] [CrossRef]
- Cortés J, Dieras V, Ro J, et al. Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncology. 2015;16(16):1700. [GoogleScholar][CrossRef]
- Elbatanony RS, Parvathaneni V, Kulkarni NS, et al. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy. Drug Del Transl Res. 2021;11(3):927-43. [GoogleScholar] [CrossRef]
- Chan MH, Huang WT, Wang J, et al. Next‐generation cancer‐specific hybrid theranostic nanomaterials: mage‐a3 nir persistent luminescence nanoparticles conjugated to afatinib for in situ suppression of lung adenocarcinoma growth and metastasis. Adv Sci. 2020;7(9):1903741. [GoogleScholar] [CrossRef]
- Chemmalar S, Intan-Shameha AR, Abdullah CA, et al. Synthesis and Characterization of Gefitinib and Paclitaxel Mono and Dual Drug-Loaded Blood Cockle Shells (Anadara granosa)-Derived Aragonite CaCO3 Nanoparticles. Nanomaterials 2021;11(8):1988. [GoogleScholar] [CrossRef]
- Buss JH, Begnini KR, Bruinsmann FA, et al. Lapatinib-loaded nanocapsules enhances antitumoral effect in human bladder cancer cell. Front. Oncol. 2019;9:203. [GoogleScholar] [CrossRef]
- Xuan Y, Guan M, Zhang S. Tumor immunotherapy and multi-mode therapies mediated by medical imaging of nanoprobes. Theranostics. 2021;11(15):7360. [GoogleScholar] [CrossRef]
- Byun DJ, Wolchok JD, Rosenberg LM. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. [GoogleScholar] [CrossRef]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295-307. [GoogleScholar] [CrossRef]
- Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med. 2015;372(4):320-30. [GoogleScholar][CrossRef]
- Knutson KL and Disis ML. Expansion of HER2/neu-specific T cells ex vivo following immunization with a HER2/neu peptide-based vaccine. Clin breast cancer. 2001;2(1):73-79.[GoogleScholar] [CrossRef]
- Bernhard H, Neudorfer J, Gebhard K, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57(2):271-80. [GoogleScholar] [CrossRef]
- Bendell JC, Varghese AM, Hyman DM, et al. A first-in-human phase 1 study of LY3023414, an oral PI3K/mTOR dual inhibitor, in patients with advanced cancer. Clin Cancer Res. 2018;24(14):3253-62. [GoogleScholar] [CrossRef]
- Priceman SJ, Tilakawardane D, Jeang B, et al. Regional Delivery of Chimeric Antigen Receptor–Engineered T Cells Effectively Targets HER2+ Breast Cancer Metastasis to the Brain. Clinical Cancer Research. 2018;24(1):95-105. [GoogleScholar] [CrossRef]
- Bedognetti D, Maccalli C, Al Bader SB, et al. Checkpoint inhibitors and their application in breast cancer. Breast Care. 2016;11(2):108-15. [GoogleScholar] [CrossRef]
- Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275-87. [GoogleScholar] [CrossRef]
- Bayraktar S. Immunotherapy in breast cancer. Breast Dis. 2019:541-52. [GoogleScholar] [CrossRef]
- Cremolini C, Vitale E, Rastaldo R, et al. Advanced Nanotechnology for Enhancing Immune Checkpoint Blockade Therap. Nanomaterials. 2021;11(3):661.[GoogleScholar] [CrossRef]
- Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Medicine. 2015;13(1):1-4. [GoogleScholar] [Crosssref]
- Hansel TT, Kropshofer H, Singer T, et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325-38. [GoogleScholar] [CrossRef]
- Shulgin B, Kosinsky Y, Omelchenko A, et al. Dose dependence of treatment-related adverse events for immune checkpoint inhibitor therapies: a model-based meta-analysis. Oncoimmunology. 2020;9(1):1748982. [GoogleScholar] [CrossRef]
- Galstyan A, Markman JL, Shatalova ES, et al. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10(1):1-3. [GoogleScholar] [CrossRef]
- Schmid D, Park CG, Hartl CA, et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8;1747. [GoogleScholar] [CrossRef]
- Chen H, Luan X, Paholak HJ, et al. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine. 2020;15(1):77-92. [GoogleScholar] [CrossRef]
- Jafari S, Lavasanifar A, Hejazi MS, et al. STAT3 inhibitory stattic enhances immunogenic cell death induced by chemotherapy in cancer cells. DARU J Pharm Sci. 2020;28(1):159-69. [GoogleScholar] [Cross Ref]
- Liu H, Yang Q, Guo W, et al. CoWO4-x-based nanoplatform for multimode imaging and enhanced photothermal/photodynamic therapy. Chem Eng J. 2020;385:123979. [GoogleScholar] [CrossRef]
- Song Y, Wang Y, Wang S, et al. Immune-adjuvant loaded Bi2Se3 nanocage for photothermal-improved PD-L1 checkpoint blockade immune-tumor metastasis therapy. Nano Res. 2019;12(8):1770-80. [GoogleScholar]
- Wang R, He Z, Cai P, et al. Surface-Functionalized Modified Copper Sulfide Nanoparticles Enhance Checkpoint Blockade Tumor Immunotherapy by Photothermal Therapy and Antigen Capturing. ACS Appl Mater Interf. 2019;11(15):13964-72. [GoogleScholar] [CrossRef]
- Dong X, Yang A, Bai Y, et al. Dual fluorescence imaging-guided programmed delivery of doxorubicin and CpG nanoparticles to modulate tumor microenvironment for effective chemo-immunotherapy. Biomaterials. 2020;230:119659.[GoogleScholar] [Cross Ref]
- Xu J, Yu S, Wang X, et al. High Affinity of Chlorin e6 to Immunoglobulin G for Intraoperative Fluorescence Image-Guided Cancer Photodynamic and Checkpoint Blockade Therapy. ACS Nano. 2019;13(9):10242-60. [GoogleScholar] [CrossRef]
- Hao Y, Zhou X, Li R, et al. Advances of functional nanomaterials for cancer immunotherapeutic applications. WIREs Nanomed. Nanobiotechnology. 2020;12(2):1574.
[GoogleScholar] [Cross Ref] - Saeed M, Xu Z, De Geest BG, et al. Molecular Imaging for Cancer Immunotherapy: Seeing Is Believing. Bioconjugate Chem. 2020;31(2):404-15. [GoogleScholar] [CrossRef]
- Yang Shi and Twan Lammers, Combining Nanomedicine and Immunotherapy. Acc Chem Res. 2019;52:1543-54. [GoogleScholar] [CrossRef]
- Li L, Wang J, Radford DC, Kopeček J, Yang J. Combination treatment with immunogenic and anti-PD-L1 polymer-drug conjugates of advanced tumors in a transgenic MMTV-PyMT mouse model of breast cancer. J Control Release. 2021;332;(10): 652-9. [GoogleScholar] [CrossRef]