Ubiquitous and Astronomical- COVID -19
Received: 03-Sep-2022, Manuscript No. puljcdt-22-5506; Editor assigned: 05-Sep-2022, Pre QC No. puljcdt-22-5506(PQ); Accepted Date: Sep 23, 2022; Reviewed: 15-Sep-2022 QC No. puljcdt-22-5506(Q); Revised: 21-Sep-2022, Manuscript No. puljcdt-22-5506(R); Published: 28-Sep-2022, DOI: 10.37532/puljcdt.22.4(5).6-8
Citation: Bajaj A., ALS: Ubiquitous and Astronomical- COVID -19.J. clin. diagn. Treat. 2022; 4(5)
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
COVID-19 pandemic is an infective condition engendered by a novel subtype of coronavirus designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) demonstrating progression into severe acute respiratory syndrome along with pneumonia and acute respiratory distress syndrome. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19 is induced by a positive sense, single stranded RNA virus demonstrating genetic similarity to bat coronaviruses. Diffuse alveolar damage (DAD) concurs with disease phase and is designated as exudative phase with hyaline membrane or alveolar proliferation of fibroblasts and fibrotic phase with dense collagenous fibrosis. Fatal COVID-19 exhibits immune mediated organ injury and mortality independent of viral implication along with tissue inflammation and organ dysfunction, non-concurrent with tissue and cellular distribution of SARS-CoV-2. SARSCoV-2 can be optimally discerned with a nasopharyngeal swab, oropharyngeal swab, sputum or Broncho-alveolar lavage subjected to reverse transcription polymerase chain reaction (RT-PCR).
Key Words
coronavirus pneumonia, reverse transcription polymerase, pneumocytes, gastrointestinal symptoms
Introduction
COVID-19 pandemic commenced as an infectious respiratory disease or pneumonia of obscure aetiology from Wuhan, Hubei Province, China. The infective condition is engendered by a novel subtype of coronavirus designated as severe acute respiratory syndrome coronavirus2 (SARS-CoV-2). COVID-19 exemplifies as a viral infection induced by coronavirus SARS-CoV-2 with progression into severe acute respiratory syndrome along with pneumonia and acute respiratory distress syndrome. COVID-19 disseminates through person to person transmission via respiratory droplets as a cougher sneeze. COVID-19 is additionally designated as novel coronavirus pneumonia whereas severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be nomenclated as 2019 novel coronavirus (2019- nCoV). Morphologically, COVID-19 enunciates diffuse alveolar damage corresponding to disease phase as acute or fibrotic with emergence of three distinct configurations of pulmonary injury designated as epithelial, vascular or fibrotic [1-2]. Widespread investigations are recommended in order to identify and isolate suspected or incriminated individuals, a manoeuver which decelerates disease dissemination. Besides, variants of SARS-CoV-2 vaccines can be employed. Definite disease discernment is contingent to isolation of viral RNA by reverse transcription polymerase chain reaction (RTPCR) (1,2).Infection control guidance for healthcare professionals, basics of personal protective equipment (PPE) basics and autopsy guidelines can be obtained from Royal College of Pathologists (RCPath), College of American Pathologists (CAP) and Centre for Disease Control (CDC).
Mild instances of SARS-CoV-2 commonly incriminate upper respiratory tract whereas severe disease is encountered within bilateral pulmonary lobes.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19 is induced by a positive sense, single stranded RNA virus demonstrating genetic similarity to bat coronaviruses. In contrast to SARS-CoV-1 virus, spike surface glycoprotein of SARSCoV-2 virus adheres to host through receptor binding domains of angiotensin converting enzyme 2 (ACE2) abundantly available within type II alveolar cells or pneumocytes and enhances binding affinity. Following adherence of SARS-CoV-2 into target cell, virion expunges RNA into incriminating cell with consequent viral replication and dissemination, thereby inducing infection of additional cells. SARS-CoV-2 engenders various virulence factors which promote viral shedding from incriminated host cells and subsequent inhibition of immune response.
Morbid instances of fatal COVID-19 may exhibit immune mediated organ injury and mortality independent of viral implication along with tissue inflammation and organ dysfunction, which appears non concurrent with tissue and cellular distribution of SARS-CoV-2[1-2]. Onset of clinical symptoms occurs within an average of 5 days. Majority (>90%) of incriminated subjects display clinical symptoms within ~11 days. An estimated ~46% infected individuals are asymptomatic. COVID-19 is exceptionally discerned in children wherein the condition enunciates mild clinical symptoms with decimated hospitalization. Hospitalized subjects commonly depict symptoms as fever, dry cough, dyspnoea, fatigue, myalgia, nausea, vomiting, diarrhea, headache or weakness.
Non classical symptoms manifest as isolated gastrointestinal symptoms, isolated anosmia or ageusia.
COVID-19 may engender diverse phenotypes of severe acute respiratory syndrome with pneumonia or acute respiratory distress syndrome.
Severity of disease encountered is categorized as
1. Mild or absent disease (81%)
2. Severe disease (14%)
3. Critical disease (5%) survival will be longer than anticipated.
Supportive therapy within intensive care units is necessitated in subjects with acute respiratory distress syndrome, acute cardiac injury, acute renal injury and shock. Aforesaid manifestations are accompanied with proportionate mortality of ~ 15 %.
Hospitalized instances commonly delineate complications such as pneumonia, acute respiratory distress syndrome, acute hepatic injury, cardiac injury, elevated troponin, acute heart failure, dysrhythmia or myocarditis.
Pro-thrombotic coagulopathy may induce venous and arterial thromboembolic events such as acute renal injury, acute cerebrovascular disease or shock.
Multisystem inflammatory syndrome simulating Kawasaki’s disease may exceptionally be encountered within children or young adults < 21 years. Grossly, pulmonary oedema or pulmonary consolidation may be observed. Lung weight appears elevated. Focal pulmonary hemorrhage is observed. Pulmonary emboli may be discerned. Pleurisy or pleural inflammation is common. Superimposed secondary bacterial infection is associated with purulent inflammation.
Examination of Broncho-alveolar lavage (BAL) specimen exemplifies an abundance of activated plasma cells.
Alveolar macrophages may display optically clear nuclei or intracytoplasmic nuclear inclusions.
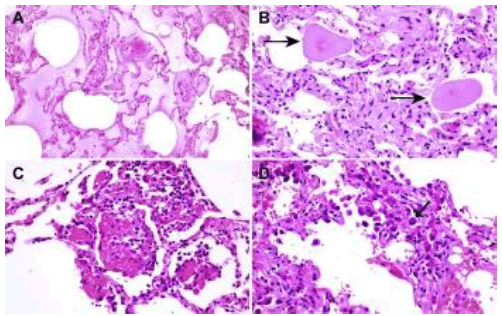
Upon microscopy, COVID-19 predominantly exhibits nonspecific pulmonary manifestations (Figure 1 A-D) [1-2].
Diffuse alveolar damage (DAD) may concur with disease phase and is designated as
1. Exudative phase demonstrating configuration of hyaline membrane, desquamation of pneumocytes, cellular or proteinaceous exudates, hemorrhage within alveolar spaces and fibrinoid necrosis of miniature vascular articulations
2. Organizing phase exemplifies interstitial and intraalveolar proliferation of fibroblasts, infiltration of lymphocytes, hyperplasia of type II pneumocytes and fibrin deposition fibrotic phase denominates densecollagenous fibrosis along with architectural remodeling.
1. Patterns of pulmonary injury are designated as
2. epithelial configuration displaying diffuse alveolar damage with variable organization, denudation and hyperplasia of pneumocytes
3. vascular articulations depicting diffuse intra-alveolar fibrin,microvascular damage, micro-thrombi, acute fibrinous and organizing pneumonia
4. fibrotic variant demonstrating fibrotic diffuse alveolar damage or interstitial fibrosis viral infection induced modifications with intra-nuclear inclusions, enlarged, multinucleated pneumocytes imbued with enlarged nuclei, amphophilic cytoplasm and prominent nucleoli confined within alveolar spaces and superimposed bacterial pneumonia.
5. Extra-pulmonary modifications are denominated as cardiovascular manifestations composed of mild pericardial oedema, serosanguinous pericardial effusion, mild myocardial oedema, low grade interstitial infiltration of mononuclear cells or endotheliitis
6. Widespread systemic vasculitis with accompanying thrombo-emboli may be infrequently discerned.
7. Hepatobiliary lesions delineate hepatic congestion, mild steatosis, patchy hepatic necrosis, Kupffer cell hyperplasia, lymphocytic infiltration within portal tracts and sinusoids or endotheliitis.
8. Renal manifestations are described as variable acute tubular injury, lymphocytic infiltration of tubules and interstitium, fibrin or hyaline thrombi within vascular articulations, dilatation of glomerular capillaries or lymphocytic endotheliitis.
9. Gastrointestinal features are composed of epithelial damage, prominent endotheliitis or ischemic enterocolitis.
10. Spleen depicts decimated lymphocytes, splenic necrosis, atrophy, congestion, haemorrhage or infarction.
11. Bone marrow exhibits histiocytic hyperplasia or haemophagocytosis. Additional manifestations may appear within cutaneous surfaces or prostate along with inflammation and blood clots confined to placenta with funisitis.
Ultrastructural examination depicts spherical particles of 60 nm to 140 nm magnitude. Virion surface exhibits distinctive spikes of 9 nm to 12 nm demonstrating a ‘solar corona’ compatible with Coronaviridae family. Respiratory epithelial cells depict intracytoplasmic inclusion bodies incorporating viral particles confined to membrane bound vesicles.
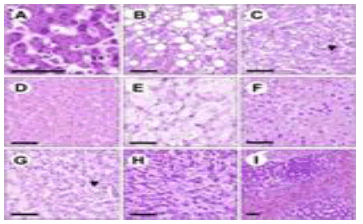
SARS-CoV-2 antigen confined to pneumocytes, ciliated or epithelial cells of upper respiratory tract within the acute phase can be appropriately discerned with In Situ Hybridization (ISH), Fluorescent In Situ Hybridization (FISH) and RNA In Situ Hybridization (RISH). Diffuse alveolar damage induced on account of SARS-CoV-2 infection simulates diffuse alveolar damage engendered due to diverse infections as Cytomegalovirus (CMV), Respiratory Syncytial Virus (RSV) or Herpes Simplex Virus (HSV) inducing viral pneumonias. Besides, segregation is required from conditions such as severe acute respiratory syndrome, acute respiratory distress syndrome of varied aetiology or idiopathic acute interstitial pneumonia. SARS-CoV-2 can be optimally discerned with the recommended nasopharyngeal swab. Alternatively, an oropharyngeal swab, sputum or broncho-alveolar lavage may be employed for viral detection, samples which are subjected to reverse transcription polymerase chain reaction (RT-PCR) Figure 2 (A-I)).
Infection with SARS-CoV-2 is associated with decreased serum albumin, elevated C reactive protein, (CRP), elevated lactate dehydrogenase (LDH), elevated D-dimer, elevated erythrocyte sedimentation rate (ESR) and lymphopenia. Plain radiographs frequently depict ground glass opacities, an aberrant ‘paving’ pattern and consolidation within bilateral pulmonary lobes. Preliminary radiographs may be normal. Radiographic anomalies are discernible within two weeks following disease onset. COVID-19 can be appropriately managed at home which is recommended in individuals with mild clinical symptoms. Optimal duration of home isolation is variable although period of viral shedding necessitates isolation, at around ~ 20 days following disease onset within hospitalized subjects. Instances of severe infection necessitate hospitalization along with oxygenation which may be low dose oxygen supplementation or invasive ventilation with extracorporeal membrane oxygenation (ECMO). Besides, antiviral drugs as ribavirin, favipiravir or remdesivir may be beneficially adopted. Dexamethasone may be optimally utilized in individuals upon ventilator or requiring supplemental oxygen [3-6]. Factors contributing to emergence of acute respiratory distress syndrome and inferior disease outcomes are Age of incriminated subject > 65 years Comorbid conditions as diabetes mellitus or secondary infection. Factors contributing to disease progression are incrimination of male subjects beyond > 65 years and cigarette smoking.
Factors inducing disease associated mortality are
1. cardiovascular disease
2. respiratory disease
3. diabetes mellitus
4. Hypertension .
Conclusion
SARS-CoV-2 antigen confined to pneumocytes, ciliated or epithelial cells of upper respiratory tract within the acute phase can be appropriately discerned with in situ hybridization (ISH), fluorescent in situ hybridization (FISH) and RNA in situ hybridization (RISH). Diffuse alveolar damage induced on account of SARS-CoV-2 infection requires segregation from diverse infections as cytomegalovirus (CMV), respiratory syncytial virus (RSV) or herpes simplex virus (HSV) inducing viral pneumonias and conditions such as severe acute respiratory syndrome, acute respiratory distress syndrome of varied aetiology or idiopathic acute interstitial pneumonia. Infection with SARS-CoV-2 is associated with decreased serum albumin, elevated C reactive protein, (CRP), elevated lactate dehydrogenase (LDH), elevated D-dimer, elevated erythrocyte sedimentation rate (ESR) and lymphopenia. Severe infection necessitates hospitalization and oxygenation as low dose oxygen supplementation or invasive ventilation with extracorporeal membrane oxygenation (ECMO). Antiviral drugs as ribavirin, favipiravir or remdesivir may be therapeutically employed.
References
- Cascella M, Rajnik M, Aleem A et al. Features, evaluation, and treatment of coronavirus (COVID-19)
- Biancolella M, Colona VL, Mehrian-Shai R et al. COVID-19 2022 update: transition of the pandemic to the endemic phase. Human Genomics. 2022;16(1):1-2.
- Alexandridi M, Mazej J, Palermo E et al. The coronavirus pandemicâ??2022: viruses, variants & vaccines. Cytokine & growth factor reviews. 2022.
- Aimrane A, Laaradia MA, Sereno D et al. Insight intoCOVID-19â??s epidemiology, pathology, and treatment. Heliyon. 2022:e08799.
- Borczuk AC, Salvatore SP, Seshan SV et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Modern Pathology. 2020;33(11):2156-68.
- Hou YJ, Okuda K, Edwards CE et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020; 182(2):429-46.