Unusual Presentation of a Uterine Leiomyosarcoma with Predominant Metastasis to the Liver: A Case Report with Gross and Histological Validation
2 Des Moines University, College of Osteopathic Medicine, United States
3 University of Nebraska Medical Center, College of Medicine, Department of Genetics, Cell Biology, and Anatomy, United States
Received: 04-Sep-2023, Manuscript No. ijav-23-6713; Editor assigned: 06-Sep-2023, Pre QC No. ijav-23-6713; Accepted Date: Sep 29, 2023; Reviewed: 20-Sep-2023 QC No. ijav-23-6713; Revised: 25-Sep-2023, Manuscript No. ijav-23-6713; Published: 29-Sep-2023, DOI: 10.37532/1308-4038.16(9).300
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Uterine Leiomyosarcoma (uLMS) are malignant mesenchymal tumors known to metastasize preferentially to the lungs. The present study concerns a female human body donor of postmenopausal age with known diagnosis of uLMS. Upon dissection and gross examination, an enlarged uterus and tumor-ridden liver were observed, however the lungs appeared typical except for a single palpable nodule on the superior lobe of the left lung. Immunohistochemical analysis of the liver tumors confirmed their myogenic origin and thus suggested uLMS distant metastasis, but the analyses of the lung nodule showed typical tissue architecture with no presence of myosin or actin. Illustrating gross and histological analyses of this unusual case with the relevant clinical information may assist biomedical research scientists, inform medical educators, and help physicians better understand uLMS with this metastatic pattern.
Keywords
Uterine Leiomyosarcoma; Metastasis; Immunohistochemistry; Myosin and actin; Gross anatomy
INTRODUCTION
Uterine Leiomyosarcoma (uLMS) are rare, aggressive tumors that arise from smooth muscle in the uterine wall. They are the most common malignant gynecologic mesenchymal neoplasm [1], affecting an estimated 0.36-0.86 per 100,000 women [2-4] and accounting for approximately 1% of all uterine malignancies [5-7]. Age (≥ 50 years) [2, 5] [8-10] and postmenopausal state [11] are known risk factors for uLMS, and a tumor size of less than 5 cm is associated with improved survival rate [12]. A confident preoperative diagnosis of uLMS is challenging however, even in characteristically typical cases, due to the disease’s histopathological and symptomatic similarities with uterine leiomyomas (benign fibroids of the myometrium) [1, 2, 4, 7] and smooth muscle tumors of uncertain malignant potential (STUMP) – a broad group of uterine neoplasms that do not meet the current histological criteria for a diagnosis of either benign or malignant tumor [10, 13, 14]. While some individuals with uLMS are asymptomatic [10], others have reported symptoms of uncharacteristic vaginal bleeding, palpable pelvic masses, and pelvic pain [6, 12] each of which can also be associated with uterine leiomyomas and STUMP. Resistance to treatment, recurrence, and dormancy also contribute to the generally poor prognosis of uLMS [1], and its mimicry with uterine leiomyomas often leads to delayed diagnosis and treatment for over 50% of affected women [2]. Relatedly, research has also shown that uterine leiomyomas can transition into uLMS over time [6], and although this manifestation is quite rare, it has been shown to occur in 0.2% of cases [15].
Distant metastasis of uLMS is common even in its early stages [8], and the predominant site for initial uLMS metastasis is the lung [5]. The relatively direct vascular connection between the uterus and lungs readily allows for metastatic spread via hematogenous route [5, 7] which also explains the strong correlations of successive metastasis from lung to other hematogenous sites [8] such as the liver [5]. Therefore cases of uLMS that metastasize via the hematogenous route but do not affect the lungs pose particular interest and value to educational and clinical literature. The purpose of this article is to report about a non-typical case of uLMS metastasis that predominantly metastasizes to the liver via hematogenous route without affecting the lungs. Accompanied by gross and histological validation, this report provides additional depth to the existing literature about uLMS that may serve to benefit scientists, educators, students, clinicians, and patients.
METHODS
The uLMS, liver tumors, and lung nodule were discovered during routine academic dissection of a human body donor acquired from an ethically approved anatomical donor program. The pathologies were photographed (iPad Pro, Apple Inc.). Tissue samples (10 mm3) were collected from the uterus, liver, and lung pathologies, embedded in paraffin, sectioned at 5 μm, and deparaffinized (Safe Clear, Fisher HealthCare). Each histological section was stained with hematoxylin & eosin (H&E) to examine tissue organization, cell shapes and sizes, mitotic figures, and vascularity. Immunohistochemical stains for smooth muscle actin (SMA), smooth muscle myosin (SMM), and nuclear protein Ki67 were also performed on the identified pathologies to determine uLMS metastasis and intensity of cell proliferation. Tissue sections were imaged at 200X and 400X with a Zeiss Axio Observer.Z1 light microscope, Axiocam 105 color camera, and ZEN 2.3 pro imaging software.
CASE REPORT
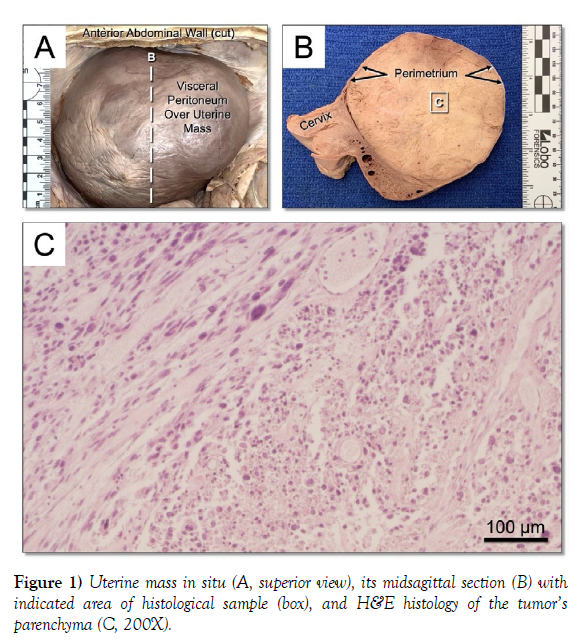
During dissection, a large uterine mass was observed occupying most of the pelvic cavity (Figure 1A). Removal and mid-sagittal sectioning of the uterus revealed a 10.5 cm spherical mass beneath the visceral peritoneum, contained by the perimetrium (Figure 1B). The tumor’s parenchyma showed atypical cell shapes and sizes, mitotic figures, and dense disorder (Figure 1C). Gross evaluation of the cervix, vagina, bladder, rectum, overlying intestines, and contiguous anatomy of the pelvic cavity was unremarkable, suggesting no involvement of the uLMS disease on these tissues. Additionally, gross and histological inspection of the obturator, internal iliac, external iliac, and periaortic lymph nodes, which are known to carry lymph from the uterus, were also unremarkable.
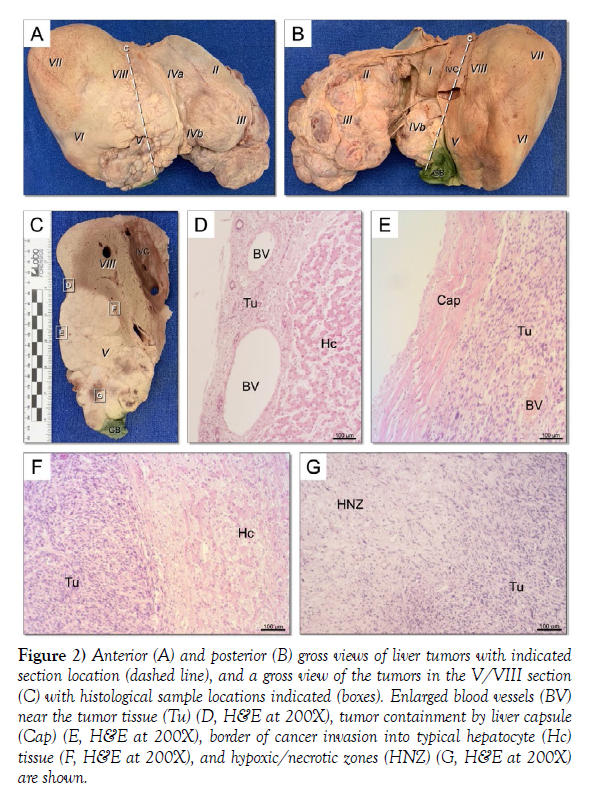
Also noted during dissection were many tumors on the periphery of the liver which primarily localized to segments II, III, IVb, and V (Figure 2 A - B). Many of the tumors were inwardly invasive (Figure 2C). The tumors were inconsistent in size, shape, color, and vascularity; some were dense while others were cyst-like, containing what appeared to be pockets of blood. Histological inspection showed large peripheral blood vessels (Figure 2D) and tumor containment by the liver capsule (Figure 2E). Regions of disorganized cellular tumor invasion into the organized liver tissue were clear (Figure 2F), as were hypoxic/necrotic zones within the tumors where the cancer had apparently starved the region of necessary elements (Figure 2G). The surrounding structures in the abdominal cavity including the gallbladder, inferior vena cava, associated ligaments, diaphragm, abdominal wall, and adjacent parts of the gastrointestinal tract were carefully and thoroughly inspected for disease impact but were unremarkable.
Figure 2) Figure 2) Anterior (A) and posterior (B) gross views of liver tumors with indicated section location (dashed line), and a gross view of the tumors in the V/VIII section (C) with histological sample locations indicated (boxes). Enlarged blood vessels (BV) near the tumor tissue (Tu) (D, H&E at 200X), tumor containment by liver capsule (Cap) (E, H&E at 200X), border of cancer invasion into typical hepatocyte (Hc) tissue (F, H&E at 200X), and hypoxic/necrotic zones (HNZ) (G, H&E at 200X) are shown.
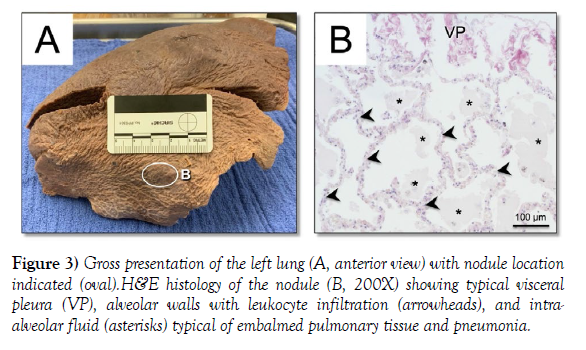
Upon initial inspection, no tumors were noted during initial dissection of the thoracic cavity. Given the substantial size and number of tumors found in the uterus and liver and how typical hematogenous metastasis characteristically involves the lung, the lungs were thoroughly reexamined with gross visual assessment, palpation, serial sectioning, and histological inspection for any signs of metastasis from the uterus or liver tumors. During this process, a single 2 cm nodule was discovered on the superior lobe of the left lung (Figure 3A). Despite the nodule being palpably denser than its surrounding tissue, its cross section revealed sponge-like consistency typical of lung tissue. Histological inspection of the nodule revealed typical post-embalmed alveolar tissue and leukocyte infiltration from pneumonia (Figure 3B) which was known as a contributor to the donor’s death. Gross and histological examination of serial sections of both lungs confirmed no additional pathologies of interest were present.
Figure 3) Gross presentation of the left lung (A, anterior view) with nodule location indicated (oval).H&E histology of the nodule (B, 200X) showing typical visceral pleura (VP), alveolar walls with leukocyte infiltration (arrowheads), and intraalveolar fluid (asterisks) typical of embalmed pulmonary tissue and pneumonia.
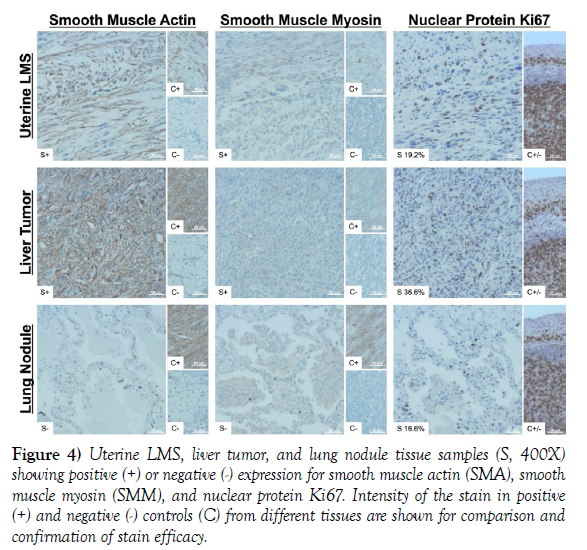
To validate suspected uLMS hematogenous metastasis to the liver without affecting the lungs, immunohistochemical analysis was performed on the uLMS tumor, liver tumor, and lung nodule. As liver and lung tissues are not myogenic, smooth muscle actin (SMA) and smooth muscle myosin (SMM) were targeted to validate if the liver tumor and/or lung nodule were derived from uLMS metastasis. Nuclear protein Ki67, a nuclear antigen marker used to ascertain tumor proliferation rate, was also quantified to determine intensity of cell proliferation in the uLMS tumor, liver tumor, and lung nodule. Results of the immunohistochemical analyses showed that the liver tumor cells expressed SMA and SMM and were highly proliferative, whereas the lung nodule was negative for SMA and SMM. High cellular proliferation was observed in the lung tissue but was consistent with leukocyte infiltration due to pneumonia (Figure 4).
Figure 4) Uterine LMS, liver tumor, and lung nodule tissue samples (S, 400X) showing positive (+) or negative (-) expression for smooth muscle actin (SMA), smooth muscle myosin (SMM), and nuclear protein Ki67. Intensity of the stain in positive (+) and negative (-) controls (C) from different tissues are shown for comparison and confirmation of stain efficacy.
DISCUSSION
The regular functions of the liver and uterus would have undoubtedly been diminished by the invasive uLMS. The cancer had invaded and destroyed much of the liver, and while some liver tissue appeared spared, its function could have been affected by obstructed vessels that carried arterial blood to or venous blood and bile from the tissue. As the donor was of postmenopausal age (≥ 51 years in the United States) [11] the sheer size of the tumor on the uterus was more concerning than its effect on uterine function. As the pelvis is enclosed by bone and contains many important blood vessels (e.g., internal iliac arteries/veins and their branches) and passageway organs (e.g., cervix, vagina, ureters, bladder, urethra, rectum), an expansive tumor in this confined area may obstruct these critical structures and consequently affect upstream and downstream tissues. The size of the initial uLMS tumor in the present study (10.5 cm) was consistent with that reported by Tirumani et al. who reported a mean tumor size of 10.9 cm among 113 patients with uLMS [5]. In the present study, the authors noted some disfiguration in the anatomy immediately adjacent to the uterus and liver due to the space demanded by the uLMS masses; however, their lumens were patent, their tissues contained no visible deposition of uLMS metastasis, and no upstream or downstream tissues appeared to be affected. Lastly, the single nodule on the lung would have presumably caused no significant changes in overall lung function. However, it is noteworthy that uLMS metastases to the lung (74% common) are known to appear as nodules [5].
It is well recognized that uLMS is associated with poor prognosis even without metastasis [6]. Diagnostic imaging techniques such as trans-vaginal ultrasound (typically the first-choice imaging modality) [3] or computed tomography (primarily used for staging purposes and to exclude distant recurrence post-therapy) [3, 14] have limited contribution to uLMS diagnosis. Specific MRI set ups may allow distinction between uLMS and benign fibroids. Generally, uLMS presents as a solitary heterogeneous and poorly demarcated mass [12, 15], much like it did grossly in the present study. The utilization of diffusion-weighted imaging can potentially delineate malignant lesions as hyper intense areas with improved tissue contrast [14, 15]. However, it is suggested that contrast-enhanced MRI produces improved diagnostic accuracy and specificity in differentiating uterine LMS, uterine leiomyomas, and STUMP compared to diffusion-weighted imaging [14]. Although radiological methods used to characterize uLMS have improved, overlap between lesions still occurs [15]. Improved histological analyses of uLMS and standardized diagnostic measurements may help determine malignancy and improve prognosis [16].
Histological inspection of the liver and uterus tumors in the present case revealed similarly disorganized tissues with many unidentifiable cells in variable shapes, sizes, and states of mitosis. Mitotic index is an important histological measure for diagnosing uLMS and a strong prognostic parameter in early tumor stage [17]. The Stanford criteria for histological diagnosis of uLMS requires the presence of at least two of the following characteristics: mitotic index ≥ 10 per 10 high power fields, moderate to severe cellular atypia, and presence of areas of coagulative tumor cell necrosis [6, 10, 14, 18]. The latter two were evident in the present case report, and while the authors were unable to attain the mitotic index for the tumors, a generally high state of cellular proliferation was clear, and high levels of Ki67, as shown in the present study, have also been shown to indicate uLMS in comparative studies [6, 14].
Aside from the given diagnosis of uLMS, validation of uLMS metastasis was gained with immunohistochemical analyses. While the uterus is myogenic in origin but the liver and lungs are not, positive findings for SMA and SMM in the liver tumors confirmed that metastatic uLMS is a plausible and probable cause of the liver tumors but not the lung nodule. If the uLMS had first spread via direct route to a contiguous tissue in the peritoneal cavity as reported as most common in a study by Rose et al. [8], hematogenous spread to the liver could have occurred via continued direct route or via the portal system without passing through the heart and lungs. While peritoneal uLMS metastasis was not apparent anywhere other than the liver, it is worth acknowledging that tumor development and aggressiveness is known to be influenced by the preferential relationship between cancer cell signaling mechanisms (e.g., Ki67, p53, caspase 9) and host tissue microenvironments [1] which could have conceivably prevented large tumor development despite cancer presence in the tissues. Even so, the authors ruled out direct-route cancer cell migration from the conclusion of this case since careful and thorough inspection of contiguous anatomy to the uterus, liver, and lung was unremarkable for any signs of uLMS metastasis. Spread via lymphatic channels was also ruled out after unremarkable gross and histological inspections of the major lymph nodes known to carry lymph from the uterus.
This case therefore demonstrates a particularly rare and interesting case where uLMS aggressively metastasized to the liver via validated hematogenous route after passing through the predominant site for distant metastasis [5] and first-pass organ for subsequent hematogenous metastases [5, 8] – the lung – without affect.
Standard treatment of uLMS involves hysterectomy [2, 5, 7, 9] with adjuvant pelvic radiation and/or chemotherapy [7, 9]. Bilateral saplingo-oophorectomy [5, 7], lymphadenectomy and omentectomy [2] are also considered. For the present case, however, no evidence of surgical treatment was indicated by postmortem inspection. Metastatic sarcomas generally respond poorly to radiation and chemotherapy, and approximately 20% recur [19]. Even if the cancer has not metastasized, the risk or recurrence within 2-5 years is 40-70% [6, 18]. Five-year survival rates are generally poor (15% to 35%) [2, 5], but prognosis can be influenced by demographic factors (e.g., age [2, 5, 8, 9], race [5]), histopathological factors (e.g., FIGO tumor stage [5, 9], tumor grade [5], tumor size [5, 6], mitotic index [5, 9], tumor necrosis [5, 9], lymphovascular invasion [5]), treatment (e.g., modality, success) [2, 9], and combinations therein. Expression of estrogen receptors, progesterone receptors, and androgen receptors has also been reported in approximately 30-70% of cases and may have prognostic significance [6, 20].
The underpinning of oncogenic mechanisms that develop uterine Leiomyosarcoma involves a vast field of dedicated research, yet because of the wide variety of sarcoma subtypes and variability in response to therapies, treatment of sarcomas continues to pose a large therapeutic obstacle [6, 19]. Gross and histopathological analyses of relevant cases are scarcely reported, especially for rare, atypical presentations of uLMS distant metastasis. The information in this report provides additional gross and histological depth to the existing uLMS literature and may assist biomedical research scientists, inform medical educators, and help physicians diagnose uLMS with this metastatic pattern and choose the most-effective patient treatment options.
ACKNOWLEDGEMENTS
Ethical statement
The human body donor was obtained from an ethically approved Anatomical Donor Program. The donor provided personal, conscious and cognizant, premortem informed consent to anatomy education and research. The study of cadaveric donors at the University of Nebraska Medical Center is classified IRB exempt, UNMC Policy 8007. The authors elected to not disclose the exact age of the donor to protect the integrity of the donor and since only a descriptive reference of age was relevant to the study.
Authorship contributions
Each author has made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Conflict of Interest
The authors have no conflicts of interest.
Gratitude
The authors wish to sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can improve patient care and increase mankind’s overall knowledge. Therefore, these donors and their families deserve our highest gratitude.
REFERENCES
- Singh Z. Leiomyosarcoma: A rare soft tissue cancer arising from multiple organs.J Cancer Res Pract. 2018; 5(1):1-8.
- Skorstad M, Kent A, Lieng M. Uterine Leiomyosarcoma – incidence, treatment, and the impact of morcellation. A nationwide cohort study.Acta Obstet Gynecol Scand. 2016; 95(9):984-990.
- Sun S, Bonaffini PA, Nougaret S, Fournier Let al.How to differentiate uterine Leiomyosarcoma from leiomyoma with imaging.Diagn interv imaging. 2019; 100(10):619-634.
- Wang L, Li S, Zhang Z, Jia J et al. Prevalence and occult rates of uterine Leiomyosarcoma.Medicine (United States). 2020; 99(33).
- Tirumani SH, Deaver P, Shinagare AB, Tirumani Het al.Metastatic pattern of uterine leiomyosarcoma: Retrospective analysis of the predictors and outcome in 113 patients.J Gynecol Oncol. 2014; 25(4):306–312.
- D’Angelo E, Prat J. Uterine sarcomas: A review.Gynecologic Oncology. 2010; 116(1):131–139.
- El-Khalfaoui K, Bois A, Heitz F, Kurzeder C et al. Current and future options in the management and treatment of uterine sarcoma.TherAdvMedOncol. 2014; 6(1):21–28.
- Rose PG, Steven Piver M, Tsukada Y, Lau T. Patterns of metastasis in uterine sarcoma. An autopsy study.Cancer. 1989; 63(5):935–938, 1989.
- Momtahan M, Emami F, Aslani FS, Akbarzadeh-Jahromi M. Evaluation of treatment results and prognostic factors of uterine sarcoma: A single-center experience.J Chin Med Assoc. 2020; 83(1):84–88.
- Juhasz-Böss I, Gabriel L, Bohle RM, Horn LC et al. Uterine Leiomyosarcoma.Oncol Res Treat. 2018; 41(11):680–686.
- Zhang G, Yu X, Zhu L, Fan Q et al. Preoperative clinical characteristics scoring system for differentiating uterine leiomyosarcoma from fibroid.BMC Cancer. 2020; 20(1).
- Huang YT, Huang YL, Ng KK, Lin G. Current status of magnetic resonance imaging in patients with malignant uterine neoplasms: A review.KJR. 2019; 20(1):18–33.
- Hughes L, Roex A, Parange A. STUMP, a surprise finding in a large fibroid uterus in a 20-year-old woman.Int J Womens Health. 2018; 10:211–214.
- Cui RR, Wright JD, Hou JY. Uterine leiomyosarcoma: a review of recent advances in molecular biology, clinical management and outcome.BJOG: Int. J Obstet Gynaecol. 2017; 124(7):1028–1037.
- Santos P, Cunha TM. Uterine sarcomas: Clinical presentation and MRI features.Diagnostic and Interventional Radiology. 2015; 21(1):4–9.
- Cree IA, Tan PH, Travis WD, Wesseling Pet al.Counting mitoses: SI (ze) matters!Modern Pathology. 2021; 34:1651–1657.
- Mayerhofer K, Obermair A, Windbichler G, Petru Eet al.Leiomyosarcoma of the uterus: A clinicopathologic multicenter study of 71 cases.Gynecol Oncol. 1999; 74(2):196–201.
- Arend RC, Toboni MD, Montgomery AM, Burger RAet al.Systemic Treatment of Metastatic/Recurrent Uterine Leiomyosarcoma: A Changing Paradigm.Oncologist. 2018; 23(12):1533–1545.
- Damerell V, Pepper MS, Prince S. Molecular mechanisms underpinning sarcomas and implications for current and future therapy.Signal Transduction and Targeted Therapy. 2021; 6(1).
- George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine Leiomyosarcoma.Journal of Clinical Oncology. 2018; 36(2):144–150.
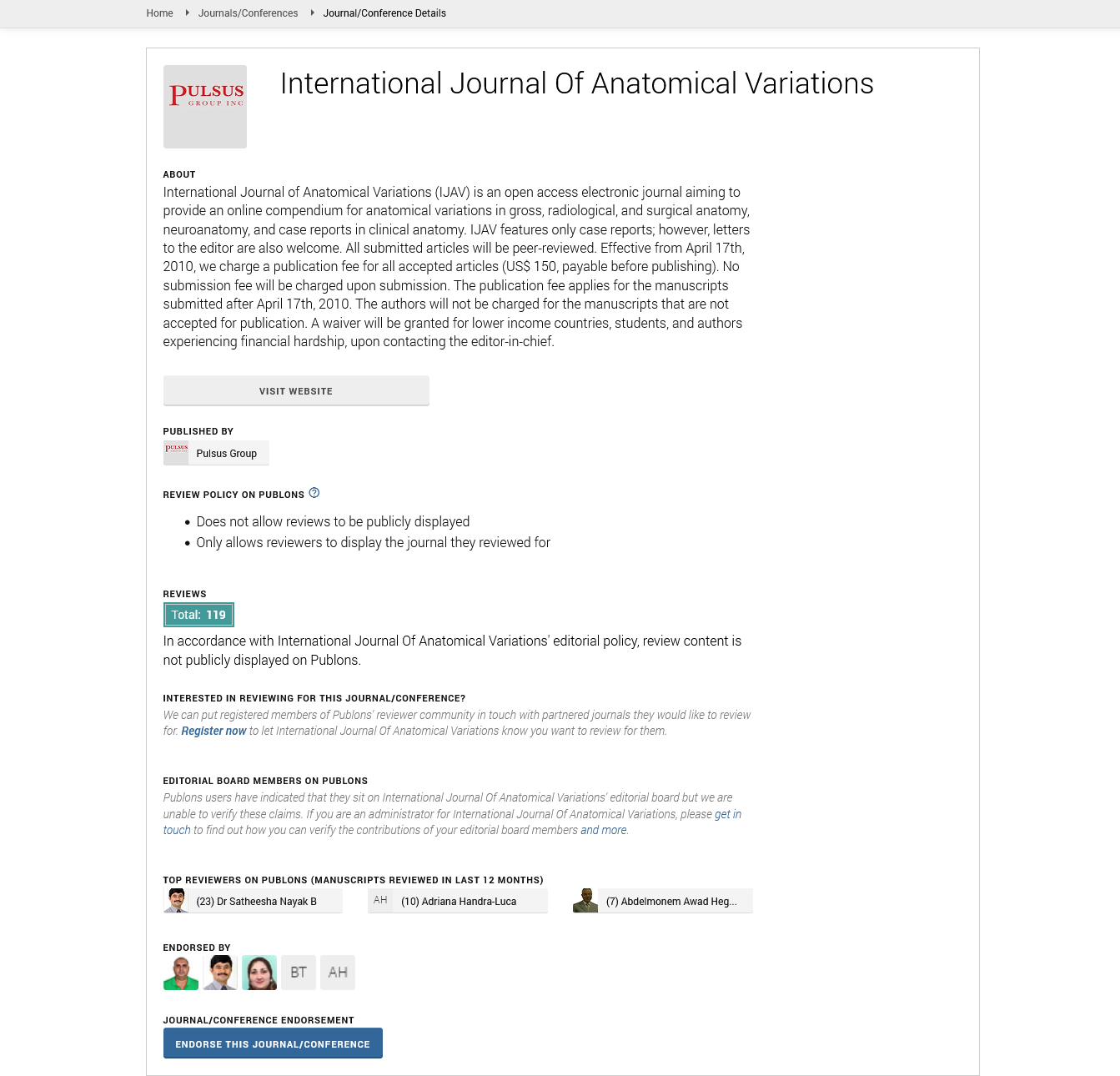
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref