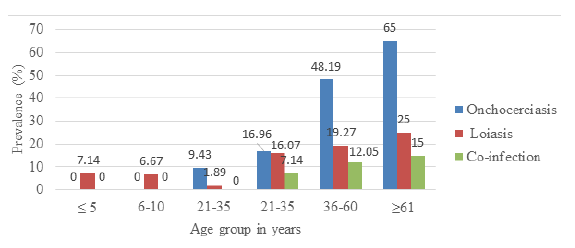Onchocerciasis and Loiasis in the Population of Widikum, Northwest Cameroon: Prevalence and Implication of Myeloperoxidase and C reactive proteins in Clinical Diseases’ indices
Received: 27-Jan-2023, Manuscript No. PULJMBR-23- 6145; Editor assigned: 29-Jan-2023, Pre QC No. PULJMBR-23- 6145 (PQ); Accepted Date: Mar 23, 2023; Reviewed: 15-Feb-2023 QC No. PULJMBR-23- 6145 (Q); Revised: 17-Feb-2023, Manuscript No. PULJMBR-23- 6145 (R); Published: 25-Mar-2023, DOI: 10.37532/puljmbr.2023.6(2).28-35
Citation: Mahamat O, Chifu NB, Abongwa LE, et al. Onchocerciasis and loiasis inthe population of widikum, Northwest Cameroon: Prevalence andimplication of myeloperoxidase and C reactive proteins in clinical diseases indices. J Mic Bio Rep. 2023;6(2):28-35.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Onchocerciasis and loiasis represent the major filarial parasites in Central and West African countries. This study sought to assess the prevalence of onchocerciasis and loiasis, its effects on inflammation and various clinical signs. METHODS: The diagnosis of onchocerciasis and loiasis was done using skin snips and blood samples respectively from 311 patients. C-reactive proteins and myeloperoxidase were determined using ELISA. RESULTS:Results obtained showed that the prevalence of onchocerciasis was 24.76% while that of loiasis was 13.83% and co-infection was 6.75%. Females were significantly (p=0.002) more infected with onchocerciasis (29.90%), while males registered an insignificant (p=0.535) higher prevalence of loiasis (14.02%). The most infected age group for onchocerciasis (48.19%), loiasis (19.278%) and co-infection (12.05%) was the 36 years-60 years age group and the results were statistically significant at p=0.027, p=0.027 and (p=0.025)respectively.
Females were at significantly higher risk of having onchocerciasis (p=0.005) than males. The 21 years-35 years age group was at significantly high risk of infection with onchocerciasis (p=0.0001) while for loiasis, the 21 years-35 years and 36 years-60 years age groups were more at risk of infection although there was no significant relationship between age and co-infection. Onchocerciasis was significantly prevalent in people with skin thickening (100% p=0.0001) while the prevalence of loiasis was highly significant in patients with skin depigmentation (29.85%; p=0.0001). Participants who were infected with onchocerciasis (2.568 µg L−1 ± 0.259 µg L−1) and loiasis (2.469 µg L−1 ± 0.238 µg L−1) had lower serum myeloperoxidase concentrations while participants with skin depigmentation showed a significantly higher level of Myeloperixidase as compared to those without skin depigmentation (p=0.032). Participants with skin depigmentation (p=0.014) and blurred vision (p=0.047) had significantly high levels of C- reactive proteins. CONCLUSION: This study has established that there is the production of CRP and Myeloperoxidase as inflammatory markers among patients with Onchocerciasis and Loiasis in Widikum and the levels of CRP and myeloperoxidase increases with their clinical manifestations.
Key Words
Onchocerciasis, loiasis
Introduction
Onchocerca volvulus and Loa loa responsible for onchocerciasis and loiasis respectively represent the major filarial parasites in Central and West African countries [1]. Onchocerciasis is rated as the second leading infectious cause of blindness in the world only preceded by blinding trachoma [2], while loiasis is a disease of public health importance as it is concern with neurological complications, which can be exacerbated with Ivermectin [2,3]. According to, an estimated 20.9 million people are infected with O. volvulus, with over 1.15 million having visual impairment [4]. Loa loa is often referred to as the African eye worm because the adult worm can sometimes be seen moving through the sclera of the eye [5]. In Cameroon, onchocerciasis is endemic in all ten regions with approximately six million people infected and about 60% of the population is said to be living in highrisk areas of the disease [6,7]. However, the prevalence of filariasis varies from one part of the country to another in the endemic areas, and recommends a regular epidemiological study for a better database about these diseases.
Patients with filariasis are a heterogeneous group in terms of the progression rate of the disease, the severity of clinical lesions, and the occurrence of acute syndromes. Oxidative stress and inflammation play a direct role in the initial and progression of disease [8,9]. In response to an early infection of filarial nematodes, there is the initiation of innate defense mechanisms characterized by an accumulation of neutrophils and eosinophils at the site of the infection. Through the stimulation of Tolllike receptors, filarial parasites cause the release of inflammatory cytokines such as IL-6 and TNF-α [10]. C-reactive Protein (CRP) and Myeloperoxidase (MPO) are also two inflammatory mediators. However, little is known about their implications in the progress of filariasis signs. CRP is an inflammatory biomarker synthesized in hepatocytes in response to primary stimulation by Interleukin 6 (IL-6) [11]. Various studies reported that CRP impaired endothelial vasoreactivity [12]. This may be by inhibiting endothelial nitric oxide synthase (eNOS) activity, reducing NO availability, and leading to an increase in total vascular resistance or systemic inflammation [11]. Myeloperoxidase, a product of systemic inflammation plays an important role both in the process of oxidative stress and inflammation and promotes the oxidation of lipoproteins [13]. It is a leukocyte-derived enzyme that catalyzes the formation of oxidative reactants and participates in innate immunity against infections. Its concentration predicts adverse clinical outcomes in setting acute inflammatory syndromes, but the prognostic role of MPO in filariasis with apparently visible signs is poorly understood. Unfortunately, there is little information indicating the relation between the MPO and CRP levels and their influence on the stability of inflammatory diseases in patients with filariasis.
This study that was carried out in Widikum was therefore designated to assess the epidemiology of onchocerciasis and loiasis and their impact on C-reactive proteins and myeloperoxidase.
Materials and Methods
Description of the study area
Widikum is a town in the Northwest region of Cameroon made up of villages including Bifang, Ambelle, Tiben, Ebendi, Oche, Bamben, Oche 1 and 2, Abedum, Ambelle, Ngalla, Dinku, Eka, Mbullam, Olorunti and Ambombo [14]. It is located along the Trans-African Highway, 31 miles west of Bamenda and about 100 miles east of Nigeria. It is located at Latitude 6.470°E and Longitude 10.4397°N. The people of Widikum are primarily found in the Momo Division of the Northwest region of Cameroon. Widikum has a population of about 30,000 people with 90% of them being actively engaged in farming, rearing pigs, and pisciculture [15]. The vegetation is a degraded forest in which the primary rain forest has been completely replaced by the oil palm, which constitutes the main commercial crop. The climate is tropical with two seasons; the rainy season which lasts from mid-March to mid-November and the dry season which lasts from mid-November to midMarch. The main rivers found in this area are river Momo and river Tanjo, which are tributaries of river Manyu [16].
Ethics statement
Ethical clearance (2021/101H/UBa/IRB) for the study was obtained from the institutional review board of the Faculty of Health Science of the University of Bamenda, Cameroon. An introductory letter was obtained from the Head of the Department of Biological Sciences, The University of Bamenda. A meeting was held with the heads of the health centers in Widikum concerning the objectives of the study, design, benefits, and risks involved, and also about the venues of the collection of data and samples. A signed consent form was used to indicate voluntary participation in the study and each participant was given the freedom to withdraw consent at any time.
Study design, study population, and selection criteria
The study was a hospital-based cross-sectional study conducted at the Widikum district hospital. The study was conducted from March 2021 to May 2021. Well-structured questionnaires were used to screen the natives of Widikum and those who had lived there for six months and above. Skin snips and venous blood were collected from consented participants for onchocerciasis and loiasis determination and immunological analysis. The study involved a cohort of both out and patients of both sexes and all ages that were present at the Widikum hospital. Visitors, those having severe diseases (such as those in the reanimation and those having cancer), and those who refused to sign the consent form were excluded from the study.
Demographic and clinical data
A well-structured questionnaire written in the English language was used in this study to screen and collect demographic information (sex, age, occupation, and educational level), disease awareness, and treatment of participants. For those who were not able to read or understand English, the questions were translated into pidgin English’ or their mother tongue. The patient’s medical and clinical history was included in the questionnaire.
Clinical examination
Each participant was examined for skin disease and nodules. The searched cutaneous signs were depigmentation, rashes (papular), worms in the eyes, and skin thickening. Nodule (onchocercomata) palpation was performed in a closed but well-illuminated room paying particular attention to bony prominences of the torso, iliac crests, and upper trochanter of the femurs. Onchocercal nodules were identified clinically as mobile masses beneath the skin, firm and painless and if present were recorded alongside the patient’s information [17].
Skin snip collection
Skin snips were collected as described by Thiele et al. [18]. Each patient’s skin (iliac crests) was cleaned using cotton and alcohol. Two skin snips were taken with a sterile blade and vacutainer (by lifting the skin with the vacutainer and cutting a portion of skin with the blade) from the two posterior iliac crests of each participant. The skin snips were placed in two separate wells of a microtitration plate containing 200 µl of normal saline (0.9%) and incubated for 24 hours at room temperature.
Blood sample collection
For blood collection, the skin was cleaned using alcohol, an elastic band (tourniquet) was tied above the area to get the veins to swell with blood, and then a needle was inserted into the vein (usually in the arm inside the elbow or at the back of the hand). Blood was then pulled into a tube (anticoagulant) taking into consideration the diurnal periodicity of Loa loa [19]. Part of the blood sample was used to examine L. loa and the remaining one was used for immunological studies.
Assessment of Onchocerca volvulus infection
The fluid of each well of the microtitration plate was examined for Onchocerca microfilariae using Bright-Field microscopy under low magnification (40X)[20]. The number of microfilariae in the fluid was counted (when positive) and the microfilariae densities were computed as the arithmetic mean number of microfilariae in the two skin snips (mf/ss).
Assessment of Loa loa infection
The presence of Loa loa was determined as described by Mogoung-Wafo with a few modifications by making a thick blood film (1 per individual) using 30 mL of blood. Each Giemsa-stained smear was then examined under a microscope at 100X and also, a wet mount was observed. Each sample was centrifuged at 3000 rpm for 10 minutes, buffy coat was picked using a pipette and observed under bright-field microscopy at10X and 40X and all the L. loa mfs present on the slide were counted [6].
Determination OF C -reactive protein
C- reactive protein was determined using the reagent as directed by the manufacturer (Biosciences PTE Ltd). One drop (40 µL) of the test sample was pipetted onto a slide using a disposable pipette provided with the kit. One drop of SBio ASO latex reagent was added to the drop of the test sample on the slide. Using a mixing stick, the serum and SBio ASO latex reagent was mixed uniformly over the entire circle. A stopwatch was immediately started, and the slide was then rocked gently back and forth observing for agglutination macroscopically after two minutes. Each positive sample was quantified as either 1/20, 1/40 1/80, 1/160, or 1/320 depending on the degree of agglutination.
Determination of myeloperoxidase
Myeloperoxidase activity was evaluated following the protocol reported by Oumar et al. with few modifications. Briefly, 100 µL of o-phenylenediamine (0.4 g/mL) and 0.002% H2O2 in phosphate-citrate buffer (pH=5.0) were mixed with equal volumes of serum using a microtitration plate [21]. The reaction was stopped after 10 minutes using 0.1 M H2 SO4 (100 µL) and Optical Density (OD) was measured at 490 nm as displayed on the BioELISA machine screen.
Data analysis
Data collected from questionnaires and other laboratory analyses were entered into excel and coded properly to allow for proper analysis. Data were checked for completeness, outliers, and missing values, using Statistical Package For Social Sciences (SPSS) version 22 according to the different variables. Using the Chi-square test, data obtained in continuous form were summarized as mean and standard deviation. Data obtained in categorical form were summarized as percentages and proportions. Data was displayed in the form of contingency tables P<0.05 was considered statistically significant.
Results
Characteristics of the study population
A total of 311 out and inpatients participated in the study out of which 65.95% (204/311) were females and 34.41% (107/311) were males. The participants were divided into 6 age groups out of which the most represented was the 21 years-35 years age group (112/311; 36.01%) while the least represented was the 6 years-10 years age group (15/311; 4.82%) as shown in Table 1.
TABLE 1. Socio-demographic data of the study population
| Characteristics | Frequency(N=311) | Proportion (%) |
|---|---|---|
| Gender | ||
| Females | 204 | 65.59 |
| Males | 107 | 34.41 |
| Total | 311 | 100 |
| Age group (years) | ||
| ≤ 5 | 28 | 9 |
| 10-Jun | 15 | 4.82 |
| 20-Nov | 53 | 17.04 |
| 21 - 35 | 112 | 36.01 |
| 36 - 60 | 83 | 26.69 |
| ≥ 61 | 20 | 6.43 |
| Total | 311 | 100 |
Influence of age and sex on the prevalence of onchocerciasis and loiasis in the study population
The overall prevalence of onchocerciasis and loiasis was 24.76% (77/311)and 13.83% (43/311) respectively. Out of a total of 120 participants having onchocerciasis or loiasis, 21 of them were co-infected giving a prevalence of 6.75% (21/311). In Figure 1, the most prevalent age group for onchocerciasis was the ≥ 61 years age group 65% (13/20) while none of the participants in the ≤ 5 years and 6 yeras-10 years age groups were infected. Equally, the age-related prevalence of loiasis showed that the most infected age group was the ≥ 61 years age group 25% (5/20) while the least infected age group was the 11 years-20 years age group (1.89% (1/53). These results were statistically significant for both Onchocerciasis (p=0.027), loiasis (p =0.027), and co-infection (p=0.025)
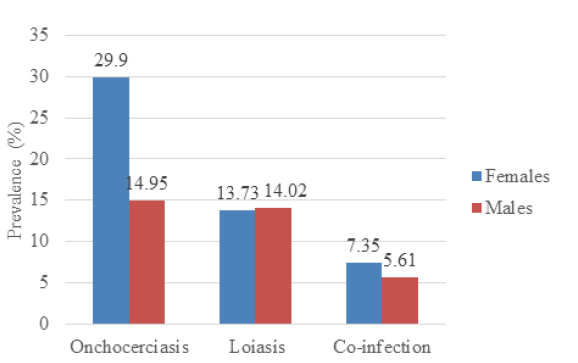
Sex-related prevalence for onchocerciasis showed that females were more infected (29.90%; 61/204) than males (14.95%; 16/107) and the results were statistically significant at p=.002. The prevalence of loiasis in females (13.73%; 28/204), was slightly lower than that of males (14.02%, 15/107), and the result was not statistically significant (p=0.535). There was equally no significant variation in co-infection with sex (p=0.375) as shown in Figure 2.
Multivariate analysis of the influence of age and sex on the prevalence of onchocerciasis and loiasis in the study area
Multivariate analysis revealed that there was a significant association between the ages of the participants and onchocerciasis (Table 2). The age group 21years-35years (OR=13.846; 95%CI=4.166, 46.016; p=0.0001) and 36years-60years (OR=2.297; 95%CI=0.745, 7.084; p=0.148) were at the highest risk of getting infected while those of age group 11years-20years (OR = 17.032; 95%CI=4.284, 67.724; p=0.0001) were significantly at low risk of infection amongst the age groups in the study population. Females were at significant risk of having onchocerciasis (OR=0.345; 95%CI=4.284, 67.724; p=0.005).
TABLE 2. Multivariate analysis of the influence of age and sex on the prevalence of onchocerciasis in the study are
| No. Examined | No. infected(%) | Onchocerca volvovus | |||||
|---|---|---|---|---|---|---|---|
| Subclasses | Unadjusted p value | OR | 95% C. | ||||
| Lower | Upper | ||||||
| Age group(years) | ≤ 5 | 28 | - | - | - | - | - |
| 10-Jun | 15 | - | - | - | - | - | |
| 20-Nov | 53 | 5 (9.43) | 0.0001 | 17.032 | 4.284 | 67.724 | |
| 21-35 | 112 | 19 (16.96) | 0.0001 | 13.846 | 4.166 | 46.016 | |
| 36-60 | 83 | 40 (48.19) | 0.148 | 2.297 | 0.745 | 7.084 | |
| ≥ 61 | 20 | 13 (65.00) | Reference | ||||
| Total | 311 | 77 (24.76) | |||||
| Gender | Female | 204 | 61 (29.90) | 0.005 | 0.345 | 0.165 | 0.72 |
| Male | 107 | 16 (14.95) | Reference | ||||
| Total | 311 | 77 (24.76) | |||||
Multivariate analysis showed that there was no significant association between the ages of the participants with loiasis (Table 3). Participants of age group 11 years-20 years were significantly at lower risk of infection (OR=14.751; 95%CI=; p=0.039) compared to those and age group 0 years-5 years (OR=4.030; 95%CI=0.465, 0.465; p=0.206); 6-10 years of age (OR=4.340; 95%CI =; p=0.267) while the age groups of 21 years-35 years and 36-60 years (OR=2.616) and (OR=2.112) were more at risk of infection but with no significant difference (p=0.276) and (p=0.412) respectively.
TABLE 3. Multivariate analyses of the association between loiasis and age and gender of the patients
| No. Examined | No. infected | Loa loa | |||||
|---|---|---|---|---|---|---|---|
| ( %) | Unadjusted p value | OR | 95% CI. | ||||
| Lower | Upper | ||||||
| Age group(years) | ≤ 5 | 28 | 2 (7.14) | 0.206 | 4.03 | 0.465 | 0.465 |
| 10-Jun | 15 | 1 (6.67) | 0.267 | 4.34 | 0.325 | 57.929 | |
| 20-Nov | 53 | 1 ( 1.89) | 0.039 | 14.751 | 1.153 | 188.77 | |
| 21-35 | 112 | 18 (16.07) | 0.276 | 2.616 | 0.463 | 14.784 | |
| 36-60 | 83 | 16 (19.278) | 0.412 | 2.112 | 0.354 | 12.61 | |
| ≥61 | 20 | 5 (25) | Reference | ||||
| Total | 311 | 43 (13.83) | |||||
| Gender | Females | 204 | 28 (13.73) | 0.762 | 1.148 | 0.469 | 2.809 |
| Males | 107 | 15 (14.02) | Reference | ||||
| Total | 311 | 43 (13.83) | |||||
There was no variation in the risk of infection with sex (p=0.762). The multivariate analysis revealed that there was no significant relationship between the ages and having confection with onchocerciasis and loiasis (p>0.05). At the same time, females were more at risk of co-infection than males even though it was not statistically significant (OR=1.607; p=0.524) as shown in Table 4.
TABLE 4. Multivariate analyses of the influence of age and gender on co-infection of onchocerciasis and Loiasis
| No. Examined | No. infected(%) | Co- infection | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted p-value | OR | 95% C. | |||||
| Lower | Upper | ||||||
| Age group(years) | ≤ 5 | 28 | - | - | - | - | - |
| 10-Jun | 15 | - | - | - | - | - | |
| 20-Nov | 53 | - | - | - | - | - | |
| 21-35 | 112 | 8 (7.14) | 0.122 | 0.161 | 0.016 | 1.626 | |
| 36-60 | 83 | 10 (12.05) | 0.473 | 0.434 | 0.044 | 4.24 | |
| ≥61 | 20 | 3 (15) | Reference | ||||
| Total | 311 | 21 (6.75) | |||||
| Gender | Females | 204 | 15 (7.35) | 0.524 | 1.607 | 0.373 | 6.925 |
| Males | 107 | 6 (5.61) | Reference | ||||
| Total | 311 | 21 (6.75) | |||||
Microfilariae load in the patients
Participants of the age group 11years -20years had the highest onchocerciasis parasite load (1500.0 mf/ss) while onchocerciasis and loiasis co-infection parasite load was highest (1343.8 mf/ss) in the 21years -35years age group. On the other hand loiasis parasitic load was higher (1350.0 mf/ss) in 21-35years age group and among those with onchocerciasis and loiasis coinfection, the parasitic load was highest (1375.0 mf/ss) in the ≥61 years age group. However all the differences were not significant (p >0.05). Although parasitic load showed no significant difference between gender, males had a higher parasitic load of onchocerciasis (1527.8 mf/ss) than females (1288.0 mf/ss). In co-infected patients, females had a higher parasite load of onchocerciasis (1230.8 mf/ss) compared to males (1062.5 mf/ss). This difference was not significant (p = 0.26). Equally, males had a higher load of infection with Loa loa microflariae (1375.0 mf/ss) than females (1166.7 mf/ss). Also co-infected males had a higher load of Loa loa microfilariae (1071.4 mf/ss) than females (1066.7 mf/ss) however the difference was not significant (p = 0.97) as shown on Table 5.
TABLE 5. Age and sex-related intensity of infection of onchocerciasis and loiasis, in the study area
| Category | No. Examined | Load of onchocerciasis (mf/ss) | Load of loiasis (mf/ml) | |||||
|---|---|---|---|---|---|---|---|---|
| single infection | Co-infection | No co-infection | Co-infection | |||||
| Mean ± SD | Mean ± SD | *p value | Mean ± SD | Mean ± SD | *p value | |||
| (N) | (N) | (N) | (N) | |||||
| Age group (years) | ≤ 5 | 28 | - | - | - | 1000.0 ±00 | - | |
| (2) | ||||||||
| 10-Jun | 15 | - | - | - | 550.0 ± 636.4 | - | ||
| (1) | ||||||||
| 20-Nov | 53 | 1500.0 ± 395.28 | - | - | 500.0±00 | - | ||
| (5) | (1) | |||||||
| 21-35 | 112 | 1022.7 ± 453.52 | 1343.8 ± 399.5 | p>0.05 | 1350.0 ± 241.52 | 1000.0 ± 267.26 | p>0.05 | |
| (11) | (8) | (10) | (8) | |||||
| 36-60 | 83 | 1379.3 ± 420.57 | 1125.0±377.31 | p>0.05 | 1083.3 ± 376.39 | 1000.0 ± 333.33 | p>0.05 | |
| (24) | (10) | (6) | (10) | |||||
| ≥ 61 | 20 | 1425 ± 354.53 | 1187.5 ± 239.36 | p>0.05 | 1000.0 ± 00 | 1375.0 ± 250 | p>0.05 | |
| (10) | (3) | (2) | (3) | |||||
| Gender | Females | 204 | 1288.0 ± 434.51 | 1230.8 ± 320.11 | p>0.05 | 1166.7± 408.25 | 1066.7±258.20 | p>0.05 |
| (46) | (13) | (15) | (15) | |||||
| Males | 107 | 1527.8 ± 384.15 (8) | 1062.5 ± 320.43 (8) | p>0.05 | 1375.0 ± 209.17 (6) | 1071.4 ± 449.87 (7) | p>0.05 | |
| 0.129 | 0.26 | 0.25 | 0.97 | |||||
| *p-value was calculated using an unpaired t-test | ||||||||
Clinical manifestations and prevalence of onchocerciasis and loiasis in patients in Widikum, Northwest Cameroon
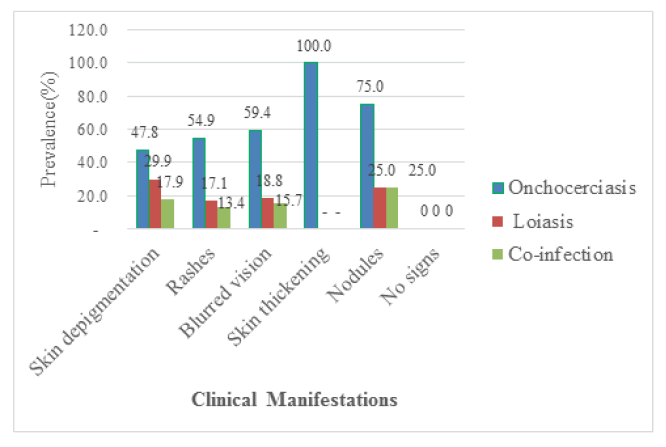
Out of the 311 participants recruited for the study 191 of them were positive for clinical manifestations giving a prevalence of 61.41%. Among these participants, 26.37% (82/311) of them presented with rashes, 21.54 (67/311) had skin depigmentation, and 1.29% (4/311) had nodules as shown in Figure 3. Onchocerciasis was significantly prevalent in people with skin thickening (100% (6/6): p=0.0001), in those with blurred vision (59.38% (19/32): p=0.0001), rashes (54.88% (45/82): p=0.0001) (29.85% (20/67); p=0.0001), but there was no significant difference concer-ning rashes, blurred vision, nodules, and skin thickening (p>0.05). The prevalence of co-infection was and skin depigmentation (47.76% (32/67): p=0.048) compared to their counterparts without the signs. On the other hand, the prevalence of loiasis was highly significant in patients with skin depigmentation higher in patients with rashes (13.41% (11/82): p=0.008) and skin depigmentation (17.91% (12/67; p=0.001) as compared to their counterparts without rashes and skin depigmentation respectively. No significant difference was observed between the prevalence of loiasis in patients having blurred vision, skin thickening, and nodules compared to their counterparts without these clinical signs as shown in Figure 3.
Multivariate analysis
Multivariate analysis revealed that onchocerciasis was not significantly associated with skin depigmentation (OR=0.879; p=0.662), rashes (OR=1.649; p=0.06), blurred vision (OR=1.298; p=0.53), skin thickening (OR=0.044; 0.994) and nodules (OR=1.320; p=0.512) (Table 6). However, there was a significant association between loiasis and its presence in patients with rashes (OR=0.336; p=0.001). It was found that the association between loiasis and the presence of blurred vision was (OR=0.400; p=0.07), skin depigmentation was (OR=0.873; p=0.672), skin thickening was (OR=0.0001; p=0.998) and nodules were (OR = 0.751; p=0.811) in patients. Despite the low risk, a significant relationship was found between co-infection and the presence of skin depigmentation (OR=0.449: p=0.026) and rashes (OR=0.275; p=0.0001) in patients. In contrast, the association was not significant between co-infection and the presence of blurred vision in patients (OR=0.491; p= 0.191), skin thickening (OR=0.0001; p=0.998), and nodules (OR=1.061; p=0.96)
TABLE 6. Multivariate analyses to determine the association between clinical manifestations and onchocerciasis, loiasis, and co-infection.
| Clinical Manifestations | N | Positive cases | Unadjusted p-value | OR | 95% CI. | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Onchocerciasis | ||||||
| Skin depigmentation | 67 | 32 | 0.662 | 0.879 | 0.494 | 1.566 |
| Rashes | 82 | 45 | 0.06 | 1.649 | 0.978 | 2.779 |
| Blurred vision | 32 | 19 | 0.53 | 1.298 | 0.575 | 2.931 |
| Skin thickening | 6 | 6 | 0.994 | 0.044 | 0.0001 | . |
| Nodules | 4 | 3 | 0.512 | 1.32 | 0.215 | 21.902 |
| Loiasis | ||||||
| Skin depigmentation | 67 | 20 | 0.672 | 0.873 | 0.466 | 1.636 |
| Rashes | 82 | 14 | 0.001 | 0.336 | 0.18 | 0.626 |
| Blurred vision | 32 | 6 | 0.07 | 0.4 | 0.148 | 1.078 |
| Skin thickening | 6 | 0 | 0.998 | 0.0001 | 0.0001 | - |
| Nodules | 4 | 1 | 0.811 | 0.751 | 0.072 | 7.869 |
| Co-infection of onchocerciasis and loiasis | ||||||
| Skin depigmentation | 67 | 12 | 0.026 | 0.449 | 0.222 | 0.909 |
| Rashes | 82 | 11 | 0.0001 | 0.275 | 0.138 | 0.546 |
| Blurred vision | 32 | 5 | 0.191 | 0.491 | 0.17 | 1.424 |
| Skin thickening | 6 | - | 0.998 | 0.0001 | 0.0001 | . |
| Nodules | 4 | 1 | 0.96 | 1.061 | 0.103 | 10.921 |
| *p-value was calculated using Chi-square. OR: Odds ratio; 95%CI: 95% confidence interval | ||||||
Serum myeloperoxidase concentration and its relationship with onchocerciasis and Loiasis in patients
Myeloperoxidase level did not vary with the patient’s status (p>0.05) as shown in Table 7. About the microfilariae load, there was a moderate and positive correlation between microfilariae load and serum levels of myeloperoxidase in single infected patients [onchocerciasis (r=0.41) and loiasis (r=0.50)] as well as in co-infected patients [loiasis (r=0.40); onchocerciasis (r=0.13)].
TABLE 7. Serum level of myeloperoxidase and linear correlation with microfilariae load in single infections and co-infection
| Category | Disease status | N | MPO (OD490 nm) | ||
|---|---|---|---|---|---|
| Mean ± SD | r | AR squared | |||
| Filariae free | 35 | 2.630 ± 0.229 | - | - | |
| Single infection | Onchocerciasis | 52 | 2.568 ± 0.259 | 0.41 | 0.15 |
| Loiasis | 21 | 2.469 ± 0.238 | 0.5 | 0.21 | |
| Co-infection | Onchocerciasis | 22 | 2.605 ± 0.238 | 0.13 | -0.03 |
| Loiasis | 0.4 | 0.12 | |||
| p value | 0.114 | ||||
As shown in Table 8, except for participants with skin depigmentation (p=0.032), there was no significant difference in the MPO level of participants with other clinical manifestations compared to their counterparts without the clinical manifestations (p>0.05). Participants with skin depigmentation showed a significantly higher level of MPO (2.638) as compared to those without skin depigmentation (2.540).
TABLE 8. Variation of myeloperoxidase level in patients following the clinical manifestations of onchocerciasis and loiasis
| Clinical manifestations | Category | Number of cases | OD492nm Mean ± SD | p-value |
|---|---|---|---|---|
| Skin depigmentation | Negative | 84 | 2.540 ± 0.250 | 0.032 |
| Positive | 46 | 2.638 ± 0.235 | ||
| Rashes | Negative | 76 | 2.566 ± 0.264 | 0.653 |
| Positive | 54 | 2.586 ± 0.226 | ||
| Blurred vision | Negative | 108 | 2.566 ± 0.253 | 0.405 |
| Positive | 22 | 2.615 ± 0.222 | ||
| Skin thickening | Negative | 124 | 2.576 ± 0.249 | 0.794 |
| Positive | 6 | 2.549 ± 0.235 | ||
| Nodules | Negative | 126 | 2.571 ± 0.247 | 0.323 |
| Positive | 4 | 2.696 ± 0.282 | ||
| SD: standard deviation | ||||
Serum C reactive protein concentration and relationship with onchocerciasis and Loiasis in patients
As shown in Table 9, patients with onchocerciasis (0.031) showed a significantly higher level of CRP (p<0.05) while patients with loiasis (0.037) and co-infection (0.030) showed no significant levels of CRP (p>0.05) as compared to uninfected participants (0.015). There was a moderate positive correlation between microfilariae load and serum levels of CRP with onchocerciasis (r=0.61) and a strong positive correlation with loiasis (r=0.89) in patients with single infection. In coinfected patients, the correlation with the microfilariae load was moderate in loiasis (r=0.43) and onchocerciasis (r=0.63).
TABLE 9. Serum level of C-reactive protein and linear correlation with microfilariae load in single and co-infection
| Diseases | Category | No. positive | CRP (IU/ml) | Linear correlation | ||
|---|---|---|---|---|---|---|
| Mean± SD | p-value | r | AR squared | |||
| Filariae free | 35 | 0.015 ± 0.007 | - | |||
| Single infection | Onchocerciasis | 52 | 0.031 ± 0.015 | p =0.032 | 0.61 | 0.34 |
| Loiasis | 21 | 0.037 ± 0.014 | p =0.643 | 0.89 | 0.77 | |
| Co-infection | Onchocerciasis | 22 | 0.030 ± 0.017 | p =0.581 | 0.63 | 0.36 |
| Loiasis | 0.43 | 0.13 | ||||
| p-value | 0.02 | |||||
Participants with skin depigmentation (0.035 IU/ml) and blurred vision (0.038 IU/ml) had significantly (p=0.014 and p=0.047) higher levels mean CRP than those without the clinical manifestations (0.024 IU/ml ± 0.013 IU/ml and 0.026 IU/ml ± 0.015 IU/ml) respectively as seen in Table 10. With regards to participants with rashes, skin thickening, and nodules, the level of CRP did not change significantly as compared to their counterparts with non clinical signs (p>0.05) (Table 10).
TABLE 10. Variations of CRP levels in patients with clinical manifestations of onchocerciasis and loiasis
| Clinical manifestations | Category | Number infected | CRP (IU/ml ) | |
|---|---|---|---|---|
| Mean ± SD | p-value | |||
| Skin depigmentation | Negative | 29 | 0.024 ± 0.013 | 0.014 |
| Positive | 21 | 0.035 ± 0.017 | ||
| Rashes | Negative | 24 | 0.028 ± 0.015 | 0.887 |
| Positive | 26 | 0.029 ± 0.017 | ||
| Blurred vision | Negative | 41 | 0.026 ± 0.015 | 0.047 |
| Positive | 9 | 0.038 ± 0.017 | ||
| Skin thickening | Negative | 48 | 0.028 ± 0.016 | 0.466 |
| Positive | 2 | 0.037 ± 0.017 | ||
| Nodules | Negative | 48 | 0.028 ± 0.016 | 0.466 |
| Positive | 2 | 0.037 ± 0.017 |
Discussion
The overall prevalence of onchocerciasis and loiasis in Widikum was 24.76% and 13.83% respectively. The climate and the presence of river Momo are favorable for the development of Simulum and Chrysops spp and thus the infection of the population. These results are in line with those of Aza’ah who found a relationship between the prevalence of onchcerciasis and the presence of rivers [22]. However, the prevalence obtained in Widikum was higher than that obtained in other parts of the country such as 7.0% obtained by Aza’ah et al. in Ndikinimeki [22]. This high prevalence in Widikum could be because most of the participants were farmers and often slept on their farms for days without good shelter, coupled with the socio-political crisis in the region which might have caused the villagers to abandon their homes to seek refuge in nearby bushes, thus exposing them to the vectors. Also, due to the socio-political crisis, ivermectin which was normally distributed in the area yearly had not been distributed for some time since access to the villages was almost impossible. The prevalence of loiasis obtained in Widikum was however lower than that obtained by Tatuene et al. in the Akonolinga center region of Cameroon and that obtained by Mogoung-Wafo et al. [6,22].
Onchocerciasis was higher in females than males. These results are in contrast with those reported by Kamga et al. and Aza’ah et al. in which onchocerciasis was more common in males [22]. This could be because, in most parts of the Northwest Region, females are the main group of persons involved in farming activities and so are those sleeping in poorly constructed tents on farms and thus neglect to adequately protect themselves from vector bites. Loiasis was more prevalent in males than females. The higher prevalence of loiasis in males than females could be a result of their daily activities since they leave their homes very early in the morning and evenings to the bush (for palm wine taping and palm oil production which are the major activities of men in Widikum) and these periods corresponds to the peak biting periods of Chrysops flies. This is in line with previous findings by Pion et al. and Lakwo et al. [25-27]. The study equally showed that the most infected age group was the 36 years-60 years age group. These results are similar to those of MogoungWafo et al. in the Mbalmayo Health District. This could be justified by the fact that people of this age group are more involved in farming activities.
The prevalence of onchocerciasis was found to be significantly higher among participants with clinical manifestations such as skin depigmentation, rashes, blurred vision, skin thickening, and nodules. This might probably be because patients with different onchocercal skin pathology have different O. volvulus-specific antibody isotopic responses and the differences could also be due to the amount of Wolbachia (a bacteria living in adult worms with a symbiotic relationship) found in each strain. This is in line with the findings of Higazi et al., [28]. The presence of skin depigmentation was more highly associated with onchocerciasis and loiasis than other clinical manifestations while participants with loiasis were at high risk of developing rashes and blurred vision with itching. This is probably because, as microfilariae circulate in blood and tissues, they develop from one stage to another and these different stages are marked by different clinical manifestations. As the larvae mature, the patient may experience vague symptoms such as pains or temporal swellings, and itching as the larvae move away under the skin and molt. These findings are in line with those reported by Lupi et al., [29]. Patients with co-infection showed a higher risk of developing skin depigmentation and rashes. This high risk in patients could be a result of both microfilariae and adult worm activities in tissues and the blood. This could also be because skin depigmentation, Rashes, and blurred vision were found to be the most important clinical sign of Onchocerciasis and Loiasis.
In the same line, patients with skin depigmentation had significantly higher myeloperoxidase and CRP than other clinical manifestations, and also, patients with blurred vision equally presented significantly higher levels of CRP. This might probably be due to the high immune reactions which result in high levels of inflammatory reactions and thus higher levels of myeloperoxidase and CRP which are makers of inflammation. The presence of microfilariae may induce inflammation. This is probably because the immune system recognizes the microfilarae as foreign and thus macrophages are activated to fight back by secreting toxic chemicals such as nitric oxide which eventually leads to the killing of microfilariae and inflammatory responses as well.
Results also showed a higher serum myeloperoxidase concentration in uninfected than infected individuals which was however not statistically significant indicating that the presence of microfilariae could induce inflammation through the production of myeloperoxidase which is a leucocyte-derived enzyme mainly secreted by activated neutrophils but this inflammatory response might have been limited by the production of Interleukin 10 (IL-10) which is a cytokine with anti-inflammatory properties limiting host immune response to pathogens by suppressing Th1 response thus preventing host’s tissue damage and decreased production of MPO and this could also be influenced by the presence of other unknown infections such as infection with lymphatic filariae (Wuchereria bancrofti).
Also, microfilariae might stimulate the production of MPO as they circulate in tissues and blood causing increased inflammatory reactions and damage to tissues leading to the production of anti-inflammatory cytokines.
Conclusion
The presence of onchocerciasis and loiasis co-infection in this study suggests that loiasis may pose an epidemiological threat to the persistent use of ivermectin for the treatment of onchocerciasis. As such, there is a need for the government to reconsider the continuous use of ivermectin in the study area. In addition, low levels of myeloperoxidase and high levels of CRP in patients with onchocerciasis and loiasis demonstrate that filariasis may induce inflammation and damage of host tissues leading to the production of anti-inflammatory cytokines such as IL-10.
References
- Akue JP, Nkoghe D, Padilla C, et al. Epidemiology of concomitant infection due to Loa loa and Mansonella perstans in Gabon. PLoS Negl Trop Dis. 2011;5(10): e1329.
- Etya'ale D. Onchocerciasis and trachoma control: what has changed in the past two decades?. Community Eye Health. 2008;21(67):43.
- Kemp C, Roberts A. Infectious Diseases: Filariasis—Bancroftian filariasis, Malayan filariasis, Loiasis (loa loa), Onchocerciasis (river blindness). J Am Acad Nurse pract. 2001;13(9):391-4.
- Higgins A, Morriss WW, Gelb AW, et al. 72nd World Health Assembly, Geneva, Switzerland, 2019. Anesthesia & Analgesia. 2020;130(3): e92-4.
- Ratmanov P, Mediannikov O, Raoult D. Vectorborne diseases in West Africa: geographic distribution and geospatial characteristics. Trans R Soc Trop Med Hyg. 2013;107(5):273-84.
- Mogoung-Wafo AE, Nana-Djeunga HC, Domche A, et al. Prevalence and intensity of Loa loa infection over twenty-three years in three communities of the Mbalmayo health district (Central Cameroon). BMC infectious diseases. 2019;19(1):1-7.
- Zoure HG, Noma M, Tekle AH, et al. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control:(2) pre-control endemicity levels and estimated number infected. Parasites & vectors. 2014; 7:1-5.
- Meribo K, Kebede B, Feleke SM, et al. Review of Ethiopian onchocerciasis elimination programme. Ethiopian medical journal. 2017;55(Suppl 1):55.
- Hassan S, Isyaku M, Yayo A, et al. Adult Loa loa filarial worm in the anterior chamber of the eye: A first report from savanna belt of Northern Nigeria.2016.
- Hise AG, Gilletteâ?Ferguson I, Pearlman E. The role of endosymbiotic Wolbachia bacteria in filarial disease. Cellular Microbiology. 2004;6(2):97-104.
- Buljubasic D, Drenjancevic I, Kibel A, et al. Myeloperoxidase (MPO) and high sensitivity C-reactive protein (hsCRP) as inflammatory biomarkers of endothelial and leukocyte activation in overweight hypertensive patients. Arterial Hypertension. 2021;25(1):15-21.
- Thim T. Human-like atherosclerosis in minipigs: a new model for detection and treatment of vulnerable plaques. Dan Med Bull. 2010 ;57(7): B4161.
- Kimak E, Zieba B, Duma D, et al. Myeloperoxidase level and inflammatory markers and lipid and lipoprotein parameters in stable coronary artery disease. Lipids in health and disease. 2018;17(1):1-7.
- Wanji S, Tendongfor N, Esum M, et al. Combined utilisation of rapid assessment procedures for loiasis (RAPLOA) and onchocerciasis (REA) in rain forest villages of Cameroon. Filaria journal. 2005;4(1):1-0.
- Ngwa DF. Power, Symbolism and Conflict in Bafut, Cameroon. Adv Hist Stud. 2022;11(3):141-68.
- Debrah LB, Nausch N, Opoku VS, et al. Epidemiology of Mansonella perstans in the middle belt of Ghana. Parasites & vectors. 2017;10(1):1-8.
- Kamga GR, Dissak-Delon FN, Nana-Djeunga HC, et al. Important progress towards elimination of onchocerciasis in the West Region of Cameroon. Parasites & vectors. 2017;10(1):1-2.
- Thiele EA, Cama VA, Lakwo T, et al. Detection of Onchocerca volvulus in skin snips by microscopy and real-time polymerase chain reaction: implications for monitoring and evaluation activities. Am J Trop Med Hyg. 2016;94(4):906.
- Kamgno J, Pion SD, Mackenzie CD, et al. Loa loa microfilarial periodicity in ivermectin-treated patients: comparison between those developing and those free of serious adverse events. Am J Trop Med Hyg. 2009;81(6):1056.
- Moreau JP, Prost A, Prod'Hon J. An attempt to normalize the methodology of clinico parasitologic surveys of onchocerciasis in West-Africa (author's transl). Med Trop: Rev du Corps Sante Colon. 1978;38(1):43-51.
- Mahamat O, Christopher T, Gilbert A, et al. Immunological in vivo and in vitro investigations of aqueous extract of stem bark of pterocarpus erinaceus poir (fabaceae). The American Journal of the Medical Sciences. 2018;356(1):56-63.
- Aza’ah RA, Sumo L, Ntonifor NH, et al. Point prevalence mapping reveals hotspot for onchocerciasis transmission in the Ndikinimeki Health District, Centre Region, Cameroon. Parasites & Vectors. 2020;13(1):1-8.
- Tatuene JK, Fotsing RG, Nkoa T, et al. Epidemiology of Loa loa and Mansonella perstans filariasis in the Akonolinga health district, Centre Region, Cameroon. Health Sci Dis. 2014;15(1).
- Kamga GR, Dissak-Delon FN, Nana-Djeunga HC, et al. Still mesoendemic onchocerciasis in two Cameroonian community-directed treatments with ivermectin projects despite more than 15 years of mass treatment. Parasites & vectors. 2016; 9:1-2.
- Pion SD, Clarke P, Filipe JA, et al. Co-infection with Onchocerca volvulus and Loa loa microfilariae in central Cameroon: are these two species interacting?. Parasitology. 2006;132(6):843-54.
- Pion, SD, Gardon J, Kamgno J, et al. Structure of the microfilarial reservoir of Loa loa in the human host and its implications for monitoring the programmes of Community-Directed Treatment with Ivermectin carried out in Africa. Parasitology. 2004;129(5):613-26.
- Barrow A, Mbowe F. State of knowledge and challenges in the control and eradica-tion of Onchocerciasis in Africa: a mini scoping review. J Biomed Life Sci. 2022:1-6.
- Higazi TB, Filiano A, Katholi CR, et al. Wolbachia endosymbiont levels in severe and mild strains of Onchocerca volvulus. Mol biochem parasitol. 2005;141(1):109-12.
- Lupi O, Downing C, Lee M, et al. Mucocutaneous manifestations of helminth infections: Nematodes. J Am Acad Dermatol. 2015;73(6):929-44.