Biosynthesis of silver nanoparticles as a platform for biomedicinal application
2 Department of Pharmacy, University of Science and Technology, Ibb, Yemen, Email: ayazchem09@gmail.com
3 Aligarh Muslim University, Aligarh, 202 002, India, Email: m.asif.ec@amu.ac.in
4 Department of Applied Physics, ZHCET Aligarh Muslim University, Aligarh, 202 002, India, Email: brijjadon@yahoo.co.in
Received: 01-Aug-2018 Accepted Date: Sep 26, 2018; Published: 30-Sep-2018
Citation: Mashrai A, Dar AM, Sherwani MA, et al. Biosynthesis of silver nanoparticles as a platform for biomedicinal application. J Nanosci Nanomed. September- 2018;2(1):25-33.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The biosynthesis of nanoparticles has been proposed as a cost effective and environmentally benevolent to chemical and physical methods. In the present study, one pot facile green synthesis of silver nanoparticles (AgNPs) has been demonstrated by using powder extract of dry ginger rhizome (Zingiber officinale). The structural analysis has been carried out by using Ultra Violet-visible spectroscopy, X-ray diffraction, scanning electron microscopy, energy dispersive X-ray analysis, transmission electron microscopy, atomic force microscopy, Fourier transform infrared spectroscopy and photoluminescence. The thermal stability has been also investigated by using thermo-gravimetric analysis. The biosynthesized nanoparticles investigated for their in-vitro antimicrobial activity in a dose-dependent manner against different pathogenic organisms under different temperatures and pH. It was noteworthy that AgNPs depicted promising antimicrobial activity. The reduction in the amount of reactive oxygen species (ROS) produced within the micro-organism cells treated with AgNPs and apoptosis have been investigated.
Keywords
AgNPs; In-vitro; Antimicrobial; Apoptosis; ROS
Introduction
Nanotechnology is a multidisciplinary scientific field undergoing explosive development. Promoting nanomaterials for diagnosis, prevention and treatment of diseases caused by microbes is the focus of the recently developing multifunctional nanotechnology [1]. Several classes of antimicrobial nanoparticles have proven their effectiveness for treating infectious diseases caused by the various genera of the pathogenic microorganisms in-vitro as well as in animal models [2]. Although, these metals can generate reactive oxygen species (ROS) in the biological systems [3], ROS are generated in all aerobic microorganisms and are indispensable for signal transduction pathways that regulate cell growth and redox status [4]. However, excess production of these ROS can initiate lethal chain reactions that involve oxidation of various bio-molecules and damage cellular integrity and survival [5-7]. If they can be selectively over produced in pathogenic microbial cells, ROS may exert remarkable antimicrobial potential due to their reaction with vital cellular targets [8]. The strategy of treatment of pathogenic microorganism by the generation of ROS selectively in infected cells (causing maximum pathogen killing without affecting the normal tissues) has been termed “oxidation therapy” [9]. As a broad spectrum antimicrobial agent, silver nanoparticles (AgNPs) are currently one of the most widely used nanomaterials. They are increasingly used in medicinal and consumer products [10,11] including household antiseptic sprays and antimicrobial coatings for medical devices that sterilize air and surfaces [12,13].
Numerous studies are specifically designed for assessing the antimicrobial activity of silver nanoparticles but due to the lack of standardized synthesis protocols, solubilization ligands, dose, coatings and cell systems, it is hard to obtain a straight response. Nevertheless, from this diversity, an implication can securely be made that the protection of silver nanoparticles surface plays a key role in their antimicrobial activity and their action may be because of generation of ROS [14-16]. The plant ginger belonging to the family of Zingeiberaceae and is a common condiment for various foods and beverages. This contains many phytochemicals such as alkaloids, saponins, tannins, and flavonoids which includes Pantothenic acid, Vit B6, Folate, Vit C, Calcium, Iron, Magnesium and Manganese [17].
Keeping in view the aforementioned facts, herein we report green synthesis and structural properties of AgNPs with their in-vitro evaluation against a panel of gram-positive, gram-negative strains of bacteria and some fungal strains under different condition. The role of ROS as well as apoptosis produced by AgNPs has been also studied in this report.
Experimental
Reagents
All reagents were of analytical grade. Silver nitrate and other chemicals used in this study were purchased from SRL, India. The nutrient media and Sabouraud's dextrose (SD) broth were obtained from the HiMedia Laboratories, Mumbai, India. Histidine, 2,7-dichloro fluorescin diacetate (DCFH-DA) was obtained from Sigma-Aldrich (St. Louis, Missouri, USA).
Biosynthesis of AgNPs using plant extract
AgNPs was synthesized following a previous report with slight modifications [17]. Briefly, dry ginger is made into fine powder and 15 g of the powder is taken. The 60 ml of water is added to it and stirred for an hour in a magnetic stirrer. The extract is filtered using Whatman qualitative filter paper, Grade 1, again 40 mL of water is added, stirred again and then filtered. The extract obtained was about 50 mL and light yellow in colour. The extract is heated by stirrer-heater and when it is at 57°C, 5 g of silver nitrate (AgNO3) are added. Soon it turned to yellow then boiled and stirred until it is reduced to a brown. It was carefully collected in a ceramic crucible and heated in an air heated furnace at 400°C for 2 h. A brown colour powder was obtained and collected for characterization purposes.
Characterization
Absorption spectra were measured with UV-Vis spectrophotometer (Perkin Elmer Life and Analytical Sciences, CT, USA) in the wavelength range of A300 to 600 nm. The X-ray diffraction (XRD) patterns were recorded on MiniFlex™ II benchtop XRD system (Rigaku Corporation, Tokyo, Japan) operating at 40 kV and a current of 30 mA with Cu Kα radiation (λ=1.54 Ǻ). The diffracted intensities were recorded from 20° to 80° 2θ angles. The crystalline size (D) of AgNPs was calculated following the Debye- Scherrer formula:
D=0.9λ/β cosθ
Where λ is the wavelength of X-ray, β is the broadening of the diffraction line measured half of its maximum intensity in radians and θ is the Bragg’s diffraction angle. The crystalline size of the AgNPs was determined by employing the full width at half maximum (FWHM) value. Scanning electron microscopy (SEM) was performed using the fine powder of the synthesized AgNPs on a carbon tape in a JSM-6510LV scanning electron microscope (JEOL, Tokyo, Japan). The elemental analysis was determined using the Oxford Instruments INCAx-sight energy dispersive X-ray (EDAX) spectrometer equipped SEM. TEM images were obtained with ultra-high resolution FETEM (JEOL, JEM-2100F) at an accelerating voltage of 200 kV. The thin film of the AgNPs was prepared on the borosilicate glass slide for the analysis of surface morphology. The prepared thin film was analyzed on the Atomic Force Microscope (AFM; Innova SPM, Veeco) in tapping mode. The commercial etched silicon tips as scanning probes with typical resonance frequency of 300 Hz (RTESP, Veeco) were used. The microscope was placed on a pneumatic anti-vibration desk, under a damping cover and analysis was performed using the SPM Lab software. The electron, EDAX and AFM images were obtained and converted into an enhanced meta file format.
For the FTIR spectroscopic measurements AgNPs powder was mixed with spectroscopic grade potassium bromide (KBr) in the ratio of 1:100 and spectra were recorded in the range of 400-4000 wave number (cm-1) on Perkin Elmer FTIR Spectrum BX (PerkinElmer Life and Analytical Sciences, CT, USA). Photoluminescence was measured using a microplate reader (Bio-Rad laboratories Inc., Hercules, CA, USA). The thermal analysis was carried out using thermogravimetric analysis (TGA) (Sieco SII, SSC5100, Instrument) at a heating rate of 20°C min-1 from ambient temperature to 800°C under nitrogen atmosphere.
Antimicrobial activity
In the context of our studies, AgNPs was screened in-vitro for antibacterial activities against the culture of Corynebacterium xerosis (ATCC-373), Streptococcus pyogenes (ATCC-29213), Pseudomonas aeruginosa (ATCC-27853), Kliebsiella pneumonia (Clinical isolate) and Escherichia coli (ATCC-25922) by disk diffusion method [18]. In brief, the microbes were cultured in Muller-Hinton broth at 37°C on an orbital shaking incubator (Remi, India) at 160 rpm. A lawn of bacterial culture was prepared by spreading 100 μL culture broth, having 106 c.f.u/mL of each test organism on solid nutrient agar plates. The plates were allowed to stand for 10-15 min. for culture absorption. The AgNPs was punched into the agar with the head of sterile micropipette tips. Wells were sealed with one drop of molten agar (0.8% nutrient agar) to prevent leakage from the bottom of the plate. Using a micropipette, 100 μL (50 μg) of the AgNPs solution was poured into each of five wells on every plate. After incubation at 37°C for 24 hours, the size of the zone of inhibition was measured. The minimum inhibitory concentration (MIC) was evaluated by the macrodilution test using standard inoculums of 1-2 × 107 c.f.u/mL (0.5 McFarland standards).
Serial dilutions of the AgNPs, previously dissolved in dimethyl sulfoxide (DMSO) were prepared with final concentrations of 512, 256, 128, 64, 32, 16, 8, 4, 2 and 1 mg/mL. To each tube was added 100 mL of 24 h old inoculums. The MIC was determined visually after incubation for 18 h, at 37°C. The susceptibility of the bacteria to AgNPs was determined by the formation of an inhibitory zone after 18 h of incubation at 37°C. DMSO and Ciprofloxacin were used as negative and positive controls. To obtain the MBC, 0.1 mL volume was taken from each tube and spread on agar plates. The number of c.f.u. was counted after 18-24 h of incubation at 37°C.
The in-vitro antifungal activities were carried out against Candida albicans and Aspergillus fumigates by agar diffusion method [18]. Then the minimum inhibitory concentration (MIC) was determined by broth dilution technique as performed in antibacterial activity. The Inhibition zones of biosynthesized AgNPs were compared with Nystatin used as positive control. The nutrient broth which contained serially logarithmic two fold diluted amount of AgNPs and controls was inoculated with approximately 1.6-6 × 104 c.f.u/mL. The cultures were incubated for 48 h at 37°C and the growth was monitored. To obtain minimum fungicidal concentration (MFC), 0.1 mL volume was taken from each tube and spread on agar plates. The number of c.f.u. was counted as the lowest drug concentration at which 99% of the inoculums were killed.
Fluorescence imaging of planktonic Candida albicans
The Candida albicans suspensions exposed to AgNPs were visualized under fluorescence microscope. To label cells with fluorescein, cells were resuspended to 0.1 mg FITC/mL (fluorescein isothiocyanate) in 0.05 M carbonate-bicarbonate buffer (pH 9.5). After incubation for 15 min in the dark, FITC labeled Candida albicans were washed twice with Hanks’s balanced salt solution containing 0.25% bovine serum albumin (BSA) and treated with different concentration of AgNPs and visualized under Fluorescence Microscope (Zeiss Imager M2, Gottingen) [19].
Effect of acid/alkaline treatment on the antimicrobial activity
To examine the minimum inhibitory concentration of AgNPs before and after pH treatment, AgNPs were exposed to acidic (pH 3.0) or alkaline (pH 12.0) at room temperature for 30 min, and then were neutralized by adding 1 M NaOH or 1 M HNO3. For dose response analyses, the treated AgNPs were serially diluted before incubating with bacteria, and then various concentration of these pH treated AgNPs were added to bacteria suspension. After getting again the pH 7, these treated nanoparticles at a concentration of 5 mg/L were added to microbial suspension at approximately 106 c.f.u/mL. Bacterial cultures not exposed to AgNPs were used as controls. The mixture was incubated aerobically at 37°C for 5 h. We used percent cell mortality, which calculated by the c.f.u as described before, to measure the dose-response of bacteria exposed to various concentration of AgNPs treated with acidic or alkaline pH [20].
Antimicrobial activities under different temperatures
To estimate the effect of incubation temperature on the antimicrobial activity, a mixture containing fresh (Lysogeny broth) LB medium, AgNPs and microbial suspension (2.0×108 c.f.u/mL) were combined to create a final concentration at 5 mg/L and microbial cells at 106 c.f.u/mL. The microbial strains are sufficiently grown in LB medium at 37°C to produce biomass for biosynthesis and here they are grown along with AgNPs as to find the effect of AgNPs on the microbial growth. The mixtures were aerobically cultured at 4, 23 and 37°C for 5 h as this much time is sufficient for bacteria to grow tremendously. The unexposed microbial suspensions under the same temperature were used as control [20].
Production of ROS by AgNPs
The production of intracellular reactive oxygen species (ROS) was measured using (DCFH-DA) [21]. Briefly, DCFH-DA (10 mM) stock solution in methanol was diluted in culture medium to yield a working solution (100 μM). At the end of exposure Candida albicans cells were washed twice with ice-cold 1X PBS (1.5 mM KH2PO4, 6.5 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl; pH 7.4) and then incubated in 1 mL working solution of DCFH-DA at 37°C for 30 min. The Candida albicans cells were treated with different concentrations of AgNPs (2.5, 5.0, 7.5, 10.0 and 12.5 mg/L) for 40 h, lysed in alkaline solution and centrifuged at 5000 rpm for 10 min. Then, a 200 μL supernatant was transferred to the other fresh well of micro-titer plate and fluorescence was measured.
Apoptosis detection
To examine apoptosis by electrophoresis of nucleosomal fragments, a standard procedure for precipitating cytosolic nucleic acid was used. AgNPs treated Candida albicans were pelleted (200 × 5 min) and lysed at 4°C for 15 min (250 μL, 0.4% Triton-X, 20 mM Tris, and 0.4 mM EDTA). Nuclei were then pelleted (13,000 × 5 min, 4°C) and the supernatant were transferred to a clean microfuge tube. Nucleosomal fragments were precipitated overnight with an equal volume of isopropanol after adjustment to 0.5 M NaCl. The pellet was washed twice in 70% ethanol, dried briefly and resuspended in 40 μL TE (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.8). The extracted DNA was separated on a 1.5% agarose gel containing 0.5 μg/mL Ethidium bromide (EtBr) and visualized on an UVtransilluminator and photographed [22].
Stability of AgNPs in SD broth medium and during storage
We determined the stability of AgNPs (30 μg/mL) in SD broth culture medium up to 72 h at 37°C through the change in UV-visible absorbance characteristics [21,23].
Results and Discussion
UV-Vis spectral analysis
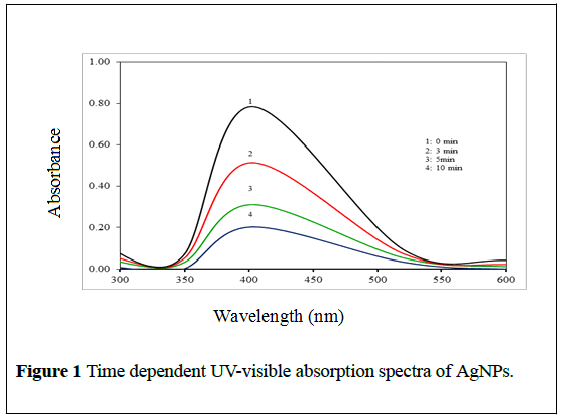
The formation of silver nanoparticles was further confirmed by using UVVis spectroscopic technique which has been frequently used to characterize the synthesized metallic nanoparticles. UV-Vis absorption spectra of AgNPs were monitored by measuring the time-dependent changes in the onset absorbance at an interval of 0, 3, 5 and 10 min at pH 7.7. As shown in Figure 1, the UV-visible spectra of the onset wavelength of AgNPs were found at ~402 nm.
The electronic band gap (Eg) was also determined by employing the following relationship:
Eg=h C/λ
Whereas, h is Planck’s constant, C is the speed of light and λ is the Cut off wavelength value of AgNPs. The (Eg) value was determined to be 3.06 eV, which shows it has good electron-transporting properties.
XRD analysis
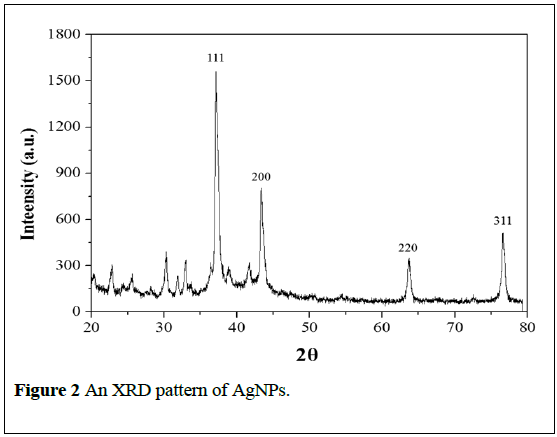
The biosynthesized AgNPs was analyzed by XRD to calculate the particle size. The data revealed that the four XRD peaks were obtained at 2θ; 37.31, 43.51, 63.68 and 76.43, which correspond to the four crystal plans of (111), (200), (220) and (311) of cubic (c) phase as depicted in Figure 2. The lattice parameters revealed that the maximum deviation between the observed and the calculated values of interplanar spacing (d) was below 0.06 Å. The full width-at half-maximum (FWHM) value for (111) plane of reflection was used for the calculation of the size of AgNPs (Figure 2). The calculated average particle size was determined to be ~24 nm. The obtained data was matched with face centered cubic structure of metallic silver nanopowders with space group of Fm-3m (JCPDS file No.04-0783).
The unassigned peaks are due to the crystallization of bioorganic phases that occur on the surface of the nanoparticles and these peaks were much weaker than those of silver, which indicates that the silver is the main material. This observation conforms the crystallization of bio-organic phase occurs on the surface of the Ag nanoparticles [1].
Microstructral studies (SEM/EDAX/TEM/AFM)
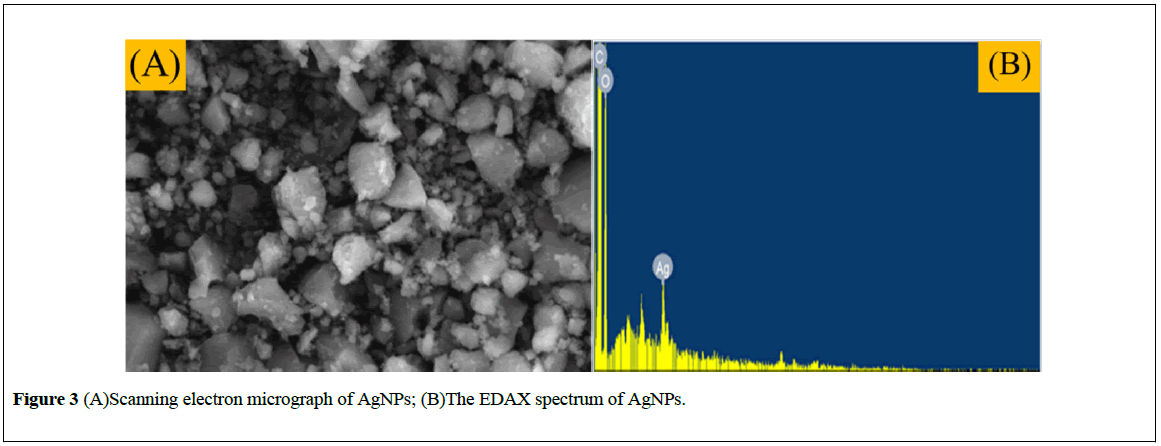
The morphology and size of the synthesized nanoparticles have been characterized on the basis of SEM technique. The SEM image of AgNPs is illustrated in Figure 3A and it is apparent that the formation of spherical shape particles and most of the particles are below 30 nm. Observed during SEM analysis may be attributed to the aggregation of the smaller silver nanoparticles during SEM measurements.
Moreover, the EDAX analysis (Figure 3B) displayed a peak at the energy of 3 keV for silver and also some of peaks for C and O which may have originated from the biomolecules bound to the surface of the silver nanoparticles. The emission energy at 3 keV indicates the reduction of silver ions to the elemental silver (AgO) [24]. TEM was used to provide further insight into the size, shape and morphology of the biosynthesized silver nanoparticles. In the TEM image the exhibited dark areas indicating the presence of the AgNPs, while the bright area represents the organic material encapsulated in the AgNPs. The capping of silver nanoparticles by organic materials further enhances their life time in solution [25,26].
The TEM image clearly showed the AgNPs to be spherical in shape with a size range of 10-20 nm (Figure 4), which is consistent with the results obtained by XRD and SEM (Figures 1 and 2).
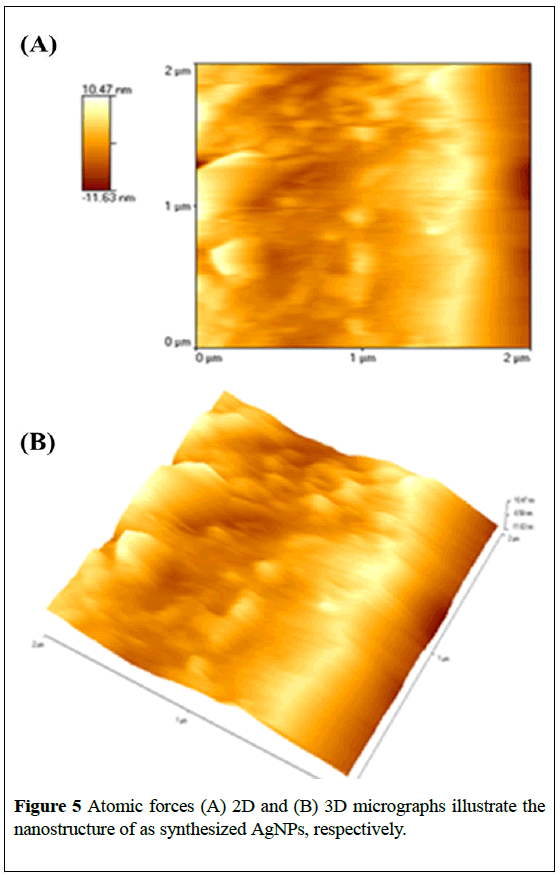
The AFM images also show the spherical morphology of AgNPs in the size range of 18-29 nm (Figure 5).
The vertical and horizontal line analysis of AFM images for AgNPs showed roughness parameters such as minimum and maximum surface value. The Mean roughness (Ra) values were found to be 12.22 nm and 11.16 nm, respectively. The other roughness parameters like mid-value (average of maximum and minimum), mean, peak to valley of the line (Rpv, difference between minimum and maximum), root-mean-squared roughness, ten point average roughness area (Rz, is the arithmetic average of the five highest and five lowest valleys peaks in the line calculated by ten point average), skewness (Rsk) and kurtosis (Rku) values of line are given in Table 1. The overall data have confirmed the biosynthesis of AgNPs with spherical shape and average particle size of ~24 nm.
| Line | Min. | Max. | Mid | Mean | Rpv | Rq | Ra | Rz | Rsk | Rku |
|---|---|---|---|---|---|---|---|---|---|---|
| Horizontal | -20.24 | 15.10 | -4.12 | 0.00 | 40.75 | 12.23 | 8.51 | 21.15 | 0.25 | 2.02 |
| Vertical | -12.12 | 14.20 | -2.13 | 0.00 | 23.60 | 4.59 | 4.18 | 21.08 | 0.70 | 3.12 |
Figure 1: Time dependent UV-visible absorption spectra of AgNPs.
FT-IR spectroscopy
The FTIR spectra of AgNPs provide useful information about the biosynthesis. The peaks observed at 603, 801 and 1059 cm-1 are ascribed to C-OH vibration in plane, methylene rock, and C-O stretching vibrations. The peaks at 1242 and 1300 cm-1 correspond to C-H out-ofplane and C-H in plane bends, whereby the symmetric and asymmetric bends of CH group are detected at 1370 and 1490 cm-1, respectively. The peak at 1650 cm-1 derives from C=C stretching vibration. The peaks in the range of 2600-2900 cm-1 belong to olefinic C-H and alkyl C-H stretching region, whereby the broad absorption band in the range of 3200-3700 cm-1 corresponds to OH stretching vibrations present in the alkaloids 6- gingerol, 6-Shogal, α-Zingeberene of Zingiber officinale. Decrease in the intensities and broadening of the OH band indicates involvement of -OH group in reduction process [27,28]. Together the characterization data revealed the successful biosynthesis of AgNPs.
Photoluminescence study
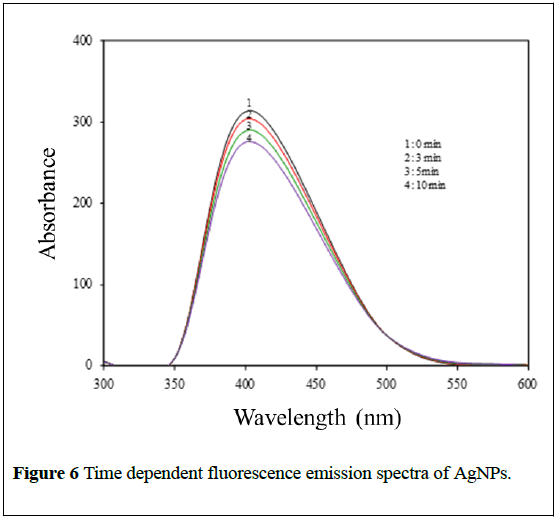
Photoluminescence finds wide applications in the branches of biochemical, medical and chemical research fields [29]. Figure 6 shows photoluminescence spectra of biosynthesized AgNPs formed at the pH 7.7 and monitored by measuring the time-dependent changes in the intensity at an interval of 0, 3, 5 and 10 min. The emission spectra of AgNPs have a broad band with a maximum emission at 537 nm, when exited at ~400 nm.
Thermal analysis
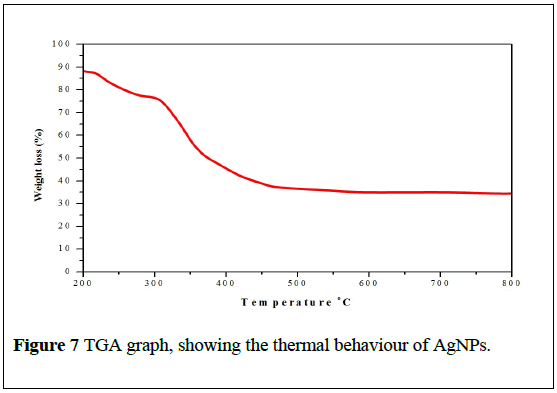
The TGA analysis of biosynthesis AgNPs is illustrated in (Figure 7). The TGA curve of the AgNPs reveals a weight loss of ~35% at 250°C because of the evaporation of water. The major weight loss occurs between 300°C and 520°C, which is around 40% of the original weight due to the removal and decomposition of organic groups present in the sample during the biosynthesis [30].
Possible mechanism for formation of AgNPs
For the biosynthesis of AgNPs there are two possible mechanisms. First one is OH group which has unshared pair of electrons on oxygen and thus might bind with the AgNPs. The second possible mechanism can be explained be Ag+-olefin interactions (Dewar-Chatt-Duncanson model), the free s-orbital in Ag+ can bond with the electron-rich π-orbital of olefins. Likewise, a π-bond is obtained by the interaction of the filled d-orbitals of Ag+ and the vacant molecular π*-orbitals. Although alkenes are good species to bind with metals in comparison of OH ligand but the alkene must be free from a ring or any hindrance, so it does not bind here with Ag. Hydroxy group, though a weak ligand but it will bind with Ag due to ease of access which has been proved by our IR studies.
Antimicrobial activity of AgNPs
The necessity for the development of new antimicrobial agents is causing a rapid surge in research due to the ever increasing threat from microbes. For this reason, researchers are increasingly turning their attention to nanomaterials for looking into new leads to develop better nanoantimicrobial drugs against microorganisms [31].
In the current study we have tested in-vitro antimicrobial activity of AgNPs by using disk diffusion and agar diffusion methods against two gram-positive Corynebacterium xerosis and Staphylococcus pyogenes and three gram-negative bacteria Pseudomonas aeruginosa, Kliebsiella pneumoniae and Escherichia coli and two fungi Candida albicans and Asperergillus fumigatus to determine its potentials in biomedicinal applications (Tables 2 and 3).
| Strain | Ch@AgNPs-Nc | Ciprofloxacin | Nystatin | DMSO |
|---|---|---|---|---|
| [Gram-positive] | ||||
| C. xerosis | 18.1 ± 0.3 | 26.8 ± 0.5 | NT | - |
| S. pyogenes | 14.5 ± 0.4 | 22.4 ± 0.4 | NT | - |
| [Gram-negative] | ||||
| P. aeruginosa | 15.0 ± 0.5 | 21.1 ± 0.2 | NT | - |
| K. pneumonia | 13.2 ± 0.5 | 25.2 ± 0.8 | NT | - |
| E. coli | 14.1 ± 0.5 | 20.0 ± 0.2 | NT | - |
| [Fungi] | ||||
| C. albicans | 20.1 ± 0.2 | NT | 29.0 ± 0.5 | - |
| A. fumigates | 18.7 ± 0.3 | NT | 27.0±0.2 | - |
Table 2: Antimicrobial Activity (zones of inhibition) of Ch@AgNPsNc.
| Strain | Ch@AgNPs-Nc | Ciprofloxacin | Nystatin |
|---|---|---|---|
| [Gram-positive] | MIC MBC/MFC | MIC MBC | MIC MBC |
| C. xerosis | 20 50 | 12.5 12.5 | - - |
| S. pyogenes | 25 50 | 12.5 12.5 | - - |
| [Gram-negative] | MIC MBC/MFC | MIC MBC | MIC MBC |
| P. aeruginosa | 30 50 | 12.5 25 | - - |
| K. pneumonia | 25.25 30 | 6.25 25 | - - |
| E. coli | 20 25.5 | 6.25 12.5 | - - |
| [Fungi] | MIC MBC/MFC | MIC MBC | MIC MBC |
| C. albicans | 12.5 25.25 | - - | 6.25 25 |
| A. fumigates | 20.25 25 | - - | 12.5 12.5 |
Table 3: MIC and MBC results of Ch@AgNPsNc against bacterial and fungal strains.
In our study, AgNPs showed remarkable antibacterial activity against both gram-positive and gram-negative bacteria. AgNPs presents excellent activities against gram-positive bacteria and possesses also moderate antibacterial activities against gram-negative Pseudomonas aeruginosa, Kliebsiella pneumoniae and Escherichia coli strains with MIC values of 20-30 μg/mL. In general, we can say that the antibacterial activity in the case of gram-positive bacteria was little higher as compared to gramnegative bacteria. Such can be explained by the presence of an extra layer of lipopolysaccharide protection in addition to the peptidoglycan membrane in gram-negative bacteria [32]. The investigation of antifungal screening data revealed that AgNPs was found to be almost equally potent as the reference drug Nystatin in the case of Candida albicans. The obtained antimicrobial results of AgNPs in the current study are corroborated with findings which have been published recently on nanomaterials activity [33,34]. AgNPs showed efficient antimicrobial property due to their extremely large surface area, which provides better contact with microorganisms. It is also possible that AgNPs not only interact with the surface of membrane, but can also penetrate inside the bacteria [1].
MIC (μg/mL)=minimum inhibitory concentration, i.e the lowest concentration of AgNPs to inhibit the growth of bacteria completely; MBC (μg/mL)=minimum bacterial concentration, i.e., the lowest concentration of AgNPs for killing the bacteria completely. MFC (μg/ mL)=minimum fungicidal concentration, i.e. the lowest concentration of the AgNPs for killing the fungus completely.
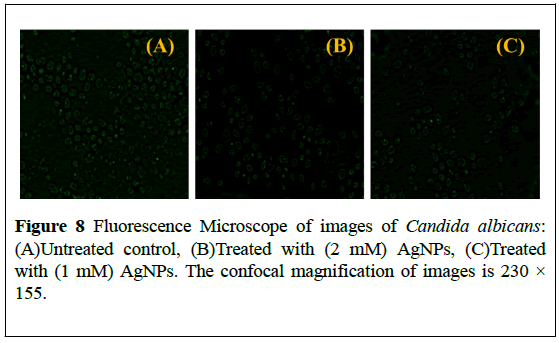
Candida albicans treated with AgNPs at (2 mM) and (1 mM) were observed under fluorescence microscope. Candida albicans cells exposed to AgNPs exhibited a significant reduction in their population. As, exhibited by (Figure 8), AgNPs inhibit the fungal cells considerably when compared to untreated control.
Effect of acid/alkaline treatment on the antimicrobial activity
The alkali treatment showed no significant changes on the antimicrobial activity. While the antimicrobial activity of AgNPs treated with acid solution at a pH of 3 showed decrease in the antimicrobial activity, down to below 10% at 5 mg/L. This may be explained in terms of possible damage on the surface functional groups at low-pH environment, leading to the aggregations of the compounds. The aggregates would have more difficulty in penetrating the peptidoglycan layer of bacterium [35].
Antimicrobial activities under different temperatures
Microbes grow at various conditions and most pathogens grow at human body temperatures. Thus, we studied the effect of various temperatures on the antimicrobial activities of AgNPs. The antimicrobial activity of AgNPs was proportional to the incubation temperature. At 4ºC and 23ºC, no obvious effect was achieved. On the other hand, at the maximum temperature tested, 37ºC, the antimicrobial activity was found to be most effective. It is possible that at higher temperature, the cell membrane were leaky allowing entry of AgNPs which resulted in higher mortality.
More apparently, it can be concluded that at higher incubation temperature, resulted in membrane fluidity that makes the transport of AgNPs easier, resulted inm higher mortality. This will be in agreement with studies that state that transport into the plasma membrane is essential for inhibiting microbial growth [36].
Production of ROS by AgNPs
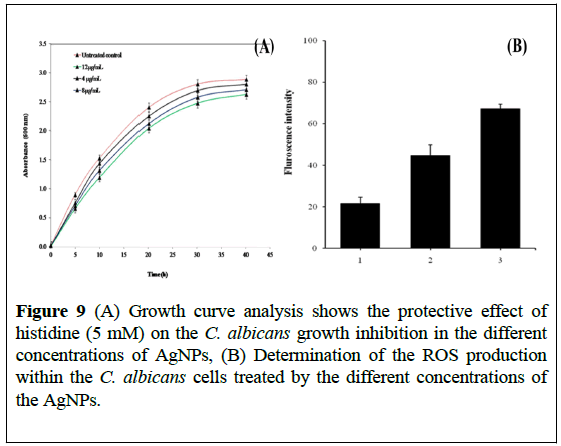
We expect that the antimicrobial effect of AgNPs can be mediated through ROS. Moreover, oxidative stress induced by reactive oxygen species (ROS) is a well-known inducer of cytotoxicity and apoptotic cell death in microorganism [35]. Therefore, we tested whether the ROS produced by the AgNPs are responsible for inhibiting Candida albicans growth. The protective effect of histidine, a known scavenger of hydroxyl radicals and singlet oxygen on antimicrobial activity of AgNPs, was tested by adding histidine (5 mM) to Candida albicans culture containing AgNPs. Figure 9A shows a protective effect of histidine in terms of growth enhancement of Candida albicans in the presence of AgNPs.
Figure 9: (A) Growth curve analysis shows the protective effect of histidine (5 mM) on the C. albicans growth inhibition in the different concentrations of AgNPs, (B) Determination of the ROS production within the C. albicans cells treated by the different concentrations of the AgNPs.
Histidine (5 mM) almost completely protects the Candida albicans from anticandidal activity of AgNPs. The fact that histidine diminished the effect of AgNPs on Candida albicans is mediated by the ROS production. This conclusion is supported by the ROS results shown in Figure 9B, exhibiting a reduction in the amount of ROS produced within the Candida albicans cells treated with AgNPs, following the addition of histidine as a ROS quencher.
Apoptosis detection
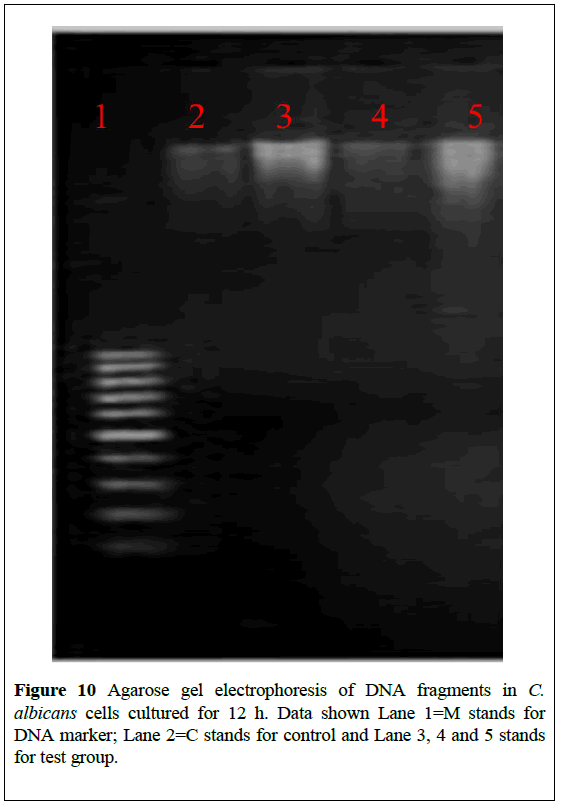
The photo-induced DNA cleavage by with and without modified AgNPs were studied. The electrophoretic mobility of DNA was followed in agarose gel. The gel electrophoresis diagram is shown in Figure 10. Lanes 1 and 2 are the irradiated DNA with and without AgNPs under UV light and dark conditions respectively, and these lanes serve as controls. Lanes 3 and 4 indicate the (2 μM) and (1 μM) concentration of the irradiated AgNPs with DNA. An intensity decrease is observed in lane 3 compared to lane 4, showing dose dependent cleavage of plasmid DNA.
Figure 10: Agarose gel electrophoresis of DNA fragments in C. albicans cells cultured for 12 h. Data shown Lane 1=M stands for DNA marker; Lane 2=C stands for control and Lane 3, 4 and 5 stands for test group.
Lane 5 contains AgNPs, DNA with sodium azide (NaN3), where the cleavage is reduced due to the presence of sodium azide which scavenges the 1O2, confirming the role of photo-generated singlet oxygen (1O2) in photocleavage of DNA.
These observations correlate well with singlet oxygen generating efficiency of AgNPs. The singlet oxygen generated upon may cause damage to DNA [22]. Fragmented DNA, shown as DNA ladder in the gel, indicates apoptotic cell death [22].
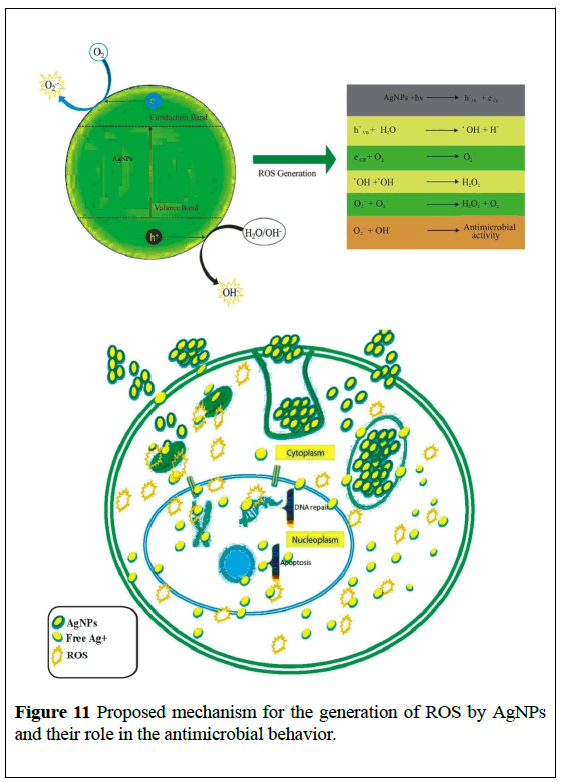
With these excellent results in our hand, we are now in a position to propose the mechanism of the antimicrobial activity of AgNPs. AgNPs excited with energy higher than the (Eg), the electrons (e-) of AgNPs were promoted across the band gap to the conduction band (Ec), which creates a hole (h+) in the valence band (Ev). The electrons in the (Ec) and holes in the (Ev) exhibit high reducing and oxidizing power [37,38]. The (e-) reacted with molecular oxygen to produce superoxide anion (O•-2) through reductive reactions. The (h+) can extract electrons from water and/or hydroxyl ions to generate (•OH) which believe to be responsible for the antimicrobial behaviour of AgNPs as presented in Figure 11.
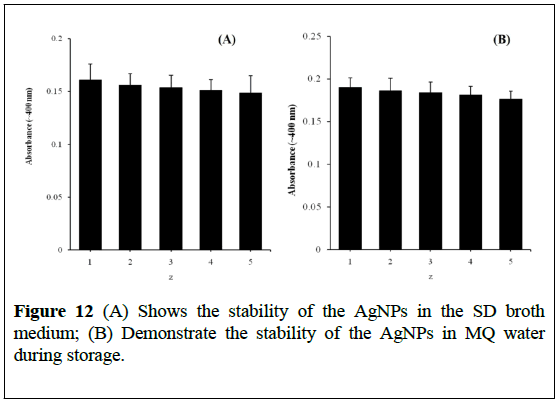
Stability of AgNPs in SD broth medium and during storage
We determined the stability of AgNPs (30 μg/mL) in SD broth culture medium up to 72 h at 37ºC through the change in UV-visible absorbance characteristics (Figure 12A) [21,23]. The significant changes such as agglomeration and absorbance of AgNPs were not observed with cell culture medium incubation, suggesting that SD broth culture medium does not significantly affect AgNPs stability, size and integrity. It was also observed that the colloidal solution of AgNPs remained stable for 90 days and the significant change in the absorbance does not decrease (Figure 12B).
Conclusion
The rapid biological synthesis of AgNPs using a powdered extract of dry ginger rhizome provides an ecofriendly, simple and efficient route for synthesis of nano particles. The structural analysis has been carried out by using UV-Vis spectra, XRD, SEM, EDAX, TEM, AFM, FTIR and photoluminescence. The synthesized AgNPs were found to have a crystalline structure with face centered cubic geometry. The particles are mostly spherical and average size around 24-30 nm. AgNPs was tested for in-vitro antimicrobial activity against different bacterial and fungal strains. The role of ROS produced by AgNPs and apoptosis has been investigated.
The results suggest that silver nanoparticles would be promising leads for the further development of smart biomaterials in future.
Acknowledgement
We are thankful to the Chairperson, Department of Pharmacy, University of Science and Technology, Ibb, Yemen.
REFERENCES
- Ashokkumar S, Ravi S, Kathiravan V, et al. Synthesis of silver nanoparticles using A. indicum leaf extract and their antibacterial activity. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015;134:34-9.
- Huh AJ, Kwon YJ. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011;156:128-45.
- Hajipour MJ, FrommKM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499-511.
- Fang J, Sawa T, Akaike T, et al. Tumor-targeted Delivery of Polyethylene Glycol-conjugated D-Amino Acid Oxidase for Antitumor Therapy via Enzymatic Generation of Hydrogen Peroxide, Cancer Res. 2002;62:3138-43.
- AshaRani PV, Hande MP, Valiyaveettil S, et al. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells, ACS Nano. 2009;3:279-90.
- Sawai J, Kawada E, Kanou F, et al. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. JAPAN 1996;29:627-33.
- Singh BN, Singh BR, Upadhyay G, et al. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009;47:1109-16.
- Ben-Yoseph O, Ross BD. Oxidation therapy: the use of a reactive oxygen species-generating enzyme system for tumour treatment. Br. J. Cancer 1994;70:1131-5.
- Cohen ML. Changing patterns of infectious disease. Nature 2000;406:762-7.
- Kim JS, Kuk E, Park SJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007;3: 95-101.
- Quadros ME, Marr LC. Silver nanoparticles and total aerosols emitted by nanotechnology-related consumer spray products. Environ. Sci. Technol. 2011;45:10713-9.
- Chen X, Schluesener HJ. Nanosilver:a nanoproduct in medical application. Toxicol. Lett. 2008;176:1-12.
- Faunce T, Watal A. Nanosilver and global public health: international regulatory issues. Nanomedicine(Lond). 2010;5:617-32.
- Díez I, Eronen P, Ikkala O, et al. Functionalization of nanofibrillated cellulose with silver nanoclusters:fluorescence and antibacterial activity. Macromol. Biosci. 2011;11:1185-91.
- Chen WY, Lin JY, Luo L, et al. Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria. Nanomedicine (Lond). 2010;5:755-64.
- Gültekin A, Diltemiz SE, Ersöz A, et al. Gold-silver nanoclusters having dipicolinic acid imprinted nanoshell for Bacillus cereus spores recognition. Talanta. 2009;78:1332-8.
- Janaki AC, Sailatha E, Gunasekaran S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015;144:17-22.
- Azam A, Ahmed AS, Oves M, et al. Memic, Size-dependent antimicrobial properties of CuO nanoparticles against gram-positive and gram-negative bacterial strains. Int. J. Nanomedicine 2012;7:3527-35.
- Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 2001;69:6813-22.
- Xu H, Qu H, Xu H, et al. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157:H7. Biometals. 2012;25:45-53.
- Singh BR, Singh BN, Singh HB, et al. ROS-mediated apoptotic cell death in prostate cancer LNCaP cells induced by biosurfactant stabilized CdS quantum dots. Biomaterials 2012;33:5753-67.
- Chowdhury S, Basu A, Kundu S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 2014;9:365.
- Wang L, Nagesha DK, Selvarasah S, et al. Toxicity of CdSe Nanoparticles in Caco-2 Cell Cultures. J. Nanobiotechnology 2008;6.
- Vanaja M, Gnanajobitha G, Paulkumar K, et al. Phytosynthesis of silver nanoparticles by Cissus quadrangularis:influence of physicochemical factors. J. Nanostructure Chem. 2013;3:17.
- Shankar SS, Ahmad A, Sastry M, et al. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004;27:496-502.
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts, Bioprocess Biosyst. Eng. 2009;32:79-84.
- George J, Sajeevkumar VA, Sabapathy SN, et al. Augmented properties of PVA hybrid nanocomposites containing cellulose nanocrystals and silver nanoparticles. J. Mater. Chem. 2012;22:22433.
- Perry DA, Cordova JS, Schiefer EM, et al. Study of Adsorption of Aminobenzoic Acid Isomers on Silver Nanostructures by Surface-Enhanced Infrared Spectroscopy. J. Phys. Chem. C. 2009;113:18304-11.
- Willard HH, Merritt LLJ, Settle FAJ, et al. Instrumental methods of analysis. 1988.
- Mashrai A, Khanam H, Aljawfi RN. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2013.
- Wady AF, Machado AL, Zucolotto V, et al. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J. Appl. Microbiol. 2012;112:1163-72.
- Shome A, Dutta S, Maiti S, et al. In-situ synthesized Ag nanoparticle in self-assemblies of amino acid based amphiphilic hydrogelators:development of antibacterial soft nanocomposites. Soft Matter. 2011;7:3011.
- Macherla C, Sanchez DA, Ahmadi MS, et al. Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Front. Microbiol. 2012;3:193.
- Panácek A, Kolár M, Vecerová R, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009;30:6333-40.
- Zou H, Koh JJ, Lin H, et al. Design and synthesis of amphiphilic xanthone-based, membrane-argeting antimicrobials with improved membrane selectivity. J. Med. Chem. 2013;56:2359-73.
- Grzegorzewicz AE, Pham H, Scherman MS, et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 2012;8:334-41.
- Li H, Zhu DC, Huang X, et al. Synthesis of β-cyclodextrin conjugated superparamagnetic iron oxide nanoparticles for selective binding and detection of cholesterol crystals. Chem. Commun. (Camb). 48;2012:3385-7.
- Li Y, Zhang W, Chen Y, et al. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6:5164-73.