Amnion membrane for the management of fungal bronchial stump infection
2 Department of Economics, Tulane University, New Orleans, Louisiana, USA
3 Tuck School of Business Administration, Hanover, New Hampshire, USA
4 Department of Plastic and Reconstructive Surgery, Weill Cornell Medical College, Houston, Texas, USA
Received: 15-Jan-2018 Accepted Date: Jan 30, 2018; Published: 28-Feb-2018
Citation: Witt JG, Haugh AM, Weaver M, et al. Amnion Membrane for the Management of Fungal Bronchial Stump Infection. Surg Case Rep 2018;2(1):9-11.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The significance of wound complications and infections has become critical in modern reimbursement schema. The management of surgical site infections in high risk surgeries can be challenging, particularly when they are comprised of invasive fungal species. When faced with local tissue necrosis or even surgical dehiscence, there is a myriad of traditional surgical techniques and an expansive array of biologic products that have been described in the management of this potentially devastating complication. This case describes the use of amniotic tissue in the management of a 19-year-old renal transplant recipient with a bronchial stump necrosis after a thoracic decortication and lower lobectomy for an invasive mucormycosis empyema.
Keywords
Amnion membrane; Fungal bronchial stump infection; Mucormycosis
Surgical site infections frequently occur in high-risk surgical patients, such as immunocompromised or immunosuppressed patients [1]. Surgical decortication and lung resection in this setting represents the highest level of complexity. Post-surgical infectious complications significantly increase patient morbidity, mortality, hospital stay, and expense. Resistant and fastidious organisms, including fungal infections, represent the most challenging to eradicate [2-5]. Mucormycosis is an organism that has been increasing in incidence, invading skin, blood, the gastrointestinal tract, and the lungs with mortality rates reaching 76% in pulmonary mucormycosis [4]. The mainstay of therapy, especially in immunocompromised patients, has been long-term antifungal treatment with amphotericin B. Despite aggressive local therapy, mucormycosis can become invasive in the blood or even the pulmonary space, which may result in recurrent lobar collapse and even empyema. Lung surgery in an infected field provides no guaranteed resolution, with a significant possibility of persistent or recurrent infection, air leak, and even bronchial closure dehiscence [5]. The most devastating of these complications is bronchial stump necrosis. Over the last five decades, multiple techniques have been described to address this complication, including rotational muscle, pericardial, and even venous flaps. Despite innumerable techniques and native tissue transfer, little data exists on a superior approach to this problem. Renewed interests in biologic materials including pericardium, dermis, intestinal, urogenital, and amniotic membrane has re-examined the primary or adjuvant use of biologics in at-risk anastomoses. In this report, we review and discuss the potential beneficial antimicrobial, anti-inflammatory, and pro-wound healing properties of amniotic membrane [6-8].
Case Report
A 19-year-old cadaveric renal transplant recipient presented to clinic with nightly high-spiking fevers. His chest X-ray revealed a left lower lobe consolidation. The patient had received thymoglobulin antibody induction at the time of transplantation six months prior and was now on tacrolimus, mycophenolate, and prednisone. He was admitted to the hospital from the clinic and started on vancomycin, piperacillin/tazobactam, and fluconazole. A chest and abdomen computer tomography demonstrated a consolidated lower lobe. Pulmonology was consulted and a bronchoscopy was performed, cultures were obtained, and his post-procedure x-ray demonstrated improved parenchyma. His immunosuppression regimen was discontinued.
Persistent fevers raised concern for a primary fungal infection, so anti-fungal coverage with micafungin was started. Initial cultures identified fungal elements but required further speciation and characterization. Despite the addition of micafungin, the patient continued to spike fevers with lobar collapse. Fungal coverage was increased to amphotericin B. After a week, the fungal culture was identified as mucormycosis. Several attempts at bronchoscopy failed to open the lower lobe. The patient was started on isavuconazonium sulfate in combination with amphotericin B. After failure of repeat bronchoscopy to re-inflate the lung, CT scan re-demonstrated a complete lobar collapse.
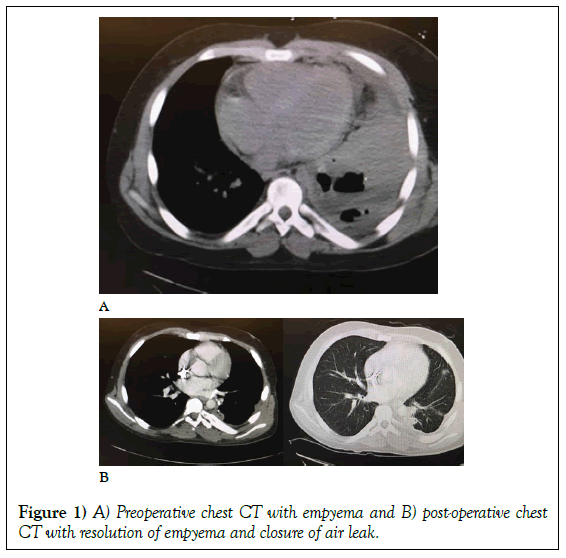
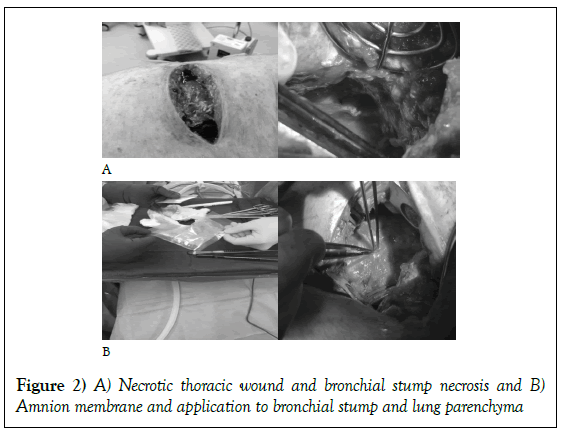
The patient was taken to the operating room for a debridement and a left lower lobectomy. Intra-operatively, the chest wall was debrided, the left lobe was resected utilizing a stapled anastomosis and irrigated with two liters of antibiotic saline. Initially, the patient improved and weaned from the ventilator with a notable decrement in his fever curve. By the sixth postoperative day, the patient began to re-spike fevers to 103°F. His thoracotomy wound had broken down and become purulent with a notable dehiscence. He was taken back to the operating room for re-exploration. His chest was grossly purulent and encased with fibrinous exudate, necrotic muscle, and bronchial stump with persistent bronchial and parenchymal air leak (Figures 1A and 1B).
The chest wall and cavity were aggressively debrided and irrigated with two liters of amphotericin. Consideration of a muscle flap was discussed, but due to the nature of the purulence and muscle necrosis a rotational flap was felt to be futile. In an attempt to control the necrosis, a 25 cm × 25 cm sheet of amniotic membrane was placed over the leaking parenchymal staple line, the necrotic bronchial stump, and the dehisced chest wound (Figures 2A and 2B).
Over the next two weeks, the patient’s fevers resolved and he was easily extubated from mechanical ventilation. The purulence and necrosis of the chest wall resolved with dramatic granulation, the air leak healed, and the chest tube drainage decreased significantly. After the third post-operative week, the patient had both chest tubes removed. His chest wound was closed secondarily and he was discharged home on a six-month course of isavuconazonium sulfate (Astellas, Osaka, Japan). At six weeks, his postdischarge CT scan showed complete resolution of his empyema (Figure 1B). After six months, he has completed his course of antibiotics and is now being considered for repeat renal transplantation.
Discussion
Aggressive localized wound infections can be a significant source of morbidity and mortality after surgery. The incidence of resistant bacterial and even fungal infections has dramatically risen over the last decade [2,9]. Postoperative lung resection patients carry the significant risk of bronchial stump closure failure, necrosis, and dehiscence. [5] Bronchial dehiscence results in a 12% incidence of bronchopleural fistula (BPF) which dramatically increases patient mortality [10]. Traditional approaches to closure and reinforcement of at-risk anastomoses included tissue autografts from the pericardium, rotation muscle flaps, and even adjacent vascular structures [11,12].
Over the last two decades, staple line reinforcement has been the mainstay of preventative strategies, including: Peri-Strips Dry, Alloderm Select, and CorMatrix. Peri-Strips were one of the first clinically available materials. The bovie pericardial patches served as buttress materials but were frequently hand loaded onto each stapler platform [13]. Alloderm Select, represented the next advancement in biologics with the allograft made from processed human dermis. The processing removes cells while retaining essential biologic components and the structural integrity of the dermal matrix. Alloderm Select, is currently produced in two basic forms, cross linked and non-cross linked, both containing metalloproteins.
The next generation biologic was CorMatrix, made of porcine small intestine submucosa, which incorporated extracellular matrix growth factors and cytokines to improve tissue integration and wound healing. Newer generations of these grafts have included antibiotics and growth factors proving to be beneficial in tissue integration and long-term integrity [14]. The most recent approach has been the reintroduction of biologic materials, including amniotic fluid and membrane. These products are derived from human placental tissue, which are comprised of concentrated cytokines, human growth factors, and natural bactericides [6-8].
Amniotic membrane has been employed in the management of ocular injuries, diabetic wounds, and surface burns [6]. It contains both collagen and an extracellular matrix with biologically active cells secreting growth factors and cytokines. The significant benefits of amniotic membrane include its ability to recruit native stem cells to sites of injury while remaining immunogenically privileged [7,8]. This immunologic state is attributed to the placenta’s expression of neonatal HLA molecules, which are not expressed externally. This immunogenicity distinguishes amniotic membrane from other biologics. A recent meta-analysis ascribed amnion’s healing activity to anti-bacterial properties of anti-ferrins and anti-inflammatory cytokines [6].
Currently, there are no randomized controlled trials comparing biologic allografts to amniotic membrane, although there are limited case reports and clinical data describing amnion in thoracic and cardiac surgery. One randomized cardiac bypass study confirmed the benefits of local application of purified reactive protein (PRP) on median sternotomies to decrease infection [15]. One animal study of bronchial stump closure failed to show significant improvement in healing between amniotic tissue and pleural patching [16]. Despite this animal data, other animal and human studies have demonstrated the effectiveness of amniotic membrane in preventing air leakage postthoracic surgery [17,18]. The advantage of amniotic membrane over bovine pericardium may be attributed to the amnion’s bioprotective properties and it’s inhibition of inflammation around the bronchial stump. Amnion has demonstrated the ability to reduce and prevent tissue inflammation, and potentially adhesion formation, in animal models [7,19,20]. Amnion is a unique tissue. The membrane possesses antimicrobial, non-inflammatory and non-immunogenic properties, potentially making this a superior tissues for the prevention or treatment of bronchial stump closure or other surgical wounds. With the diagnosis of an ALT or sarcoma referral to a cancer center with multidisciplinary care is warranted.
Conclusion
The current study is very limited in its ability to ascribe these potentially beneficial properties of amniotic tissue in all settings or patients. Our group has had limited, but successful, use of amniotic tissue and amniotic fluid in improving chronic wound healing, acute and suppurative wounds, treatment of bronchial stumps, thoracic and abdominal wall infections, and management of ischemic rotational flaps. The potentially beneficial properties of amniotic tissue should be examined and pursued as an additional technique for the management of complicated local infections.
REFERENCES
- Akhter MS, Verma R, Madhukar KP, et al. Incidence of surgical site infection in postoperative patients at a tertiary care centre in India. J Wound Care. 2016;25(4):210-2.
- Sanglard D. Emerging threats in antifungal-resistant fungal pathogens. Frontiers in Medicine. 2016;3:11.
- Abe J, Hasumi T, Takahashi S, et al. Fatal broncho-pulmonary artery fistula after lobectomy for lung cancer. J Surg Case Rep. 2015;2015(9).
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis, clinical infectious diseases. 54,1:S23–S34.
- Zajac-Lenczewska I, Czarniak E, Skokowski J, et al. Infections in patients after thoracic surgery]. Pneumonol Alergol Pol. 2003;71(1-2):36-42.
- Tenenhaus M. The use of dehydrated human amnion chorion membranes in the treatment of burns and complex wounds: current and future applications. Ann Plast Surg. 2017;78:S11-S13.
- Ueta M, Kweon MN, Sano Y, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol. 2002;129:464.
- Koob TJ, Lim JJ, Massee M, et al. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-62.
- Ventola CL. The antibiotic resistance crisis: Part 1: causes and threats. Pharmacy and Therapeutics. 2015;40(4):277-283.
- Klepetko W, Taghavi S, Pereszlenyi A, et al. Impact of different coverage techniques on incidence of postpneumonectomy stump fistula. Eur J Cardiothorac Surg. 1999;15(6):758-763.
- Fiorelli A, Frongillo E, Santini M. Bronchopleural fistula closed with cellulose patch and fibrin glue. Asian Cardiovasc Thorac Ann. 2015;23(7):880-883.
- Katoch CD, Chandran VM, Bhattacharyya D, et al. Closure of bronchopleural fistula by interventional bronchoscopy using sealants and endobronchial devices. Med J Armed Forces India. 2013;69(4):326-329.
- Vannucci J, Gervasi GL, Freddolini M, et al. Pericardium matrix buttressing hinders the stapled bronchial stump healing. J Surg Res. 2016;201(2):286-92.
- Deering TF, Chang C, Snyder C, et al. Enhanced antimicrobial effects of decellularized extracellular matrix (CorMatrix) with added vancomycin and gentamicin for device implant protection. Pacing and Clinical Electrophysiology, 2017;40:615–623.
- Serraino GF, Dominijanni A, Jiritano F, et al. Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int Wound J. 2015;12(3):260-264.
- Mohajeri G, Omid M, Melali H, et al. Bronchial stump closure with amniotic membrane in animal model. J Res Med Sci. 2014;19(3):211-214.
- Tsuda T, Nakamura T, Yamamoto Y, et al. Prevention of postoperative air leakage from lungs using a purified human collagen membrane-polyglycolic acid sheet. Ann Thorac Surg. 1999;68(2):339-342.
- Zmijewski M, Pietraszek A. The application of deep-frozen and radiation-sterilized human amnion as a biological dressing to prevent prolonged air leakage in thoracic surgery. Ann Transplant. 2005;10(3):17-20.
- Young RL, Cota J, Zund G, et al. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril. 1991;55(3):624-8.
- Massee M, Chinn K, Lei J, et al. Dehydrated human amnion/chorion membrane regulates stem cell activity in vitro. J Biomed Mater Res B Appl Biomater. 2015 Jul 14.