Plasma-lyte for intravenous fluid maintenance, replacement or resuscitation as an alternative to other intravenous fluids in paediatric patients: A systematic review
2 Honorary Senior Lecturer Public Health, Swansea University Medical School, Grove Building, Swansea University, Singleton Park, Swansea, UK, Email: brendan.w.mason@gmail.com
Received: 23-Feb-2019 Accepted Date: Mar 10, 2019; Published: 20-Mar-2019
Citation: Edwards ED, Mason BW. Plasma-lyte for intravenous fluid maintenance, replacement or resuscitation as an alternative to other intravenous fluids in paediatric patients: A systematic review. J Pedia Health Care Med 2018;1(1):23-26.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Plasma-Lyte is a licensed isotonic fluid that can be used as an intravenous (IV) fluid in children and infants. It can be used as a lower cost alternative to IV fluids containing 0.45% or 0.9% Sodium Chloride (NaCl), with or without potassium or glucose 5%. Objectives: The aim of this study is to assess evidence which compare the outcome in paediatric patients of using Plasma-Lyte for intravenous fluid maintenance, replacement or resuscitation as an alternative to other intravenous fluids. Design: This study is a systematic review of published, original research articles of any design. Subjects: Children aged 0–18 years. Method: Electronic database search was performed of MEDLINE and EMBASE. Results: Seven articles, which reported 5 studies, met the inclusion criteria. A single high quality peer reviewed randomised controlled trial (RCT)demonstrated that in comparison with 0.9 % NaCl for rehydration in children with gastroenteritis, Plasma-Lyte was well tolerated and led to more rapid improvement in serum bicarbonate and dehydration score. The remaining studies provided low grade evidence of better or equivalent biochemical or blood gas outcomes when Plasma-Lyte was used as an alternative to 0.9 % NaCl. Limitations: Small number published studies, only two RCTs, only 1 peer reviewed publication. Conclusions: Plasma-Lyte is a licensed isotonic fluid that can be used for intravenous fluid maintenance, replacement and resuscitation in children and infants. It conforms to the 2015 The National Institute for Health and Care Excellence (NICE) guideline on intravenous fluid therapy in children and young people in hospital. The limited published evidence available suggests that in children Plasma-Lyte is equivalent to
Keywords
Plasma-Lyte; Sodium chloride; Acute kidney injury; Gastroenteritis; Hyperkalemia
Introduction
Plasma-Lyte is a licensed isotonic fluid that can be used as an intravenous (IV) fluid for maintenance, replacement or resuscitation in children and infants. It can be used an alternative to IV fluids containing 0.45% or 0.9% Sodium Chloride (NaCl), with or without potassium or glucose 5%. Using Plasma-Lyte in place of currently utilised intravenous fluid has the potential for cost savings.
The early clinical trials in adults comparing 0.9% saline with Plasma-Lyte had small sample sizes and reported short term biochemical outcomes [1]. Although 0.9% saline was associated with decreased serum pH, elevated serum chloride levels and decreased bicarbonate levels in these studies, no significant difference was found between groups in renal function [1,2]. A subsequent large multicentre double-blind cluster randomised controlled trial comparing 0.9% saline with Plasma-Lyte in acutely ill adults found no significant difference between group in rates of acute kidney injury, acute kidney injury requiring renal replacement therapy, and 90 day mortality [3]. A large cluster-randomized, multiple-crossover trial conducted in critically ill adults, found that the use of balanced crystalloids (including Plasma- Lyte) for intravenous fluid administration resulted in a lower rate of the composite outcome of death from any cause, new renal-replacement therapy, or persistent renal dysfunction than the use of saline [4]. Data from adults supports the use of Plasma-Lyte for resuscitation or maintenance fluids in preference to saline [5].
The characteristics of intravenous fluids employed in the paediatric population are described by Langer et al. [6]. Plasma-Lyte contains Na+140 mEq L–1, Cl- 98 mEq L–1, Acetate 27 mEq L–1, Gluconate 23 mEq L–1, and a Tonicity of 296 mOsm L–1. In contrast 0.9% NaCl contains Na+154 mEq L–1, Cl- 154 mEq L–1, Acetate 0 mEq L–1, Gluconate 0 mEq L–1, and a Tonicity of 308 mOsm L–1. The composition of intravenous fluids determines their effect on plasma acid-base and electrolyte equilibrium [7,8]. The term ‘balanced solutions’ is used to define solutions, such as Plasma-Lyte, that have electrolyte concentrations close to those of plasma [9]. The chloride concentration of Plasma-Lyte is not only closer to that of plasma than 0.9% NaCl but also nearer to plasma than other balanced solutions. Chloriderestrictive fluid administration has been found to be associated with a lower incidence of acute kidney injury and use of renal replacement therapy in [7]. It is therefore plausible that the use of Plasma-Lyte in children will have better outcomes than alternative IV fluids.
We report a systematic review undertaken to determine the effectiveness of Plasma –Lyte compared with alternative crystalloid solutions in children.
Methods
Search strategy
The electronic database MEDLINE and EMBASE were searched using the free text terms with wildcard truncation “Plasmalyte*” and “Plasma-lyte*”. Searches were undertaken on 2nd December 2018. The searches from the two databases were combined to remove duplicate publications.
Study selection
The abstracts of all publications were screened independently by two authors (EDE and BWM) to identify all papers that potentially met the following criteria:
• The publication reported a controlled trial, quasi experimental, cohort, case-control study, or systematic review: and,
• The patients studied were paediatric (less than 18 years of age); and,
• The study compared Plasma-Lyte with an alternative intravenous fluid for maintenance, replacement or resuscitation; and,
• The study reported any outcome.
The full texts of potentially relevant publications were obtained and reviewed to determine if they met the above inclusion criteria. The findings of included publications were summarised. The methodological quality of randomised controlled trials was assessed using the Jadad five point scale [10].
Results
Study selection
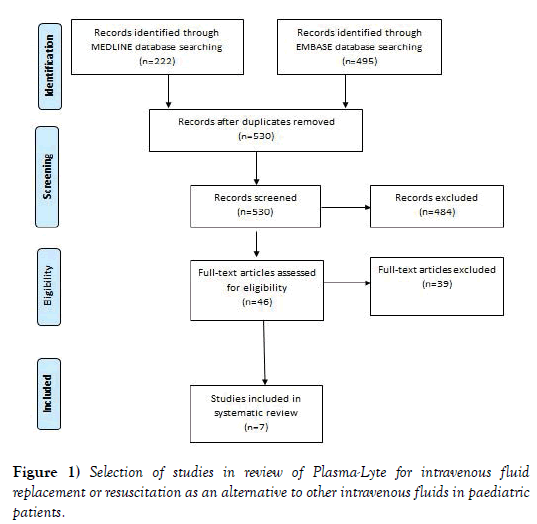
The search of electronic databases identified 222 publications from MEDLINE and 495 publications from EMBASE, with 530 unique publications from the combined searches. Screening of abstracts of these 530 publications identified 46 publications that potentially met the inclusion criteria. Review of the full text of these 46 publications identified 7 papers that met the inclusion criteria (Figure 1). The 39 papers were excluded for one or more of the following reasons: the publication did not report a controlled trial, quasi experimental, cohort, case-control study, or systematic review (6 papers); the patients studied were not paediatric (31 papers); the study did not compared Plasma-Lyte with an alternative intravenous fluid (11 papers); the fluids were not used for maintenance, replacement or resuscitation (7 papers); and, the study did not reported any outcome (7 papers).
Figure 1: Selection of studies in review of Plasma-Lyte for intravenous fluid replacement or resuscitation as an alternative to other intravenous fluids in paediatric patients.
Study characteristics
One study [11] was published in a peer reviewed journal the remaining 6 publications were non peer reviewed conference abstract (Table 1) [12-17]. The data presented was based on 5 studies, as one conference abstract [12] reported on the study the was later published in a peer review journal [11] and two conference abstracts presented data from the same study [14,15]. The 5 studies included 2 randomised controlled trials [11-13], 2 retrospective before and after studies [14,15,17] and a retrospective cohort study [16].
| Study | Study Design | Number of patients | Country | Disease / Setting | Study Finding |
|---|---|---|---|---|---|
| Allen et al. [11] | Double blind Randomised Controlled Trial. Intervention Plasma-Lyte A (PLA) or 0.9 % sodium chloride (NaCl) for intravenous fluid replacement. Primary outcome measure was serum bicarbonate level at 4 h. Secondary outcomes included safety and tolerability. |
100 children aged >=6 months to <11 years. | US and Canada. | Dehydration secondary to acute gastroenteritis / paediatric emergency departments. | At hour 4, the PLA group had greater increases in serum bicarbonate from baseline than did the 0.9 % NaCl group (mean +/- SD at 4 h: 18 +/- 3.74 vs 18.0 +/- 3.67; change from baseline of 1.6 and 0.0, respectively; P=.004. Both treatment groups received similar fluid volumes. The PLA group had less abdominal pain and better dehydration scores at hour 2 (both P=.03) but not at hour 4 (P=0.15 and 0.08, respectively). One patient in each treatment group developed hyponatremia. Four patients developed hyperkalemia (PLA:1, 0.9 % NaCl:3). |
| Allen et al. [12] | Randomised controlled trial. | 100 children aged >=6 months to <11 years. | US and Canada. | Dehydration secondary to acute gastroenteritis / paediatric emergency departments. | Conference abstract presenting findings of paper published in peer review described above [2]. |
| Lima et al.[13] | Randomised controlled trial. Intervention NaCl 0.9% (NSG) or Plasma-Lyte (PLA) from anesthetic induction until 24 hours after surgery. Outcomes electrolytes and arterial blood gas analysis at three distinct moments: anesthetic induction, immediate postoperative day and first postoperative day. |
14 children aged between 6 months and 10 years of age | Brazil | Elective neurosurgery for resection of brain tumour / Inpatient. | There was no significant variation of serum sodium on both groups and the values were kept within normal range. Serum chloride variation was significantly greater on NSG than PLA (6.57+/-5.42 vs 1.33 +/-2.42, p=0.037), as well as pH (-0.13+/-0.08 vs -0.006 +/-0.095, p=0.02) and base excess variations (-4.98+/-3.12 vs -0.86+/-2.53, p=0.018). Mean pH was significantly lower on NSG (7.25 vs 7.38, p<0.01). |
| Sutherland et al. [14] | Retrospective before and after study Change from traditional maintenance fluid (0.9% sodium chloride with 5% glucose or 0.45% sodium chloride with 5% glucose) to a new formulation (Plasmalyte-148 Glucose 5%). Retrospective audit in a Paediatric Intensive Care Unit (PICU) comparing 2010-2011 and 2011-2012 examining population serum electrolytes. |
Number children not stated | UK | Critically ill children / Paediatric Intensive Care Unit | Serum electrolytes showed no significant change between the two periods: Sodium: 141 mmol/l (2010-2011; range 95-187, median 140) to 143 mmol/l (2011-2012; range 126-190, median 141) Potassium: 3.99 mmol/l (2010-11; range 1.9-9.2, median 3.9) to 3.81 mmol/l (2011-2012; range 1.9-9.0, median 3.7) Chloride: 106 mmol/l (2010-11; range 70-142, median 105) to 106 mmol/l (2010-11; range 68-141, median 105). |
| Sutherland et al. [15] | Retrospective before and after study | Number children not stated | UK | Critically ill children / Paediatric Intensive Care Unit | Conference abstract presenting same but abridged data from above conference abstract [5]. |
| Kara et al. [16] | Retrospective cohort study Comparison outcomes of children who received plasmalyte during renal transplantation to those who received primarily normal saline. |
20 transplanted childre, 14 received plasmalyte, and 6 received predominantly 0.9% saline. | New Zealand | Renal Transplantation / Inpatient | The first post-operative potassium checked on arrival in intensive care was 4.2 mmol/l (range 3.2-5.8) in the plasmalyte group and 4.6 (range 4.2-5.3) in the saline group. The chloride at 24 hours was on average 102 mmol/l ( range 99-105) in the plasmalyte group, and 114 ( range 112-116) in the saline group. |
| Andrew et al. [17] | Retrospective before and after study All children admitted in the 18?month periods before and after the change from 0.9% NaCl to Plasma_Lyte, and receiving a fluid bolus in the first 24?hours of admission. Arterial blood gas and creatinine values for up to 5 days after bolus fluid administration were examined. Patients were stratified according to the total resuscitation volume (ml/kg). The primary outcome was plasma chloride. Secondary outcomes included blood pH and percentage change in creatinine. Clinical outcomes were length of ventilation and length of PICU stay. |
126 Children | UK | Critically ill children / Paediatric Intensive Care Unit | Children receiving 0.9% NaCl boluses tended to have a higher maximum chloride, higher average chloride, lower pH and higher percentage creatinine increase than those given Plama-Lyte. Subgroup analysis showed a statistically significant difference in average serum chloride for children give 61–90?ml/kg total resuscitation volume {Plasma-Lyte 105.59 ± 1.29?vs 0.9% NaCl 111.29 ± 2.1?mmol/L; difference: -6.21 [95% confidence interval (CI)-9.55,–2.87]}. Patients who received Plasma-Lyte tended to have a higher pH than those receiving 0.9% NaCl. A statistically significant difference was seen in children given 10–30?ml/kg total resuscitation volume [Plasma-Lyte 7.42 ± 0.49?vs 0.9% NaCLNS 7.33 ± 0.65; difference: 0.0913 (95% CI: -0.18 to -0.02)]. Significant differences were not seen in the clinical outcomes of length of stay or ventilation. |
Table 1: Summary of included studies of Plasma-Lyte for intravenous fluid replacement or resuscitation as an alternative to other intravenous fluids in paediatric patients
Patients groups studied included children: with gastroenteritis [11,12]; undergoing elective neurosurgery for resection of brain tumor [13]; treated on a Paediatric Intensive Care Unit [14,15,17]; and, undergoing renal transplantation [16].
Study quality
Only 1 randomised controlled trial (RCT) was described in full to allow the assessment of methodological quality [11]. The study scored the maximum 5 points on the Jadad scale.
Study findings
The peer reviewed RCT [11] reported that in children with gastroenteritis those given Plasma-Lyte had statistically significant greater increases in serum bicarbonate from baseline than did the 0.9 % NaCl group. Both treatment groups received similar fluid volumes. The Plasma-Lyte group had a significantly better dehydration scores at hour 2 and a non-significant better score at hour 4. No patient experienced clinically relevant worsening of laboratory findings or physical examination, and hospital admission rates were similar. One patient in each treatment group developed hyponatremia. Four patients developed hyperkalemia, 1 with Plasma-Lyte and 3 with 0.9 % NaCl.
The non-peer reviewed RCT [15] reported statistically significantly higher mean PH in children treated with Plasma-Lyte. Variation in serum chloride, pH and base excess were all significantly greater in those treated with 0.9% NaCl.
In a before and after study children receiving 0.9% NaCl boluses , as resuscitation fluid, tended to have a higher maximum chloride, higher average chloride, lower pH and higher percentage creatinine increase than those given Plama-Lyte [17]. Subgroup analysis showed a statistically significant difference in average serum chloride for children give 61–90 ml/ kg total resuscitation volume [difference: −6.21, 95% confidence interval (CI)−9.55,–2.87]. Children who received Plasma-Lyte tended to have a higher pH than those receiving 0.9% NaCl. A statistically significant difference was seen in the children given 10–30 ml/kg total resuscitation volume [Plasma- Lyte 7.42 ± 0.49 vs 0.9% NaCl 7.33 ± 0.65; difference: 0.0913 (95% CI: −0.18 to −0.02)]. Significant differences were not seen in the clinical outcomes of length of stay or ventilation.
The conference abstracts for the two remaining studies [14-16] provided no statistical tests to enable interpretation of the findings.
Discussion
A systematic review using a sensitive search strategy will identify all relevant published research. It is therefore possible to conclude with certainty that published research comparing Plasma-Lyte with other intravenous fluids in the paediatric population is very limited. Two publications were based on data that was presented in other publications, leaving only 5 original datasets. Six of the 7 included publications were conference abstracts which will not have been subject to peer review. The only paper published in a peer reviewed journal was a high quality randomised controlled trial which demonstrated the superiority of Plasma-Lyte over normal saline in paediatric patients with gastroenteritis. Conference abstracts are not only limited by the absence of peer review but often contain insufficient information to make judgments on the validity of their findings, for example it is not clear if the subgroup analysis was based on apriori hypothesis or post hoc analysis [17]. These remaining studies provide low grade evidence which suggests that Plasma-Lyte is equivalent, or possible superior in some clinical situations, to alternative intravenous fluids.
At current prices available to the NHS in Wales Plasma-Lyte costs less than 0.9% sodium chloride in 500 ml bags and for maintenance fluids has the potential for additional savings from both fluid costs and staff time if 1000 ml bags are used (Bhavee Patel, personal communication). The National Institute for Health and Care Excellence (NICE) guidance on intravenous fluid therapy in children and young people in hospital recommends glucose‑free crystalloids that contain sodium in the range 131–154 mmol/ litre for IV resuscitation, and isotonic crystalloids that contain sodium in the range 131–154 mmol/litre as initial IV fluids for routine maintenance [18]. As an isotonic crystalloid available with and without glucose that contains 140 mmol/litre of sodium [6,19], Plasma-Lyte meets the criteria in the NICE guidance for use in children and young people.
Conclusion
Plasma-Lyte is a licensed isotonic fluid that can be used as an intravenous fluid in children and infants. It conforms to the 2015 NICE guideline on intravenous fluid therapy in children and young people in hospital [20]. The limited published evidence available for children suggests that, as in adult clinical trials, Plasma-Lyte is equivalent to or possible superior in some situations to alternative intravenous fluids. Further clinical trials in children are urgently required.
Conflict of Interest
There are no conflicts of interest to disclose from all authors.
REFERENCES
- Reddy S, Weinberg L, Young P, et al. Crystalloid fluid therapy. Crit Care 2016; 20:59.
- Cortés DO, Bonor AR, Vincent JL, et al. Isotonic crystalloid solutions: A structured review of the literature. Br J Anaesth 2014; 112:968-81.
- Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA 2015; 314:1701-10.
- Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018; 378:829-39.
- Weinberg L, Collins N, Van Mourik K, et al. Plasma-Lyte 148: a clinical review. World J Crit Care Med 2016; 5:235.
- Luthar SS, Cicchetti D, Becker B, et al. The construct of resilience: A critical evaluation and guidelines for future work. Child Dev 2000;71:543-62.
- Langer T, Limuti R, Tommasino C, et al. Intravenous fluid therapy for hospitalized and critically ill children: rationale, available drugs and possible side effects. Anaesthesiol Intensive Ther. 2018; 50:49-58.
- Langer T, Ferrari M, Zazzeron L, et al. Effects of intravenous solutions on acid-base equilibrium: from crystalloids to colloids and blood components. Anaesthesiol Intensive Ther 2014; 46:350-60.
- Langer T, Carlesso E, Protti A, et al. In vivo conditioning of acid–base equilibrium by crystalloid solutions: an experimental study on pigs. Intensive Care Med 2012; 38:686-93.
- Morgan TJ. The meaning of acid–base abnormalities in the intensive care unit–effects of fluid administration. Crit Care 2004; 9:204.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Control Clin Trials 1996; 17:1-2.
- Allen CH, Goldman RD, Bhatt S, et al. A randomized trial of Plasma-Lyte A and 0.9% sodium chloride in acute pediatric gastroenteritis. BMC Pediatr 2016; 16:117.
- Allen CH, Goldman RD, Simon HK, et al. Balanced crystalloid or saline in pediatric gastroenteritis: A randomized controlled trial: 466. Acad Emerg Med 2014; 21:196-7.
- Child’s Nervous System. 25th Congress of the European Society for Pediatric Neurosurgery (ESPN) Paris-France, 8-11 May 2016. Springer Nature 2016;32: 905–12.
- Sutherland A, Playfor S, Manaf A, et al. Balanced maintenance fluids in PICU are safe and cost effective. Arch Dis Child BMJ 2013;98.
- Sutherland A, Playfor S. ABSTRACT 278- Ovid Technologies (Wolters Kluwer Health). Pediatr Crit Care Med 2014; 15:65.
- Kara T. Retrospective review of choice of intravenous fluids in pediatric renal transplantation. Pediatric Transplantation. Conference: 8th Congress on Pediatric Transplantation, IPTA 2015. San Francisco, CA United States. Conference Publication 2015; 19:144.
- Andrew W, Patrick D. P18 Plasma-lyte 148 vs 0.9% saline for fluid resuscition in children: electrolytic and clinical outcomes. Arch Dis Child BMJ 2018; 103:122–1.
- Neilson J, O’Neill F, Dawoud D, et al. Intravenous fluids in children and young people: summary of NICE guidance. BMJ 2015; 6388.
- Weinberg L, Collins N, Van Mourik K, et al. Plasma-Lyte 148: A clinical review. World journal of Crit Care medicine. 2016; 5:235.