Neonatal exchange transfusion (NET) – what is its current net value?
Received: 11-Mar-2018 Accepted Date: Apr 06, 2018; Published: 15-Apr-2018
Citation: Nandyal R. Neonatal exchange transfusion (NET) – what is its current net value? J Blood Disord Treat. 2018;1(2):9-13
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Neonatal exchange transfusion (NET) has a very special place in the history of newborns with severe hyperbilirubinemia (SHB) who are at risk for kernicterus, and other neurodevelopmental problems. NET’s primary indication was and has been the hemolytic disease of the newborn (HDN),which is less common now due to the universal screening for iso-sensitization and also because of appropriate use of antenatal anti-Rh D antibody prophylaxis. In this review, an attempt is made to describe the historical perspective, indications, complications, procedural variations, NET trend, and current status along with future perspective.
Keywords
Exchange transfusion; Hyperbilirubinemia; Neonatal jaundice
Historically, Neonatal exchange transfusion (NET) was the first intervention tried successfully for the management of severe hyperbilirubinemia (SHB), aiming to prevent kernicterus [1]. For neonates with SHB, NET is shown to decrease neurological morbidity, such as kernicterus and bilirubin induced neurological dysfunction (BIND) with several neurodevelopmental issues. It also offers additional benefits to the management of SHB, which include removal of anti-body coated red blood cells (RBC), correction of hemolytic anemia, lowering of circulating maternal antibody titers, and increase of albumin content (resulting in enhanced bilirubin binding). It has been primarily used for the management of immune mediated, and non- immune hemolytic jaundice. The use of NET for the effective control of severe hyperbilirubinemia has been decreasing during the last three decades due to the fast decline of the severity of its primary indication, hemolytic disease of the newborn (HDN), as a result of the universal screening for iso-sensitization, and also because of appropriate use of antenatal anti-Rh D antibody prophylaxis [2]. Along with the decrease in its use, the number of neonatal healthcare professionals with required expertise and experience is also dwindling [1]. Several pediatric residents are graduating now without doing a single NET or witnessing one. Despite difficulty in defining procedure specific mortality and morbidity, there is some evidence that the incidence of post -exchange complications is increasing. The number of publications about the use of NET for indications other than severe hyperbilirubinemia seems to be increasing, but slowly. In this review, an attempt is made to describe the historical perspective, indications, complications, procedural variations, NET trend and its current status along with future perspective. It is beyond the scope of this article to cover the discussion on intrauterine transfusions, comprehensive management of severe hyperbilirubinemia, sepsis and other indications for NET. Use of phototherapy, intravenous immunoglobulin, pre-exchange intravenous albumin infusion, phenobarbital (prophylaxis and treatment) and antibiotics were not discussed. This also does not address partial exchange transfusion done for polycythemia and severe anemia associated with hydrops.
Historical Perspective
The first documented description of Hemolytic Disease of the Newborn (HDN) was done in 1609 by a French midwife, but not until the 1950s was the underlying cause of HDN (Blood type incompatibility and sensitization of maternal RBC) explained (2). In October 1946 at the Boston Lying-in Hospital, Dr. Louis K Diamond was the first physician to use the umbilical vein in the exchange transfusion technique and he was also the first to provide complete details of how to perform the procedure in a paper published in 1951 [3]. As reproduced in NeoReviews 2013 by Dr. AGS Phillip, Dr. Diamond stated that “Before 1941, transfusions of anemic newborn infants with erythroblastosis foetalis (EF) were performed as a life-saving measure, but with variable results. After the discovery of the Rh factor, more success was achieved through the use of Rh-negative blood [4]. The indications for this procedure were further expanded, as shown in the following paragraphs.
Discussion
The need for NET is rapidly decreasing which is good news to the neonatologists that were trained in 1970s and 1980s, who used to do 2 to 3 NETs per day. Unfortunately, its decline in numbers is making the new millennium neonatologist less experienced, and more insecure due to less exposure. Now, let us review the indications, contra-indications, risks and complications, procedure variations, controversies, common questions and NET trend.
Indications
NET has been used for the management of severe hyperbilirubinemia [5] refractory to intensive phototherapy with or without other adjunctive treatment, such as the use of intravenous (IV) albumin infusion and/ or IV immunoglobulin (IVIG). Before performing NET, IVIG has been used frequently for severe hemolytic disease of the newborn (Rh disease) and other immune related hyperbilirubinemias, including ABO incompatibility and minor blood group incompatibility (such as anti-Kell and anti Ce sensitization) [6]. NET has been used also for severe refractory hyperbilirubinemia from non-immune hemolytic disorders such as RBC enzyme deficiencies (G-6-P-D deficiency, pyruvate kinase, glutathione reductase) and RBC membrane defects (congenital spherocytosis, congenital eliptocytosis). Other indications include [5], fulminant neonatal sepsis, with or without toxic shock [7,8], disseminated intravascular coagulation, metabolic disorders (such as galactosemia, other aminoacidopathies and hyperammonemia) [9], severe fluid and electrolyte imbalance, drug toxicity, congenital lead poisoning [10], polycythemia, and severe anemia. In the early 80s, Christensen et al showed that NET could be used as an alternative to granulocyte concentrate administration/transfusion, in neonates with bacterial sepsis and profound neutropenia [11]. Exchange transfusions have been successfully tried in cases of Neonatal hemochromatosis [12,13], Hereditary Iron Overload Disease [14], and transient myeloproliferative disorder [15]. The American Academy of Pediatrics (AAP) guidelines (2004) addresses the management of hyperbilirubinemia in the newborn Infant, 35 or more weeks of gestation. Those guidelines are based on evidence and expert opinion, for the use of exchange transfusion in the management of severe hyperbilirubinemia refractory to intensive phototherapy and other adjunctive therapy [16]. Maisels et al. [17] suggested guidelines, again based on a combination of expert opinion and evidence for the management of hyperbilirubinemia, in the preterm infant less than 35 weeks of gestation. In other words, evidence based and/or expert opinion based guidelines for the use of exchange transfusion in the management of severe hyperbilirubinemia, from hemolytic and non-hemolytic causes do exist. But still, a practicing physician is left with sparse literature and a lack of consensus supporting the use of exchange transfusion for other indications.
Risks and Complications
It seems prudent to assume that the pre-exchange clinical status of the neonate will adversely influence NET related morbidity and mortality. NET procedure involves slowly drawing blood from the infant, and slowly pushing donor blood into the infant (pull and push technique), with an inherent potential for numerous fluctuations in the blood volume and blood pressure, simultaneously happening in various organs (vital and non-vital). Also, we can expect undulations in the chemical composition (including pH, electrolytes, calcium and magnesium) and cellular components of the blood, which can happen within a short period. Morbidity and mortality from NET are much lower in otherwise healthy term infants when compared to very ill preterm infants [1]. Current information on the mortality is relatively unobtainable, as the procedures were done very infrequently. The reported overall mortality from the procedure alone or from its complications leading to death is ranging between 0.3% and 8% [1]. Keenan et al. [18] listed morbidity (apnea, bradycardia, cyanosis, vasospasm, thrombosis) which were observed in 22 (6.7%) of 328 exchange transfusions performed. Excluding minor arterial spasms, they concluded that the incidence of procedure linked morbidity is 5%. Watchko [19] listed 22 potential complications using system by system approach (19). At two perinatal centers in Cleveland (1992-2002), adverse events (thrombocytopenia, hypocalcemia and metabolic acidosis) occurred in 74% of 67 babies that had NET over a decade. Only two serious adverse events were reported from that data [19]. Post transfusion syndrome was reported with a rash, eosinophilia and thrombocytopenia in 21 out of 35 (60%) of neonates who had both intrauterine transfusions and postnatal exchange transfusions [20]. Air embolism is another potential risk and fortunately its incidence with NET is very low.
Procedure: Step by step approach to NET
Even in the best circumstances at a Level IV Neonatal Unit, it can take 3 to 6 h from the decision, to the starting of the procedure. Some common reasons for this lag time as per the author’s experience include finding the nursing staff that has had sufficient experience, finding appropriate equipment including functioning blood warmer and the time taken for the preparation of blood (antigen-antibody screen, reconstitution and irradiation). These problems get amplified, when the procedures are done infrequently, and by less experienced physicians and the team.
Initial steps
A. Once the decision to do exchange transfusion is taken, the physician leader needs to initiate the process immediately, starting with notification of the blood bank regarding the indication, type of blood, desired hematocrit of the blood to be exchanged, volume, time when the procedure is expected to start, etc. (Flow chart).

Prior to starting the procedure (team orientation and informed consent)
A. It is imperative for the physician and nursing team to explain to the parents and/or the family, the reason for doing NET, pre-exchange precautions, expected duration of the procedure, steps involved in the actual procedure including antiseptic cleansing of umbilical area, preparation and placement of catheter, exchange process, monitoring of the infant and post-exchange follow up. Expected potential immediate and long term complications, and the precautions taken to prevent or manage them, also need to be explained to the family. Informed consent from a parent or a legal guardian should be obtained at that stage. It is also essential to have the entire team oriented to the procedure. Lab should be alerted also.
B. Because of its infrequent occurrence, the author suggests to review a standard procedure manual [21] or a NICU protocol on NET, prior to the procedure. Also, reading the paragraphs on controversies will be beneficial. The physician leader needs to emphasize to the entire team that, this is a surgical procedure (usually performed in a Neonatal ICU), and extreme aseptic precautions should be followed.
Start of the procedure
A. After blood arrives to the procedure site, monitors need to be connected to the baby (if not done already); the infant needs to be restrained (gentle and effective).
B. An oro-gastric or a naso-gastric tube (5 fr for preterm and 8 fr for term infants) needs to be inserted, and stomach to be emptied [21]. May leave the NG/OG tube for gravity, and aspirate every 30 min, until the procedure is completed.
C. Having sucrose drops (per oral) available for stable late preterm and term infants frequently helps to make the procedure go smoothly.
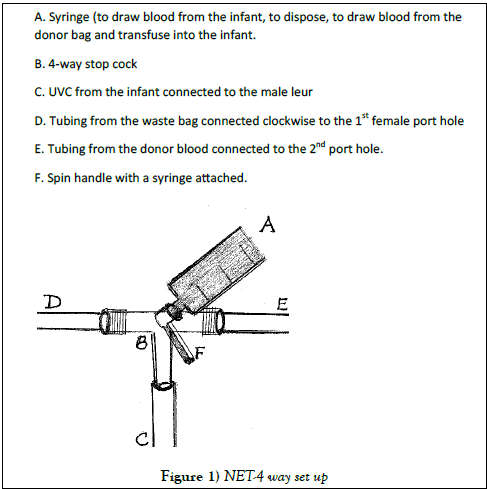
D. Under sterile conditions, an umbilical venous catheter (UVC- 5 fr for <3.5 kg infant and 8 fr for >3.5 kg infant), pre-flushed (filled) with normal saline, and screened for air bubbles is inserted into the umbilical vein [1,19]. A 4-way stopcock is attached with clockwise connections (Figure 1), starting with: i) a male luer, attached to the UVC after its placement, ii) a female luer port hole to be attached to a tubing connected to an appropriately sealed and properly hung plastic bag (waste container), to collect disposed blood drawn from the baby. Also, periodically checking for any potential for leaking of blood from the bag, and also being watchful for sudden disconnection (de-linking) of tubing from stop-cock will help, iii) the third port hole (another female luer port hole is at 90° to the previous port hole), is attached to the tubing from the donor blood (cross matched, anticoagulated, irradiated, fresh and reconstituted blood). And, iv) a 10 cc syringe is attached at the top of the spin handle. As mentioned earlier, despite minimal incidence of air embolism but, because of its severe consequences, at every step and every pass, precautions need to be taken to prevent any air bubbles getting into the system. Screening for air bubbles and making it an air tight system is essential.
E. A sterile ruler is usually provided in the exchange transfusion trays, which can be used for measuring central venous pressure (CVP). CVP trend is more important than the actual number which tends to be in the range of 10 cm +/- 4 cm and it needs to be re-measured at the completion of the procedure. Both pre and post numbers need to be close and correlate. If post NET CVP is much lower (3 cm or more) than that of Pre NET, may consider transfusing additional 5-10 ml of blood (not more than 1 aliquot/pass). CVP measurements need to be documented.
F. A thermostatically controlled blood warmer needs to be used to keep the temperature of the transfused blood in the range of 36.5 to 37.5°C. Precautions need to be taken to prevent over heating of blood (>38°C) [21].
During the procedure
A. After making sure that all connections are air tight, 5-10 ml of blood was drawn from the infant, for pre exchange lab such as complete blood count (or hemoglobin, hematocrit and platelet count), serum electrolytes, calcium (total or ionized), serum glucose and bilirubin (total and direct). Blood gases are recommended for infants on ventilator support. Blood pH, blood culture and blood lactate levels can be used judicially. In the author’s experience of more than 200 exchange transfusions, blood cultures are found to be helpful (anecdotal and not evidence based). The author recommends having lab personnel to be available to receive the first drawn blood, which needs to be placed in appropriate color tubes for various tests, to avoid “tube errors”.
B. Using only UVC catheter (no arterial catheter), a pull and push procedure needs to be done slowly. Each pass may have aliquots of 5-10 ml/kg [1] or aliquots of 3 ml for less than 1 kg infant, 5 ml for 1-2 kg infant, 10 ml for 2-3 kg infant and 10-20 ml for >3 kg infant. Each pass (starting from the drawing of the baby’s blood per UVC, disposing of that old blood, followed by drawing donor blood and transfusing that blood into the infant) takes approximately 1.5-2 min for completion. Some researchers recommended up to 3 min [1], for each pass. Usually, the procedure takes 1-2 hours to complete (1.5 +/- 0-5 h) [1]. The recorder should notify the NET performer after every 50 ml of ins and outs. Both numbers (ins and outs) should be the same. Heart rate and pulse ox readings also will reassure us.
C. Occasionally, transient complications like life threatening bradycardias are reported. Slowing down the procedure or even stopping the procedure for 10-15 min might help in managing such episodes.
D. After completing NET (after the last pass), 5-10 ml of blood needs to be drawn for post-exchange lab (same as pre-exchange lab). It needs to be replaced with equal volume of donor blood.
Post-exchange care
A. Shortly before, during, and for at least 1-2 h after NET, the neonate needs to be closely monitored for cardiovascular and respiratory complications, using a cardiac monitor and a pulse oximeter. Heart rate, respiratory rate and pulse oximeter readings need to be documented every 5-10 min and both temperature and blood pressure need to be monitored every 15 min (by the recorder/nursing staff) throughout the NET and for another 30 min post procedure.
B. Frequently the infants are kept NPO and on intravenous fluids, for 3-4 h after NET. Later, feedings including breast feeding can be resumed.
Post-NET
Intensive phototherapy is continued post-NET for another 6-12 h or longer, depending on the rebound bilirubin values and the etiology of severe hyperbilirubinemia.
Recommend neurodevelopmental follow up for all infants that get severe hyper-bilirubinemia especially those requiring NET.
Controversies and Variations in Practice
Medical literature has several studies advocating variations of NET procedure, often confusing to the busy practicing physician. They were discussed in the following paragraphs, and evidence based recommendations were made, when possible.
1. Single volume (SV) versus double volume (DV) transfusion: Currently double volume exchange is the norm, as there are few studies that addressed it. Cochrane reviews [22], looked at all randomized and quasi randomized control trials comparing single volume and double volume exchange transfusions in jaundiced newborns. Only one study fulfilled the criteria. Twenty full term infants requiring exchange transfusion for hemolytic jaundice due to ABO incompatibility were randomly allocated to receive single or double volume transfusion. Total bilirubin levels immediately after exchange transfusion were not significantly different. The reviewers concluded that there was insufficient evidence to support or refute the use of SV exchange transfusion as opposed to DV transfusion in jaundiced newborns.
2. Two stage SV exchange transfusion in severe hemolytic disease of the newborn was studied by Abbas et al. [23]. They concluded that the two stage single volume exchange transfusion, proved to be more effective in reducing serum bilirubin level post-exchange, and in decreasing the need for repeated exchange transfusions. There were no significant differences in mortality or morbidity between the two groups. It certainly needs to be addressed again. Double volume NET is still the norm.
3. Pharmacokinetic considerations in NET: Some medications such as antibiotics, cardiac drugs and CNS medications can be really crucial for neonates. It is possible that their post-NET decreased levels can add to the post-exchange morbidity. There are no clear guidelines for the management of those situations. The author suggests checking a drug level, after hemoequilibration occurs (4-6 h after the completion of the NET) and take further steps as needed. Vasopressors may need to be adjusted. In a study reported in 1978, only 3% of kanamycin was removed as a result of exchange transfusion [24]. MacDonald and Ramasethu [19] gave a list of medications with hypothetical drug loss for both single volume and double volume NET (calculated by first-order kinetics from a single compartment). The expected percent of loss due to DV NET for PCN-G, ampicillin and vancomycin is in the range of 11 to 14.7 and for gentamycin and kanamycin; it is in the range of 10.1 to 10.9. For digoxin it is only 2.4% and for phenobarbital and phenytoin, the expected percent of loss is 6.2 to 12.3.
4. Irradiation of blood used for NET: Even though the potential for graft versus host inflammatory response is very low with NET, it is advisable to use irradiated blood for NET (1). It is also recommended to give irradiated blood for follow up post-NET simple transfusions during neonatal period and also for simple blood transfusions given to infants weighing less than 1200 g using leucocyte poor or CMV negative blood [25].
5. Use of supplemental calcium during NET is discouraged as it is shown to have little effect on serum ionized calcium, and too rapid infusion of calcium may cause brady-arrhythmias and cardiac arrest (1). Pre-exchange hypocalcemia may need to be corrected (1), prior to starting NET. There are no neonatal studies regarding the use of supplemental magnesium.
6. Route of NET: Use of both umbilical venous (UVC) and umbilical arterial catheters (UAC) for NET was tried but did not get much traction, as there were more adverse events with the use of both vascular accesses, when compared to NET using UVC alone or via other routes [19]. In a retrospective study, NET via peripheral arteries and veins is reported to be efficient and effective. It was done on 102 neonates from January 1995 to December 2006, comparing 99 procedures done on peripheral vessels (mostly using radial arteries), to [24] procedures done using umbilical vein [26]. There were 8 deaths, though not attributed to NET. There was also a report in the Chinese literature, on the use of automated arteriovenous exchange transfusion for the treatment of severe hyperbilirubinemia [27]. In US, using UVC for pull and push technique is the standard. Having a peripheral intravenous access can be advantageous for the correction of electrolyte abnormalities etc.
7. Desired hematocrit of donor blood: In order to get the best results from the NET, aiming at drawing extravascular bilirubin into the plasma, and increasing bilirubin binding capacity (bilirubin-free albumin with available binding sites), it is recommended to select donor blood of high plasma volume (with a hematocrit of 40%) [1]. As done in the 90s, use of reconstituted donor blood with a high hematocrit for NET may correct the anemia and oxygen carrying capacity, but does not improve the bilirubin binding capacity. The practice of using pre-exchange albumin infusion in such situations is not evidence based.
8. Optimal result-percentage of correction of pre-NET total bilirubin and rebound: It is common to see approximately 50% decrease in the total bilirubin between pre and post-exchange labs (45 to 60%) and rebound of total bilirubin up to 65% is frequent (60 to 80%) [19].
9. Repeat exchanges for the same indication on the same baby: It is somewhat controversial, as there is no significant evidence to prove or disprove. Frequently, physicians use the same guidelines which were used for the initial NET [19]. Based on anecdotal experience, the author advises to have a safe interval of 12 to 18 h between two NETs, in order to decrease the already accelerated potential for adverse events. Based on the evidence noted in studies that showed pre-exchange (first NET) morbidity influences the outcome [1,19], it is prudent to be cautious with repeat NETs, as the infant has gone through a very complex and stressful procedure.
10. Getting a routine abdominal film for the placement of umbilical venous catheter (UVC) is controversial. UVC tend to move frequently during the procedure. So, it is hard to maintain the original x-ray confirmed position of the UVC. It is recommended to do an abdominal film, always when the UVC is left in after the procedure, for the purpose of infusing intravenous fluids, etc.
11. Other issues: Reconstituted blood (RCB) is preferred with a desired hematocrit of 40% [1,19] over citrated whole blood [28]. The size of the infant determines the amount of RCB to be requested for the double volume NET. Usually 160 to 180 ml/kg weight is recommended for double volume NET [1,19,21].
12. There is no controversy here. During the follow up, repeat hearing screen and neurodevelopmental evaluations need to be done because of the potential for hearing loss (secondary speech delay) and neurodevelopmental delays [1,19,29] from severe hyperbilirubinemia.
Net Trend
At the Royal Women’s Hospital in Melbourne, Australia 1830 exchange transfusions (ET or NET) were performed on 1160 infants from 1951-1968, averaging 101 procedures per year. At the same hospital during the period between January 1st 2001 and December 31st 2010, the ET (NET) rate came down to 6.4 per year [30]. Israel data from 1995 to 2005 comprising over 99% of all live very low birth weight infants (VLBW- less than 1500 g at birth) born in Israel, showed that 7.7% of them had total serum bilirubin (TSB) levels considered to be at a level requiring ET as per their established protocols. Only 9 to 14% of them with exchangeable bilirubin levels had undergone ET (NET) for the management of very high TSB. The authors of the study suggested that the Israel neonatologists seemed to be reluctant to perform ET on VLBW infants. The general trend is (including in US) to use more intensive phototherapy when TSB is approaching ET levels [31]. In the US, a retrospective chart review done by Steiner et al. [32] showed a sharp decline in the number of exchange transfusions (NET) performed during 1996- 2006, compared to 1986-1995, which was attributed to the improvements in perinatal and neonatal care. This decline was not associated with increase in complications which are expected with decreased procedural experience.
Conclusion
The frequency of Neonatal Exchange Transfusion (NET) either single or double volume, in the management of severe hyperbilirubinemia has been decreasing during the last few decades. And, new uses (indications) for NET are emerging. It remains as a valuable procedure for all of its indications with inherent potential for complications. One needs to exercise caution in selecting the neonate and the team, and take all the necessary precautions irrespective of the indication. It is imperative that the NET should be performed only by experienced individuals at a perinatal-neonatal center using both cardio-respiratory monitor and pulse oximeter. The team should be ready to respond to any adverse event that may arise at any stage of the procedure. Once in 3 months having the team participate in a mock drill can be helpful and may decrease the anxiety of the team members during the actual procedure. After the procedure, documentation of the suggestions that originated from the debriefing can be used for quality improvement. Communication is the key between the family and neonatologists, amongst all physicians and between physicians and other services such as blood center, nursing, respiratory therapy, and laboratory. Over all, the net value for NET during this internet age is still considerable.
REFERENCES
- Gleason CA, Juul SE. Avery’s diseases of the newborn. 10th edn. Philadelphia. Elsevier. 2018:1216-7.
- Nandyal R. Hemolytic disease of the newborn. J Hematol Thromb Dis. 2015;3:1-3.
- Diamond LK, Allen FH Jr, Thomas WO Jr. Erythroblastosis fetalis. VII: Treatment with exchange transfusion. N Engl J Med. 1951;244:39-44.
- Phillips AGS. The rise and fall of exchange transfusion. Historical perspective. NeoReviews. 2003;4:169-74.
- Murki S, Kumar P. Blood exchange transfusion for infants with severe neonatal hyperbilirubinemia. Semin Perinatol. 2011;35:175-84.
- Wagner T, Resch B, Legler TJ, et al. Severe HDN due to anti-Ce that required exchange transfusion. Immunohematology. 2000;40:570-4.
- Aradhya AS, Sundaram V, Kumar P, et al. Double volume exchange transfusion in severe neonatal sepsis. Indian J Pediatr. 2016; 83:107-13.
- Togari H, Iwanaga T, Matsumoto N, et al. Endotoxin clearance by exchange blood transfusion, in septic shock neonates. Acta Paediatr Scand. 1983;72:87-91.
- Woo HC, Phornphutkul C, Laptook AR. Early and severe indirect hyperbilirubinemia as a manifestation of galactosemia. J Perinatol. 2010;30:295-7.
- Chinnakaruppan NR, Marcus SM. Asymptomatic congenital lead poisoning – Case report. Clin Toxicol (Pila). 2010;48:563-5.
- Christensen RD, Hill HR, Anstall HB, et al. Exchange transfusion as an alternative to granulocyte administration in neonates with bacterial sepsis and profound neutropenia. J Clin Apher. 1984;2:177-83.
- Nicastro E, Iorio R. Neonatal hemochromatosis and exchange transfusion: treating the disorder as an alloimmune disease. J Pediatr Gastroenterol Nutr. 2010;50:471-2.
- Babor F, Hadzik B, Stannigel H, et al. Successful management of neonatal hemochromatosis by exchange transfusion and immunoglobulin: A case report. J Perinatol. 2013;33:83-5.
- Fellman V, von Bonsdorff L, Parkinnen J. Exogenous apotransferrin and exchange transfusions in hereditary iron overdose disease. Pediatrics. 2000;105:398-401.
- Hayasaka I, Cho K, Morioka K, et al. Exchange transfusion in patients with Down syndrome and severe transient leukemia. Pediatr Int. 2015;57:620-5.
- American Academy of Pediatrics. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1).
- Maisels MJ, Watchko JF, Bhutani VK, et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol. 2012;32:660-4.
- Keenan WJ, Novak KK, Sutherland JM, et al. Morbidity and mortality associated with exchange transfusion. Pediatrics. 1985;75:417-21.
- Macdonald MG, Seshia MK. Avery’s Neonatology. 7th edn. Wolters Kluwer. 2016;634-5.
- Chudwin DS, Ammann AJ, Wara DW, et al. Post-transfusion syndrome. Rash, eosinophilia and thrombocytopenia following intrauterine and exchange transfusions. Am J Dis Child. 1982;1367:612-4.
- MacDonald MG, Ramasethu J. Atlas of procedures in neonatology. 2nd edn. Lippincott. 2007;329-37.
- Thayyil S, Milligan DW. Single versus double volume exchange transfusion in jaundiced newborn infants. Cochrane Database Rev. 2006;18:CD004592.
- Abbas W, Attia NI, Hassanein SM. Two stage single-volume exchange transfusion in severe hemolytic disease of the newborn. J Matern Fetal neonatal Med. 2012;25:1080-3.
- Yakatan GJ, Smith RB, Leff RD, et al. Pharmocokinetic considerations in exchange transfusion in neonates. Clin Pharmacol Ther. 1978;24:90-4.
- Nandyal R. To transfuse or not to transfuse - A neonatologist’s daily dilemma. J Hematol Thrombo Dis. 2016;4:1-3.
- Chen HN, Lee ML, Tsao Ly. Exchange transfusion using peripheral vessels is safe and effective in newborn infants. Pediatrics. 2008; 4:905-10.
- Chen HW, Huang WM. Automated peripheral arteriovenous exchange transfusion for treatment of hyperbilirubinemia in neonate. Nan Fang Yi Ke Da Xue Bao. 2010;30:2396-8.
- Gherhbaghi MM, Hosseinpour SS. Exchange transfusion in neonatal hyperbilirubinemia: A comparison between citrated whole blood and reconstituted blood. Singapore Med J. 2010;51:641-4.
- Nandyal R, Beaver S, Becker W, et al. Neurologic and neurodevelopmental outcome of marked neonatal hyperbilirubinemia during infancy. Abstract presented at the National Perinatal Conference. J Perinatol. 2000;2:137-41.
- Chitty HE, Ziegler N, Savoia H, et al. Neonatal exchange transfusions in the 21st century: A single hospital study. J PaedIatr Child Health. 2013;49:825-32.
- Kuint J, Mayan-Metzger A, Boyko V, et al. Excessively high bilirubin and exchange transfusion in very low birth weight infants. Foundation Acta Paediatrica. 2011;100:506-10.
- Steiner LA, Bizarro MJ, Ehrenkranz RA, et al. A decline in the frequency of neonatal exchange transfusions and its effect on exchange-related morbidity and mortality. Pediatrics. 2007;120:27-32.