Proficiency of graphene oxide in adsorption and removal of methylene blue from water: An overview
Received: 06-Sep-2017 Accepted Date: Sep 08, 2017; Published: 12-Oct-2017
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Ever-increasing demands of dye and printing technology has resulted in uncontrolled disposal of dyes into water bodies leading to tremendous environmental pollution. Methylene blue (MB), a popular, water soluble blue dye, has been reported as a major component of water pollutant. It is carcinogenic as well as has enormous toxic effects on the aquatic organisms, thereby considerably affecting ecological food chain. The removal of these types of dyes from water is thus, an important issue and consequently modern technology strongly recommend for less expensive, facile techniques in this regard. Graphene oxide (GO), oxidized form of graphene containing oxygen functionalities, is found to exhibit superior adsorption property compared to available carbon-based materials and therefore possesses significant roles in waste water treatment technology. This overview categorically narrates recent progresses on the fabrication, performances and achievements of GO as adsorbent with a first-hand idea of plausible mechanism of adsorption of MB. A concise outline on the challenges and future prospects of this field has also been highlighted.
Keywords
Adsorption; Removal; Graphene oxide; Methylene blue; Water treatment
Industrial and technological progression has been recognized to be key steps for economic growth and development of the modern society. But it has simultaneously imposed many non-ignoring ecological problems as well! For instance, non-stop release and disposal of the toxic industrial effluents from printing, textiles, paper, leather, food, and cosmetics industries to environment has become one of the most hazardous sources of pollution in the recent times [1,2]. Major effluents mainly constitute water soluble organic dyes and metals ions [3]. Their accumulation in the water bodies, provisioned for common water supplies has huge adverse effect on the ecosystem. Though toxic metal ions can be removed to appreciable extent by ion exchangers used in water treatment plants to striking effect, organic water soluble dye elimination process is of great concern today keeping in view of large scale, cost-effective and eco-friendly degradation criteria. The presence of water soluble dyes in water bodies has several toxic effects; namely, they consume dissolved oxygen to considerable extent and thus raise the biochemical oxygen demand (BOD), which consequently destroys aquatic life. Such dye contamination not only makes the water highly unsuitable for drinking but also renders inaptness in the domestic and agricultural usage. Eye burns in humans and animals are common effects. It may also stimulate the gastrointestinal tract and cause nausea, vomiting and diarrhea if ingested. It also leads to dyspnea, tachycardia, cyanosis, methemoglobinemia; convulsions, if inhaled. Several other serious complications may also arise when contaminated with larger dozes [4-6]. Moreover, several dyes are found to be carcinogenic as well [7]. Owing to these harmful effects on environment, it is necessary to remove these dyes from aqueous solution with immediate effect. Accordingly, bold technological measures are to be adapted to control and restrict their discharge and contamination to the environment. For the last few decades, physical, chemical, and microbial biodegradation; membrane separation; bioreactors; fungal consortium; and other methods have been common techniques for removing effluents from industries [8-10]. All of these methods have their own advantages as well as limitations. The removal of pollutants onto eco-friendly materials by mode of “adsorption” has been considered to be superior to other techniques because of low cost, simplicity of design, availability, and advanced ability to treat different dyes simultaneously with the same adsorbent [11]. So it is very important and meaningful to seek new adsorbents with large specific surface area, high adsorption capacity, fast adsorption rate and special surface reactivity. Various biomaterials were initially used in these processes include rice husk, orange peel, neem leaf, red mud, bagasse fly ash, sawdust, conducting polymers, etc. but were limited by removal ineffectiveness as well as reproducibility criteria [12-18]. In this context, much attention has recently been offered to nanoscience and nanotechnology with the hope that it may open new fruitful pathways to curb the existing problems [19,20]. Organic-based nanomaterials such as activated carbons, bio-polymers, carbon nanotubes (CNT), have been widely studied for adsorption of various dyes from water because of their unique physiochemical and mechanical properties [21-25]. Moreover, they are more bio-compatible to that of materials of inorganic source. Initially, wide usage of activated carbon was achievable due to less expensive, high specific surface area, but presently limited by highly-flammable nature and difficulty to regenerate. Moreover, weak hydrophilic properties also lead to weak interaction between the adsorbent and dyes. Due to hollow and layered structures, carbon nanotubes (CNTs) have been utilized for the adsorption of a large number of different organic compounds from water but again their usage is limited by high production cost and poor efficiency on large scale [26]. In the recent past, graphene-based 2D nanomaterials such as graphene, graphene sponges, graphene oxide (GO) sheets and its derivatives, have attracted broad attention because of their unique conjugated, twodimensional (2D) structure, which exhibits superior optical, mechanical and electronic properties [27-29]. Owing to its comparatively high surface area, graphene-based systems find applications in a number of fields such as catalysis, sensor, super capacitors, adsorption, and so forth [30,31]. As far as adsorption characteristics are concerned, besides large surface area, the negative charges in the GO sheets due to various oxygen-rich functional groups (i.e., carboxy, epoxy, hydroxyl groups) allow additional strong electrostatic interactions with cationic dye molecules. Furthermore, extensive usage of GO is also attributed to its high yielding method of synthesis (using naturally abundant graphite as the starting material). Its’ water solubility/ dispersibility and stability also plays an important role in the successful formation of the complex with water soluble dyes and subsequent removal of the same for the purpose of effective water treatment applications [32]. In the recent past substantial explorations on adsorption of water soluble dyes by graphene oxide (GO) has been executed. Thus, it is the right moment to summarize, analyze and compare the results obtained till-date to understand the current status of GO as adsorbent material for water soluble dues to be employed in water treatment technologies, the ultimate aim in writing this tutorial overview.
Methods of Preparation
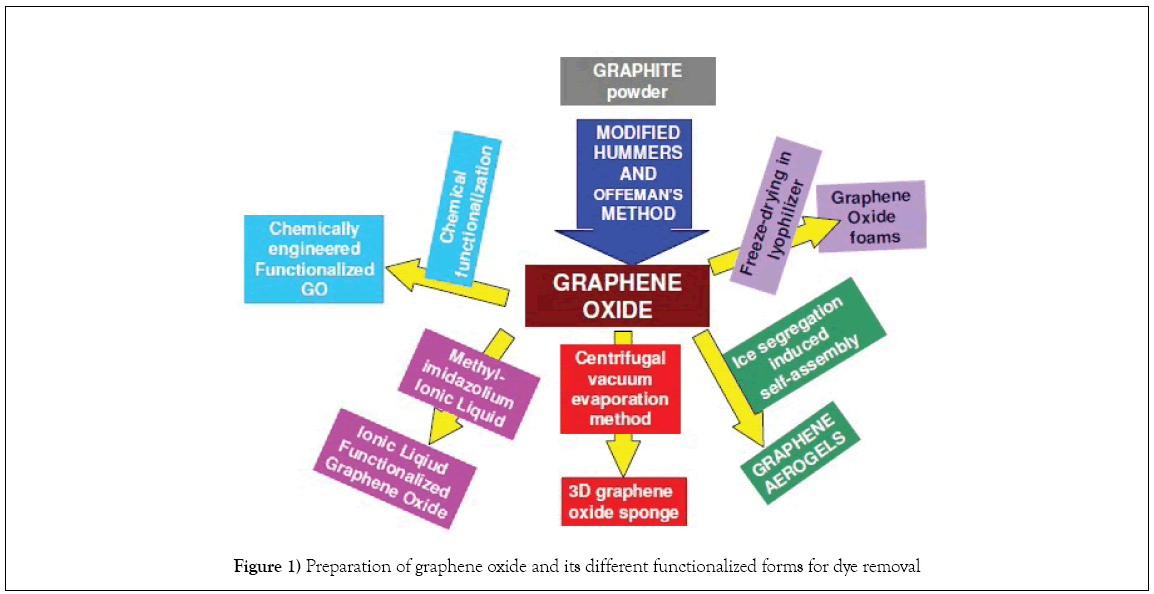
Graphene oxide has been prepared by modified Hummer’s method by most workers [33-36]. GO functionalized forms, as reported by the numerous workers, have been prepared by adopting a variety of methods that are briefly narrated in subsequent sections, are shown schematically in Figure 1 [37-41].
Results and Discussion
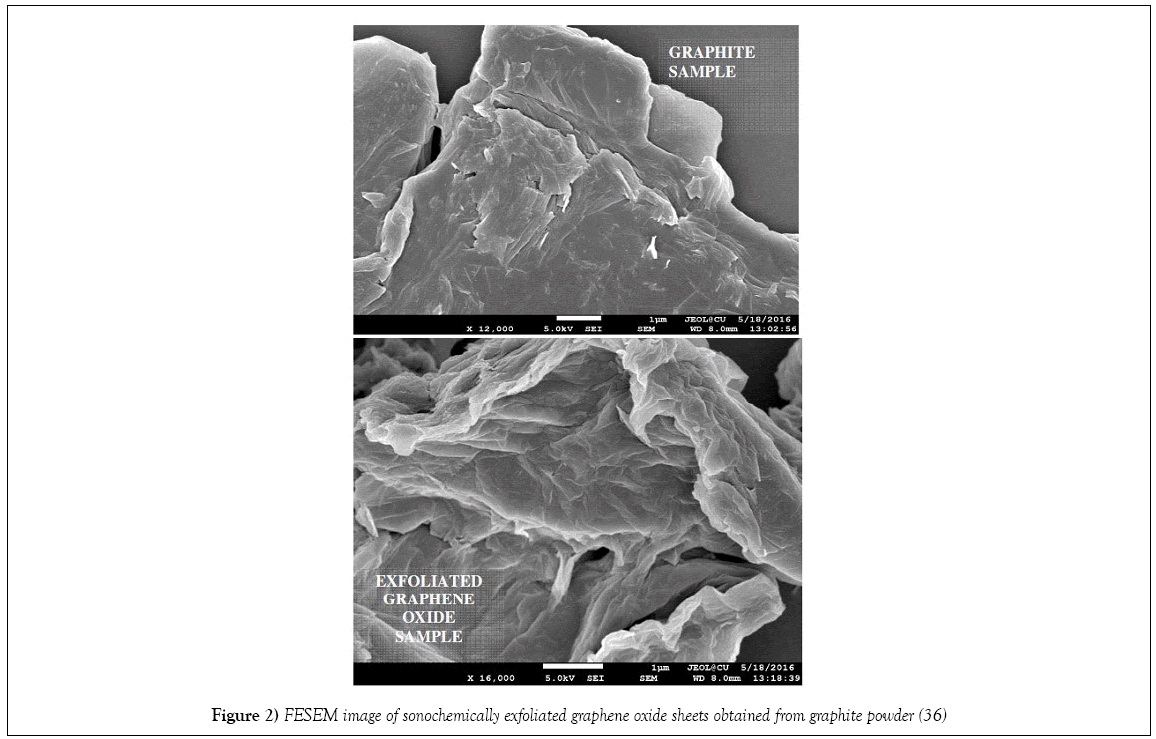
There are numerous studies of water soluble dye removal with GO as adsorbent [42-46]. Yang group, reported that GO can be directly utilized to remove methylene blue (MB) from initial concentration of 250 mgL-1 to 1.4 mgL-1, owing to its excellent adsorption performance (~714 mg/g) [33]. Succeedingly, Liu group demonstrated that a three-dimensional (3D) graphene oxide sponge could remove dyes such as MB with high efficiently [34]. Zhang et al. showed that GO prepared via modified Hummer’s method could adsorb MB very quickly but hardly release the dyes unlike previously results [35]. Our group has also obtained comparable results in the removal of MB by GO prepared by sonochemical method [36]. Morpology of the sonochemically exfoliated GO sheets obtained from graphite powder (are shown in the FESEM image of Figure 2) have g ood adsorption characteristics. MB adsorption properties on GO surface were compared with that of other forms of carbon materials. Few years later, Deng et al. [47] has reported that GO possesses adsorption efficiency of 351 mgg-1 for Methylene blue (MB) based on Langmuir isotherm which is much higher than activated carbon owing to larger surface area in the former, playing important role in adsorption phenomena. Similar comparative study of functionalized carbonaceous materials as adsorbents, namely, activated carbon (AC), graphene oxide (GO) and multi-walled carbon nanotubes (CNTs), for the removal of methylene blue dye from aqueous solution carried out Li et al. [37] The adsorption capacities of MB onto AC, GO and CNTs were 270.27, 243.90 and 188.68 mg/g, respectively and followed the order of AC>GO>CNTs. As earlier case, such adsorption characteristics were attributed to large surface area as well as electron donor acceptor interactions and electrostatic attraction between positively charged dye ions and negatively charged adsorbents [37]. Wang group in their work showed the influence of temperature, pH, ionic strength, and dissolved organic matter (DOM) content on the adsorption capacity of GO for MB [34]. Low temperature favors adsorption process as it is an exothermic process. Increase of the ionic strength of the medium, at high MB concentrations, leads to the increase of the absorption capacity. It may be due to the fact that increase of ionic strength improves the hydrophobicity of both MB and GO. Moreover, effect of dissolved organic matter (DOM) suggest that competitive absorption of MB onto GO and DOM (tannic Acid) (both GO and tannic acid are negatively charged, in this case) led to absorption inhibition. The study also reinforced the influence of electrostatic as well as interactions held mainly responsible are for adsorption of dye and its removal process by GO [38]. Very recently, Yan and co-workers synthesized a series of graphene oxides (GO) with different oxidation degrees to study the fundamental adsorption behavior of the GO series for removal of methylene blue (MB) from aqueous solutions. They reported that increasing oxidation degree of GO, gradually changed the interaction modes with MB. The parallel stacking on graphite plane through – interactions got modified to vertical standing via electrostatic interactions resulting in a significant improvement of MB uptakes [39]. Furthermore, the dye adsorption of GO increased exponentially with the increase of oxidation degree, from a Freundlich-type to a Langmuir-type adsorption, with pH independent adsorption characteristics. Similar conclusions were drawn by Kumar Group while studying adsorption behavior of graphene oxide towards cationic and anionic dyes [40]. Recently, Peng and his group reported the effects of pH, foreign ions and their concentrations on MB removal by GO [41]. They showed that the adsorption of MB in the presence of cations increased in the sequence Li+<Na+<K+, while it was reduced in the order ClO4−>NO3−>>Cl− in the presence of anions at pH range 2-12. MB removal in ClO4− was independent of pH, which may be attributed to the synergistic effect between GO and ClO4− ions. The adsorption process followed a pseudo-second-order kinetics model, and the adsorption isotherm agreed well with the Langmuir model, with adsorption capacity of 2255.35 mg/g. Previous reports about using GO for dye removal form wastewater, the initial dye concentrations were often at hundreds of ppm level, and the dye concentrations generally remain at several ppm after adsorption process. Indeed, some dye-removal strategies through advanced oxidation process and photocatalytic degradation, which succeed with ppm level dye wastewater in some experiments, require a high dosage of catalytic materials and waste the dyes. Therefore, it is still a big challenge to efficiently remove low concentration pollutants from natural water system, especially via simple and inexpensive approaches. Lately, electrochemical degradation of MB showed improved results by adopting simple heterogeneous Electro-Fenton method (in situ generation of active degrading agent H2O2 by electrochemical reduction of O2) carried out using modified graphite electrodes (GE) with Graphene oxide [41,42].
Figure 2: FESEM image of sonochemically exfoliated graphene oxide sheets obtained from graphite powder [36]
Thus the above reports convey the following points:
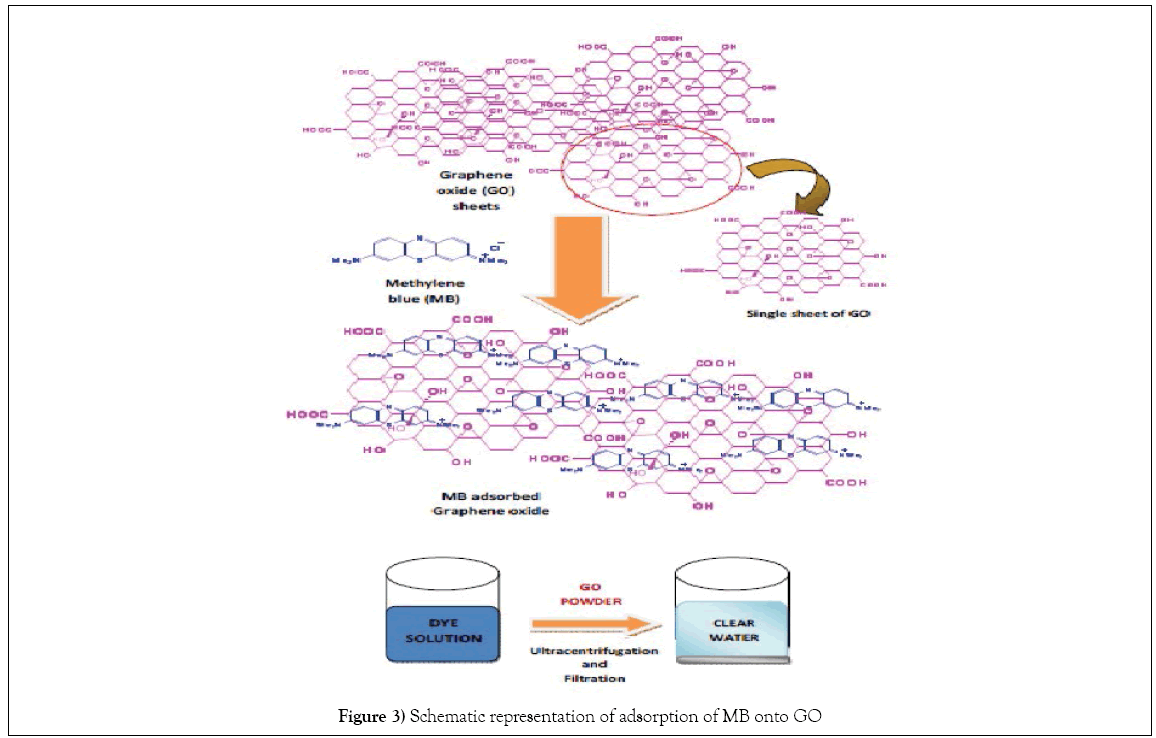
1. GO is an excellent absorbent for MB removal and the removal efficiency is higher than other carbon materials for adsorption and the solution can be decolorized to nearly colorless. Schematic representation has been shown in (Figure 3).
2. The main strength of absorption for GO towards MB is the electrostatic interaction, while the — stacking interaction also contribute to the total interaction.
3. The removal efficiency is regulated by temperature, pH, ionic strength and DOM content, extent of oxygen functionality, especially at high MB concentrations.
4. The adsorption process is found to follow pseudo-second-order kinetics in all the cases.
5. The suggested adsorption mechanisms support Langmuir model and provide the best fitting to describe the isotherms.
Though GO has several benefits as adsorbents for MB and can be effectively used for water treatment processes, it suffers some unavoidable disadvantages too that has triggered further modification of GO for addressing the above purpose. GO is highly dispersible in water which often leads to poor adsorption process. Moreover, GO-MB interaction is relatively weak and chances always creep up for reversible desorption of MB [43]. Moreover, the eco friendliness of GO is also yet to be addressed clearly. The removal efficiency of GO is dependent on pH and temperature, ionic strength of the medium that needs to be pre- optimized for dye removal [44]. Current research is aiming in functionalization of GO so as to overcome the adverse effects of GO adsorbent at the same time preserve all its effectiveness for obtaining superior results. Various composites using metal oxide, polymers, and other carbon materials have been fabricated for the purpose [44-48]. Even GO has been functionalized to obtain superior results in this area [49-51]. The idea has been attracting increasing interest in wastewater treatment because of high effectiveness, eco-friendliness, low-cost, mild reaction conditions and simple operation.
Conclusion
It is quite sure that in the very near future, GO has to play extremely vital role in water treatment technology! Thus, this short synopsis endeavors with special address to the genuine achievements as well as to the difficulties experienced in this domain of research which will surely help the newcomers to understand where to begin with! Nonetheless, it is anticipated to encourage more future opportunities to achieve the aim and promote sustainability in this field of research.
Acknowledgements
DM acknowledges Barasat Government College, Barasat, Kolkata; Department of Chemistry (Physical Chemistry Div.), Jadavpur University and Indian Association for the Cultivation of Science, Jadavpur, Kolkata, India for infrastructural facilities.
REFERENCES
- Kant R. Textile dyeing industry an environmental hazard. Nat Sci. 2012;41:22-6.
- Scott A. Cutting out textile pollution cleaning up one of the world’s dirtiest industries will require new technology and more. Chem Eng News. 2015;93(41):189.
- Stephenson RJ, Sheldon JB. Coagulation and precipitation of a mechanical pulping effluent-I, removal of carbon, colour and turbidity. Water Res. 1996;30:781-92.
- Oz M, Lorke DE, Hasan Md, et al. Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev. 2011;31:93-117.
- Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009;78(8):927-32.
- Sills MR, Zinkham WH. Methylene blue-induced Heinz body hemolytic anemia. Arch Pediatr Adolesc. 1994;148(3):306-10.
- Olliver JR, Wild CP, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;32(9381):373-4.
- Kang S, Asatekin A, Mayes, et al. Protein antifouling mechanisms of PAN UF membranes incorporating PAN-g-PEO additive. J Membr Sci. 2007;298:42-50.
- Hassan ES. Solar distillation: A promising alternative for water provision with free energy, simple technology and a clean environment. Desalin. 1998;116:45-56.
- Mark AS, Paul WB, Elimelech M, et al. Science and technology for water purification in the coming decades. Nature. 2008;452:301-10.
- Gupta VK, Carrott PJM, Suhas MMLR, et al. Low cost adsorbents: Growing approach to wastewater treatment — a review. Crit Rev Environ Sci Technol. 2009;39:783-842.
- Chowdhury S, Mishra R, Saha P, et al. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination. 2011;265:159-68.
- Arami M, Limaee NY, Mahmoodi NM, et al. Removal of dyes from colored textile wastewater by orange peel adsorbent: equilibrium and kinetic studies. J Colloid Interface Sci. 2005;288:371-6.
- Chowdhury S, Chakraborty S, Saha P. Biosorption of basic green 4 from aqueous solution by Ananas comosus (pineapple) leaf powder. Colloids Surf B. 2011;84:520-7.
- Ansari R, Raoï¬Âe F. Removal of lead ion from aqueous solutions using saw dust coated by polyaniline. E J Chem. 2006;3(10):49-59.
- Chowdhury S, Saha P. Removal of malachite green from water using hydrothermally carbonized pine needles. J Environ Eng. 2011;137:388.
- Liu ZS. Control of heavy metals during incineration using activated carbon ï¬Âbers. J Hazard Mater. 2007;142:506-11.
- Bulut Y, Tez Z. Adsorption studies on ground shells of hazelnut and almond. J Hazard Mater. 2007;149:35-41.
- Qu X, Alvarez PJJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47:3931-46.
- Bora T, Dutta J. Applications of nanotechnology in wastewater treatment - A review. J Nanosci Nanotech. 2014;14(1):613-26.
- Nam SW, Choi DJ, Kim SK, et al. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J Hazard Mater. 2014;270:144-52.
- Hameed BH, Ahmad AL. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J Hazard Mater. 2009;164:870-5.
- Li YH, Zhao YM, Hu WB, et al. Carbon nanotubes – the promising adsorbent in wastewater treatment. J Phys. 2007;61:698-702.
- Sadegh H, Ghoshekandi RS, Masjedi A, et al. A review on Carbon nanotubes adsorbents for the removal of pollutants from aqueous solutions. Int J Nano Dimens. 2016;7(2):109-20.
- Sánchez-Martín J, Beltrán-Heredia J, Gibello-Pérez P. Adsorbent biopolymers from tannin extracts for water treatment. Chem Eng J. 2011;168:1241-7.
- Ali I. New generation adsorbents for water treatment. Chem Rev. 2012;112(10):5073-91.
- Gadipelli S, Guo ZX. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog Mat Sc. 2015;69:1-60.
- Majumdar D, Baskey M, Saha SK. Mol Rapid Commun. 2011;32:1277-83.
- Fang Q, Shen Y, Chen B. Synthesis, decoration and properties. Chem Eng J. 2015;264:753-71.
- Geim AK, Novoselov K.S. The rise of grapheme. Nat Mater. 2007;6:183-91.
- Singh RK, Kumar R, Singh DP. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016;6:64993-5011.
- Si Y, Samulski ET. Synthesis of water soluble graphene. Nano Lett. 2008;8(6):1679-82.
- Yang ST, Chen S, Chang Y, et al. Removal of methylene blue from aqueous solution by graphene oxide. J Colloid Interface Sci. 2011;359(1):24-9.
- Liu F, Chung S, Oh G, et al. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl Mater Interfaces. 2012;4(2):922-7.
- Zhang W, Zhou C, Zhou W, et al. Fast and considerable adsorption of methylene blue dye onto grapheme oxide. Bull Environ Contam Toxicol. 2011;87(1):86-90.
- Pal S, Majumdar D. Book title modern trends in chemical sciences. Edited Dr. Kamala Mitra. Chapter on: Adsorption and removal of soluble methylene blue dye from water by sorbefacient graphene oxide. 2016;19-27.
- Li Y, Du Q, Liu T, et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide and carbon nanotubes. Chem Eng Res Design. 2013;91:361-68.
- Ramesha GK, Kumara AV, Muralidhara HB, et al. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci. 2011;361:270-7.
- Yan H, Tao X, Yang Z, et al. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J Hazard Mater. 2014;268:191-8.
- Minitha CR, Lalitha M, Jeyachandran YL, et al. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and pi-pi interactions. Mater Chem Phys. 2017;194:243-52.
- Peng W, Li H, Liu Y, et al. Adsorption of methylene blue on graphene oxide prepared from amorphous graphite: Effects of pH and foreign ions. J Mol Liq. 2016;221:82–7.
- Akerdi G, Es’haghzade Z, Bahrami SH, et al. Comparative study of GO and reduced GO coated graphite electrodes for decolorization of acidic and basic dyes from aqueous solutions through heterogeneous electro-Fenton process. J Environ Chem Eng. 2017;5:2313-24.
- Li N, Chena J, Shi YP. Magnetic reduced graphene oxide functionalized with cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phyto-hormones in tomatoes coupled with high performance liquid chromatography. J Chromat A. 2016;1441:24-33.
- Zambare R, Song X, Bhuvana S, et al. Ultrafast dye removal using ionic liquid − Graphene oxide sponge. ACS Sustainable Chem Eng. 2017;5:6026-35.
- Zambare RS, Dhopte KB, Patwardhan AV, et al. Polyamine functionalized graphene oxide polysulfone mixed matrix membranes with improved hydrophilicity and anti-fouling properties. Desalination. 2017;403:24-35.
- Bhattacharya G, Sas S, Wadhwa S, et al. Aloe vera assisted facile green synthesis of reduced graphene oxide for electrochemical and dye removal applications. RSC Adv. 2017;7:26680-8.
- Deng XZ, Wang YW, Peng JP, et al. Surface area control of nanocomposites Mg(OH)2/graphene using a cathodic electrodeposition process: High adsorption capability of methyl orange. RSC Adv. 2016;6:88315-20.
- Kar P, Sardar S, Liu B, et al. Facile synthesis of reduced graphene oxide–gold nanohybrid for potential use in industrial waste-water treatment. Sci Technol Adv Mater. 2016;17(1):375-86.
- Han Y, Xu Z, Gao C. Ultrathin graphene nano-filtration membrane for water purification. Adv Funct Mater. 2013;23:3693-700.
- Gao W, Majumder M, Alemany LB, et al. Engineered graphite oxide materials for application in water purification. ACS Appl Mater Interfaces. 2011;3(6):1821-6.
- Gupta K, Khatri OP. Reduced graphene oxide as an effective adsorbent for removal of malachite green dye: Plausible adsorption pathways. J Colloid Interface Sci. 2017;501:11-21.