Successful treatment of hepatitis C in young child on hemodialysis
2 Department of Gastroenterology and Hepatology, Hamad Medical Corporation, Hamad General Hospital, Qatar, Email: mostafa_elb@rocketmail.com
3 Department of Pediatric Hepatology, Hamad Medical Corporation, Hamad General Hospital, Qatar, Email: abubakrimam5625@gmail.com
4 Department of Pediatric Nephrology, Hamad Medical Corporation, Hamad General Hospital, Qatar, Email: k.abou_h@yahoo.com
Received: 15-Sep-2018 Accepted Date: Oct 15, 2018; Published: 25-Oct-2018
Citation: Derbala M, Elbaba M, Imam AB, et al. Successful treatment of hepatitis C in young child on hemodialysis. J Hepato Gastroenterol. 2018;2(2):47-8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Direct-acting Antivirals (DAAs) dramatically improve the treatment of hepatitis C virus (HCV) infections; however, the treatment of HCV in young children with chronic kidney disease is controversial because of possible adverse effects. Case summary: We report a case of sustained remission of a 9-year-old Egyptian girl with end stage kidney disease on regular dialysis was diagnosed as having chronic hepatitis C, genotype 1a. She was treated with Paritaprevir/ ritonavir/ombitasvir and dasabuvir for 12 weeks. Conclusion: Our case showed a high safety margin, efficacy and tolerability in Hemodialysis-dependent patient under the age of 12. Confirmation of these clinical observations in large clinical studies may help improve morbidity and decrease mortality outcome in pediatric with chronic kidney disease.
Keywords
HCV; Hemodialysis; DAAs
Background
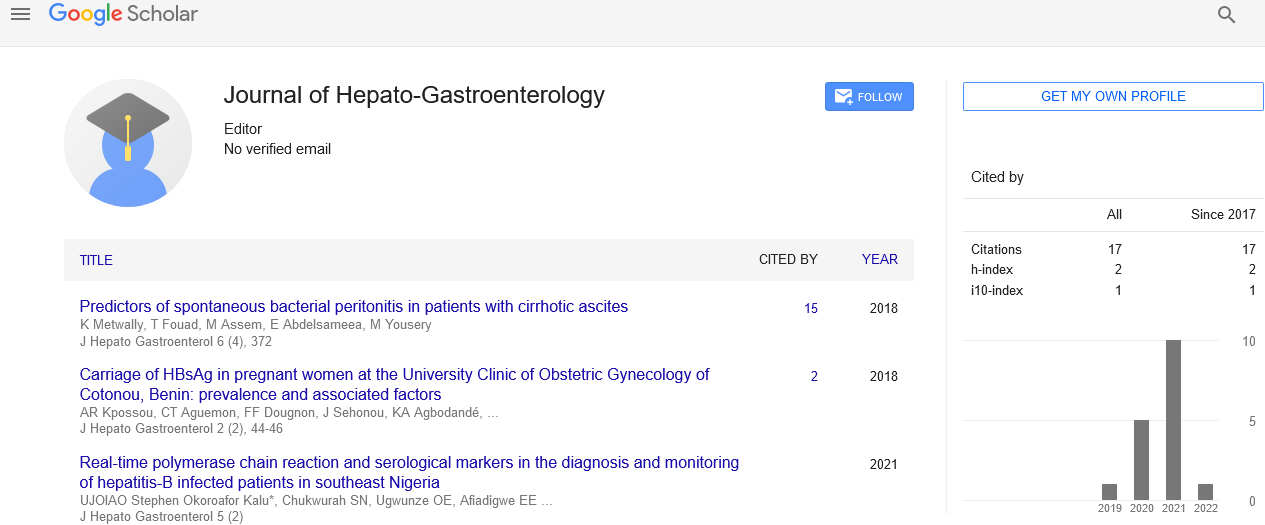
Globally, 130–150 million people have chronic hepatitis C virus (HCV) infection, 11 million of infected people are younger than 15 years of age, of whom 5 million are viraemic [1]. After the universal screening of blood products, the leading source of infection in childhood is mother-tochild transmission in developed countries, and horizontal transmission in low-income and middle-income countries [2]. While the prevalence of HCV in Qatar is 0.4-0.8%, reaching up to 8.7% in dialysis patients [3], little data are known about HCV infection in children, especially in KD subgroup. In the last 4 years, interferon-free direct-acting antiviral therapies have been approved, and several combinations have been studied in dialysis patients. The highest rate of eradication of HCV infection in patients with end-stage renal (ESRD) was reported with the second generation directlyacting antivirals (DAAs). Those patients were previously considered “difficult to treat“. In a recent report, the combination of Paritaprevir/Ritonavir/ Ombitasvir (Viekirax), which is eliminated through the biliary system, shows higher rate of efficacy and safety in patients with chronic kidney disease (CKD) stage 4 or 5, whether cirrhotic or non-cirrhotic [4]. Paritaprevir inhibits the HCV NS3/4A protease and ombitasvir is an inhibitor of NS5A, both working to prevent viral replication. Although HCV remains an important consideration in pediatric ESRD, and HCV infection may affect the renal replacement plan and outcome, the current guidelines do not apply directly and completely to this specific age-group population. The United States Food and Drug Administration (FDA) recently approved sofosbuvir and the fixed-dose combination of ledipasvir/sofosbuvir for the treatment of hepatitis C virus infection in children ages 12 to 17 [5]. Also, high rate of cure with high safety was reported in children with hepatitis C, aged from 6 to 11 years, who were treated with a half-strength tablet of sofosbuvir/ ledipasvir. Up-to-date, no satisfactory data are published on the use of Viekirax in pediatrics with severely decreased kidney function. We report the cure of the youngest hemodialysis girl with chronic HCV infection on the new medication.
Case Summary
A 9-year-old Egyptian girl with ESRD secondary to focal segmental glomerulonephritis (FSGS) and steroid resistant Nehrotic syndrome (SRNS). The diagnosis of nephrotic syndrome was in March, 2016 was not responding to 2 months full dose of steroid therapy. A renal biopsy was done and showed FSGS. Whole Exome Sequencing. (WES) did not detect a variant in genes known to cause FSGS, however CD2AP gene: heterozygous variant of uncertain significance (VUS) is inherited from the father. The patient initial course was grave; her severe edema was not responding well to albumin and diuretics. Beside the high dose corticosteroid, three potent immune suppressors were tried sequentially without any benefit; these medications included mycophenolate mofetil (MMF), Cyclosporine and Rituximab. The child progressed gradually towards chronic kidney disease with the decline of GFR to 29 ml/min/1.73 m2 (stage IV CKD) and started dialysis in 22/1/17. She has severe hypertension, which is controlled with 2 drugs Amlodipine and Enalapril and increase in cholesterol (aggravated with HCV treatment). She undergoes hemodialysis 3 times per week, each session lasts for 3 hours and her dry weight is 20.5 kg. The 24 h urine protein was 7.6 mg/24 h. Hepatitis serology was negative for both HBV and HCV till January 2017, but in May 27th,2017, her investigations showed positive serology for HCV, while HBV is negative with a post vaccination protective antibody level 213.09 mIU/ml. She did not report any history of blood transfusion or any incidents with infected needle pricks, but she has H/O of repeated albumin transfusion and her family members were tested negative for HCV. PCR was positive for HCV at a titer of 6,129,839 IU/ml, genotype 1a. Liver biopsy 9/217 showed Grade II/IV, mild to moderate inflammatory activity with some globular component, Stage I/IV fibrosis (according to Scheuer Score). Abdominal ultrasound showed Normal hepatic echotexture, right hepatic lobe measures 13 cm. No discrete focal lesions, Portal vein is patent and measures 10 mm in diameter and normal spleen, measures 8.5 cm. Liver function showed ALT ranges between 278-414 U/L, AST 289-405 U/L, bilirubin 35 umol/L (direct 22), Alkaline phosphatase 227 U/L, albumin ranging between 14- 19 g/L, cholesterol 8.4 mmol/L, Triglyceride 4.7 mmol/L, creatinine 307 umol/L, INR 0.9, Hemoglobin 10.2, platelet 337, serum ferritin 1,524 Mc GM/L. Serology for HIV, CMV, EPV, HAV, HEV were all negative and she was tested negative for cryoglobulin. In spite of clear explanation to the family that the DAAs has not yet reported safe in children below 14 years and body weight less than 25 Kg, the family insisted to try the medical treatment to proceed for kidney transplantation and hoping that she may improve, if renal impairment is HCV-related. After the family signed a consent, she started treatment with Viekirax/Exviera 17/7/17, with close monitoring 3 times/week during dialysis and weekly follow up at Hepatology clinic. The patient tolerated the treatment well, she was asymptomatic and no clinical or laboratory adverse effects were reported during the course of therapy. The patient showed decline in viral load, with negative PCR by week 4 and follow up PCR at the end of the course and 12 and 24 weeks post treatment, showed complete sustained viral eradication with improvement in liver function: bilirubin 11, albumin 27, ALT 9, AST 12, Alkaline phosphatase 337 ( mainly of bone origin), serum ferritin 300, Vitamin D 32 ng/ml. There is no change in kidney function after viral eradication, except for decrease prtoeinuria, where 24 h urine protein declined from 7.6 to 5.26 mg/24 h.
Discussion
A recent systematic review of HCV antibody seroprevalence in children, estimated that 13.2 (11.5–21.2) million children aged between 1 and 15 years are HCV infected worldwide [6], and the prevalence was higher among children with renal failure requiring hemodialysis and those treated for malignancy [7]. It was reported that the prevalence of HCV is as high up as 18% in children with CKD have hepatitis C infection in Egypt and that the longer the duration of renal disease, the more likely to be positive for HCV [8]. Also, HCV causes adverse effects leading to the poor longterm outcome in renal transplant recipients. HCV infection increases the risk of renal impairment with higher risk of morbidity and mortality and, on the other hand, renal impairment, especially stage 4-5 CKD, increases the incidence of HCV. This reciprocal negative influence emphasizes the need for priority access to oral antiviral drugs in these patients. Every HCVinfected patient waiting for kidney transplantation should be considered for antiviral therapy to reduce HCV-associated posttransplant morbidity and mortality. The EMA and the FDA, approved two DAAs combinations for treatment of children with chronic HCV infection, ledipasvir/sofosbuvir and sofosbuvir and ribavirin, and studies have shown that SVR24 corresponds to a definitive cure of HCV infection in 98% to 100% of cases [9]. Viekirax was reported to be safely and effectively used in adult patients with HCV infection and end stage chronic kidney disease [10], even those with factors predicting an unfavorable response to treatment, such as compensated cirrhosis or advanced fibrosis and nonresponse to previous treatment with PEG-IFN plus ribavirin [11]. The Hepatology Committee 0f European Society of Pediatric Gastroenterology, Hepatology and Nutrition 2018 [12], recommended that treatment of children under 12 should be individualized, based on the severity of liver disease and the presence of co-morbid disease. Accordingly, we treated our patient with viekirax, so she can proceed for kidney transplantation. Similar to, premilinary results of 100% cure rate in children aged 6 to 11 years, who was treated with the fixed-dose combination of ledipasvir/sofosbuvir, our 9 years patient showed complete sustained viral eradication [13]. We used a full dose of Viekirax/ Exvira, which is not adjusted to the bodt size which, Where the Pharmacokinetics studies in Patients with Chronic HCV Infection, reported that, no dose adjustments are needed for the 3D regimen in patients with mild or moderate renal impairment [14]. In accordance to Aby et al. [15], In spite of viral clearance, there was no significant differences in renal function decline or clinical improvement, suggests that viral eradication may not be associated with improvement in the progression of renal disease. This can be simply explained that, HCV infection causes membranoproliferative glomerulonephritis (MPGN) in the setting of cryoglobulinemia, which is not the cause in our patient with FSGS. The exact mechanism of HCV-induced kidney injury could be attributed to Immune complex deposition with HCV proteins and anti-HCV antibodies in the mesangium which associated higher proteinuria or HCV induces accelerated atheromatous disease at the kidney level. The effect of viral clearance on kidney disease progression is conflicting. While, a recent study by Emery et al. [16], showed that despite high SVR rates after DAA treatment in patients with HCV associated mixed cryoglobulinemia only 29.4% of symptomatic patients had complete cryoprecipitate clearance, our patient showed decrease in protein excretion. This similar to a meta-analysis, which showed that HCV RNA clearance with interferon based therapy was not associated with a decrease in serum creatinine, however, those who achieved SVR12 did have a decrease in protein excretion [15], +l but, it may be simply explained by the decline in urine output over a period of dialysis. The most common reported adverse events associated with Paritaprevir/ritonavir/ ombitasvir with dasabuvir therapy included nausea, pruritus, insomnia, diarrhea, asthenia, dry skin, vomiting, and anemia, but our patient did not experience any of them [17]. Although, the main concern for DAAs therapy, remains drug-drug interactions, but in our case, no drug interaction noticed. Hematologic adverse events, which were frequently observed among patients with CKD, were not seen in our case.
Conclusion
Our case report demonstrates that treatment with Paritaprevir/ritonavir/ ombitasvir (Viekirax), shows the high safety margin, efficacy and tolerability in Hemodialysis-dependent patient under the age of 12. With implications of effective HCV- DAAs therapies in children, it may be prudent to institute strategies to decrease waiting time and waitlist mortality for HCV+ candidates by using DAAs, which approved in adults, for children on waiting list, with close monitoring. Further studies are needed to increase the body of evidence related to use of the Viekrax with younger children on hemodialysis.
Financial Support
This case has been treated in HMC and all investigations and medication were part of the routine work.
Conflicts of Interest
I declare that the authors have no competing interests or other interests that might be perceived to influence the results and/or discussion reported in this paper.
REFERENCES
- El-Sayed M, Razavi H. Global estimate of HCV infection in the pediatric and adolescent population. J Hepatol. 2015;62:831-32.
- Thursz M, Fontanet A. HCV transmission in industrialized countries and resource-constrained areas. Nat Rev Gastroenterol Hepatol. 2014;11:28-35.
- Sokal E, Nannini P. Hepatitis C virus in children: the global picture. Arch Dis Child. 2017;102:668-71.
- Psaros EA, Brenndörfer ED, Frelin L, et al. Neonatal Exposure to hepatitis C virus antigens in uninfected children born to infected mothers. J Pediatr Gastroenterol Nutr. 2018;66:106-11.
- Chen DS, Hamoudi W, Mustapha B, et al. Strategies to manage hepatitis C virus infection disease burden, J Viral Hepat. 2017;24(2):44-63.
- Sanai FM, Alghamdi AS, Afghani AA, et al. High Efficacy of ombitasvir/paritaprevir/ritonavir plus dasabuvir in hepatitis C genotypes 4 and 1-infected patients with severe chronic kidney disease. Liver Int. 2017.
- Rizza SA, Nehra V, Temesgen Z. Sofosbuvir/ledipasvir fixed-dose combination for treatment of chronic hepatitis C virus infection in children. Drugs Today (Barc). 2017;53:447-51.
- Youssef DM, Abdo H, Alakhras A, et al. Hepatitis C in children with chronic kidney disease: A single-center, Egypt. Saudi J Kidney Dis Transpl. 2017;28:102-6.
- Kohli A, Alshati A, Georgie F, et al. Direct-acting antivirals for the treatment of chronic hepatitis C in patients with chronic kidney disease. Therap Adv Gastroenterol. 2016;9:887-97.
- Ponziani FR, Siciliano M, Lionetti R, et al. Effectiveness of paritaprevir/ritonavir/ombitasvir/dasabuvir in hemodialysis patients with hepatitis C virus infection and advanced liver fibrosis: Case reports. Am J Kidney Dis. 2017;70:297-300.
- Haber B, Alonso E, Pedreira A, et al. Long-term follow-up of children treated with peginterferon and ribavirin for hepatitis C virus infection. J Pediatr Gastroenterol Nutr. 2017;64:89-94.
- Indolfi G, Hierro L, Dezsofi A, et al. Treatment of chronic hepatitis C virus infection in children: A position paper by the hepatology committee of European society of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2018;66:505-515.
- Murray KF, Balistreri W, Bansal S, et al. Ledipasvir/sofosbuvir _ribavirin for 12 or 24 weeks is safe and effective in children 6- 11 years old with chronic hepatitis C infection. J Hepatol. 2017;66:S101.
- Polepally AR, Badri PS, Eckert D, et al. Effects of mild and moderate renal impairment on ombitasvir, paritaprevir, ritonavir, dasabuvir, and ribavirin pharmacokinetics in patients with chronic HCV infection. Eur J Drug Metab Pharmacokinet. 2017;42:333-9.
- Aby ES, Dong TS, Kawamoto J, et al. Impact of sustained virologic response on chronic kidney disease progression in hepatitis C. World J Hepatol. 2017;9:1352-60.
- Emery JS, Kuczynski M, La D, et al. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol. 2017;112:1298-308.
- Smith MA, Lim A. Profile of paritaprevir/ritonavir/ombitasvir plus dasabuvir in the treatment of chronic hepatitis C virus genotype 1 infection. Drug Des Devel Ther. 2015;9:6083-94.