Case report of a syndromic girl with intellectual disability having both DYRK1A and SCN1A mutation
- *Corresponding Author:
- Kanij Fatema Department of Pediatric Neurology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh Tel: +880171309775 E-mail: maiomonami@gmail.com
Received: July 24, 2019, Accepted: March 18, 2020, Published: March 25, 2020
Citation: Rahman M, Fatema K. Case report of a syndromic girl with intellectual disability having both DYRK1A and SCN1A mutation. J Clin Gen Genomics 2020;3(1):1-3.
[ft_below_content] =>Keywords
Syndromic intellectual disability; Mutation; DYRK1A; SCN1A
Introduction
Chromosomal deletions encompassing DYRK1A have been associated with intellectual disability for several years. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) is located in the chromosome 21 [1,2]. This gene plays a major role in brain development, specifically neurogenesis, neural plasticity and cellular death [3]. It is already known that protein kinases are key enzymes for the regulation of basic cellular processes in all eukaryotes [4]. It encodes a highly conserved protein that plays an essential role in the development of the central nervous system.
Haploinsufficiency of DYRK1A is responsible for a syndrome characterized by intellectual disability (ID), microcephaly and dysmorphic features. This disorder also includes impaired speech development, autism spectrum disorder including anxious and/or stereotypic behaviour problems and microcephaly. Affected individual often have clinically recognizable phenotypes including a typical facial gestalt, feeding problems, seizures, hypertonia, gait disturbances and foot abnormalities [5-7]. Individuals with this condition were initially reported with partial monosomies of chromosome 21 as detected on routine karyotype that encompassed the DYRK1A gene (21q22.13) [8-10].
Voltage-gated sodium channel SCN1A are associated with a growing number of disorders including Generalized Epilepsy with Febrile Seizures Plus (GEFS+), Severe Myoclonic Epilepsy of Infancy (SMEI) and Familial Hemiplegic Migraine (FHM) [11].
Here, we present one patient with DYRK1A mutations and SCN1A mutation with details of the phenotype and genotype of this emerging syndrome.
Case Description
A 7 year 10 month old girl, presented with inattention, hyperactivity, impaired adaptive behaviour and cognitive delay to a private chamber. On query she had sleep disturbances in the form of decreased night time sleep. Mother also complained of self-abusing habit with biting herself causing trauma of her hands along with some abnormal sexual behaviour in the form of masturbation. In addition to that she had history of febrile seizure since her 10 month of age. Till date she had 7 attacks, all with fever, generalized tonic in nature, persisting for about 2-3 minute with postictal drowsiness. She did not have any history of afebrile seizure, fever with rash, jaundice, visual or hearing impairment.
She is the only issue of her non-consanguineous parents. None of her family members had similar type of illness or history of febrile or afebrile seizure, congenital anomaly or intellectual disability. She was born by lower uterine caesarean section at term. Her birth weight was 2 kg (small for gestational age). She had history of delayed cry and neonatal jaundice for which she was given phototherapy for 4 days. She was developmentally delayed in motor, cognition and speech; she started to sit at 1 year and started to walk at 2 year. Her speech was delayed, at 7 year she could only speak few meaningful words. She could not read or write nor did she go to school. Her vision and hearing was intact. She could feed herself but could not perform her chores. Her social and communication skills were altered; she could not follow the social customs and could not mix and play with peers.
On examination, the girl was conscious, cooperative, not fully oriented to surrounding. Her vitals were within normal limit. Her height was 114 cm (-2.2 SD), weight 15 kg (-3.3SD), BMI: 11.5 (-3.5 SD). OFC 42 cm (<3SD) suggestive of microcephaly. She had bitemporal narrowing, a prominent occiput and deep set eyes (Figure 1). She was not pale, anicteric, skin survey was normal. Nervous system was normal except higher psychic function. Her speech was incoherent, not clear, memory and orientation was altered. She walked with broad-based clumsy gait. She had some stereotypes like hand flapping. Psychological assessment by Bailey scale of infant development III: suggestive that the cognition was at 24 months.
Investigation
EEG: generalized slowing with epileptic discharges in the frontal region of both hemispheres. CT scan of brain showed mild cerebral atrophy without any structural abnormality. Karyotyping was normal, next generation sequencing was done, here only related genes were sequenced. It showed there was pathogenic variant of DYRK1A gene causative of phenotype and as an additional finding there was variant of uncertain significance in SCN1A gene.
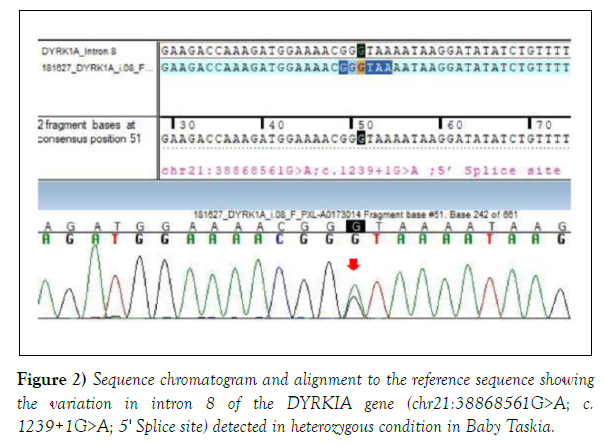
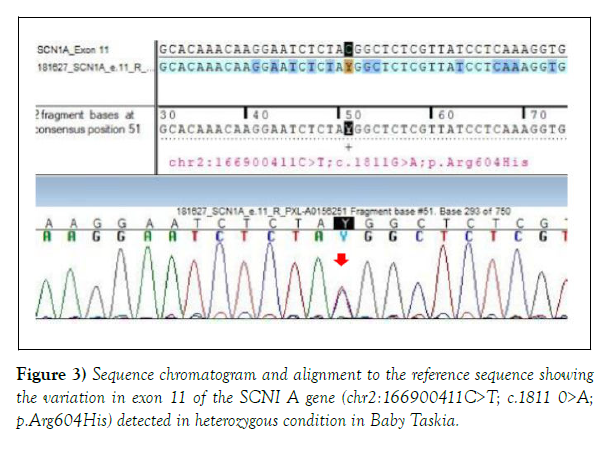
Both the variations were detected in heterozygous condition by Sanger sequencing (Figures 2 and 3). The detail of the report was as follows:
1. Gene: DYRK1A+, Location: Intron 8; Variant: C 1239+1 G>A; Zygosity: heterozygous; Disease: Mental Retardation -7; Inheritance: Autosomal dominant; Classification: Pathogenic. 2. Additional finding: Gene: SCN1A (-); Location: Exon 11; Variant: C18 11 G>A; Zygosity: heterozygous; Disease: Generalized Epilepsy with Febrile Seizure Plus type 2; Early Infantile Epileptic Encephalopathy-6 (Dravet Syndrome); Inheritance: Autosomal Dominant; Classification: Uncertain significance.
We treated the girl with speech therapy, behavioural management and physical therapy. As she had sleep problems with treated her with melatonin. Due to financial constrain gene analysis of parents could not be done. So it could not be determined whether it is de novo or not.
Discussion
DYRK1A gene has 13 exons and spans~147.8 Kb of genomic DNA. Pathogenic sequence mutations in this gene result in loss of protein function and account for around 0.5% of syndromic intellectual disability. Patients typically have global developmental delay and microcephaly with a number of other common phenotypes including delayed speech and language, growth restriction, dysmorphic facial features, eye malformations and seizures [12]. It has also been studied extensively in the last decade because of its association with neuronal deficits, dendritic atrophy, spinal dysgenesis, precocious Alzheimer-like neurodegeneration, and cognitive deficits in Down syndrome individuals [13].
Our case was diagnosed late, at the age of 7 year 10 month. In literature, the age range of diagnosis showed a wide range from 17 months to 59 years [14,15]. Onset of clinical features was early in most of the published cases. Prenatal finding were present in 77% cases in one case series published by Loco et al. The antenatal findings were oligohydroamnios, polyhydroaminios, and low birth weight. 15 There was no evidence of antenatal sonography in our case. However, our case was a small for gestational age which matches with the literature and also our patient had history of delayed cry. Like other cases in literature this girl also was born with low birth weight (78% in case series of Jiangi et al.) [13].
Our patient mainly presented with intellectual disability and behavioural issues. Loco et al. and Jiangi Ji et al. also found ID in almost all of their patients in their case series. Other features such as speech delay, global developmental delay is also consistent with the previous studies [13-15]. Our patient was born from a noncosanguineous parents with no similar positive family history. Our case has some severe behavioural issues like presented with inattention, hyperactivity, self-abusing habit and masturbation. Behavioural issues were also common in other related case series (88%) and hyperactivity in 26%. Autism spectrum disorder was present in 45% cases in one case series. Febrile seizures were reported in 67 % cases and epilepsy or non-febrile seizures were observed in 60 % in case one series [15]. This girl only had febrile seizure although her age was beyond the age of simple febrile seizure.
In our case the important physical findings were microcephaly which is a common finding of other cases (86% and 92% in similar studies) [13,15,16]. She had dysmorphic face like bitemporal narrowing, prominent occiput, and deep set eyes. Dysmorphism was also found in case series by Luco et al in 98% of the cases. Our case had broad-based clumsy gait. Majority of the described cases had a broad based ataxic gate. A stiff gate has also been described [13,15,17]. Apart from this she had stereotypes like hand flapping. In this case, CT scan revealed mild cerebral atrophy. In related cases, 76% of patients had abnormal findings namely cortical atrophy, thin brain stem, thin corpus calosum, pituitary stalk hypoplasia, neuronal migration defect, enlarged cisternal megna etc. Among them cortical atrophy was the most common finding which matches with our case [15].
The final diagnosis is done by molecular genetic approaches including single gene testing, use of multi-gene panel and more comprehensive genetic testing [17,18]. We initially did karyotyping which was normal and then did next generation sequencing and sanger sequencing which came out abnormal as DYRKIA gene mutation but the location was in intron 8 which is a rare site.
DYRK1 related intellectual disability syndrome is inherited as an autosomal dominant manner. It usually represents as simplex case: a single occurrence in a family resulting from a de novo DYRK1A pathogenic variant [17]. The majority of mutations occurred within the kinase domain or the N-terminal domain in a previous case series. The mutations were of nonsense, frame shift and 5 splice site mutations [15]. In our case, the mutation occurred in 5 splice site. There does not seem to be a clear genotype-phenotype correlation with the mutations and phenotype reported previously. This gene was initially identified for its role in intellectual disability which is clinically defines as childhood onset of significant cognitive and adaptive impairment. Here it is to mention that recurrent disruption of this gene have been found in as many as 0.5% cases of ASD [14]. In our case autistic features and intellectual disability are present.
In addition to DYRK1A gene mutation, this case also had SCN1A gene mutation, which is heterozygous in nature. The SCN1A gene belongs to a family of genes that provide instructions for making sodium channels. These channels, which transport positively charged sodium atoms (sodium ions) into cells, play a key role in a cell's ability to generate and transmit electrical signals. It provides instructions for making one part (the alpha subunit) of a sodium channel called NaV1.1. These channels are primarily found in the brain, where they control the flow of sodium ions into cells. NaV1.1 channels are involved in transmitting signals from one nerve cell (neuron) to another. Communication between neurons depends on chemicals called neurotransmitters, which are released from one neuron and taken up by neighbouring neurons. The flow of sodium ions through NaV1.1 channels helps determine when neurotransmitters will be released [19].
This mutation has been described as uncertain significance. SCNIA mutation is related to early infantile epileptic encephalopathy 6 (EIEE 6, Dravet syndrome), Generalized Epilepsy With Febrile Seizures Plus, Type 2( GEFSP2), familial hemiplegic migraine 3 (FHM3) and autism.19 Our patient has repeated attacks of febrile generalized epilepsy and autistic features which may be correlated with the mutation although the mutation was suggested as variance of unknown significance.
Outpatient had features of intellectual disability, dimorphism which coincides with DYRK1A mutation while she had features of autism and epilepsy which coincides with SCN1A mutation. But in DYRKIA mutation autistic features may occur, so it is uncertain that the child’s clinical features reflect only one or both gene mutation.
There is no reported case of mutation of both DYRK1A and SCN1A in same patient till date.
Conclusion
Any child with syndromic features, intellectual disability, and behavioural abnormality should be addressed meticulously and DYRK1A mutation should be considered. Our case is a rare case with DYRK1A mutation in intron 8 associated with SCN1A gene mutation.
Conflict of Interest
There is no conflict of interest.
Funding
No funding has been taken for this paper.
Ethical Implications
Written informed consent has been taken from the parent of the patient. Ethical clearance has been taken from the ethical review committee of the institute.
References
- Guimera J, Casas C, Pucharcos C, et al. A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum Mol Genet. 1996;5(9):1305–10.
- Iossifov I, O'Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–21.
- Bronicki LM, Redin C, Drunat S, et al. Ten new cases further delineate the syndromic intellectual disability phenotype caused by mutations in DYRK1A. Eur J Hum Genet. 2015;23(11):1482-7.
- Aranda S, Laguna A, de la Luna S. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011;25(2): 449–62.
- Hämmerle B, Vera-Samper E, Speicher S, et al. Mnb/Dyrk1A is transiently expressed and asymmetrically segregated in neural progenitor cells at the transition to neurogenic divisions. Dev Biol. 2002;246(2):259–73.
- Lagran MMD, Benavides-Piccione R, Ballesteros-Yanez I, et al. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. Cereb Cortex. 2012;22(12):2867–77.
- Soppa U, Schumacher J, Florencio Ortiz V, et al. The Down syndrome-related protein kinase DYRK1A phosphorylates p27Kip1 and Cyclin D1 and induces cell cycle exit and neuronal differentiation. Cell Cycle. 2014;13(13):2084–100.
- Chettouh Z, Croquette MF, Delobel B, et al. Molecular mapping of 21 features associated with partial monosomy 21: involvement of the APP-SOD1 region. Am J Hum Genet. 1995;57(1):62–71.
- Matsumoto N, Ohashi H, Tsukahara M, et al. Possible narrowed assignment of the loci of monosomy 21-associated microcephaly and intrauterine growth retardation to a 1.2-Mb segment at 21q22.2. Am J Hum Genet. 1997;60(4):997–9.
- Bartsch O, Hinkel GK, Petersen MB, et al. A large family with subtelomeric translocation t (18; 21)(q23; q22. 1) and molecular breakpoint in the Down syndrome critical region. Hum Genet. 1997;100(5–6):669–75.
- Melinda S. Martin, Karoni Dutt, Ligia A. Papale, et al. Altered function of the SCN1A voltage-gated sodium channel leads to GABAergic interneuron abnormalities. J Biol Chem. 2010;285(13):9823–34.
- Jochem M.G. Evers. Structural analysis of pathogenic mutations in the DYRK1A gene in patients with developmental disorders. Hum Mol Genet. 2017;26(3):519-526.
- Ji J, Lee H, Argiropoulos B, et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. Eur J Hum Genet. 2015;23(11): 1473–81.
- Earl RK, Turner TN, Mefford HC, et al. Clinical phenotype of ASD-associated DYRK1A haploinsufficiency. Mol Autism. 2017;8:54.
- Luco SM, Pohl D, Sell E, et al. Case report of novel DYRK1A mutations in 2 individuals with syndromic intellectual disability and a review of the literature. BMC Med Genet. 2016;17:15.
- Courcet JB, Faivre L, Malzac P, et al. The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. J Med Genet. 2012;49(12):731-6.
- Van Bon BWM, Coe BP, de Vries BBA, et al. DYRK1A-Related Intellectual Disability Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® 2015. Seattle (WA): University of Washington, Seattle; 1993-2019.
- Møller RS, Kübart S, Hoeltzenbein M, et al. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am J Hum Genet. 2008;82(5):1165-70.
- Hamosh A, Scoot AF, Amberger J, et al. Mendelian Inheritance in Man, OMIM. Hum Mutat. 2000;15(1):57-61.
- *Corresponding Author:
- Kanij Fatema Department of Pediatric Neurology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh Tel: +880171309775 E-mail: maiomonami@gmail.com
Received: July 24, 2019, Accepted: March 18, 2020, Published: March 25, 2020
Citation: Rahman M, Fatema K. Case report of a syndromic girl with intellectual disability having both DYRK1A and SCN1A mutation. J Clin Gen Genomics 2020;3(1):1-3.
Abstract
Background: Mutation of DYRK1A is associated with intellectual disability along with syndromic characteristics like microcephaly, developmental delay, dysmorphic face. Here we present a girl with DYRK1A mutation and SCNIA mutation with syndromic features. Case summary: This is a 7-year 10-month girl with behavioural disorder, autistic feature, speech delay and cognitive impairment, febrile seizure with distinct facial features like bitemporal narrowing, a prominent occiput and deep-set eyes. She also had microcephaly, stereotypes and ataxic gait. Her neuroimaging was non-contributory. Karyotyping was normal, a denovo mutation was noted in DYRK1A+( Location: Intron 8; Variant: C 1239+1 G>A; Zygosity: heterozygous) by next-generation sequencing. Additionally, there was the mutation of SCN1A (Location: Exon 11; Variant: C18 11 G>A; Zygosity: heterozygous). Conclusion: In a case of intellectual disability with facial features with autistic and behavioural disorder DYRK1A gene study should be considered. Our case is a rare case with DYRK1A mutation in intron site along with SCN1A gene mutation. -Keywords
Syndromic intellectual disability; Mutation; DYRK1A; SCN1A
Introduction
Chromosomal deletions encompassing DYRK1A have been associated with intellectual disability for several years. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) is located in the chromosome 21 [1,2]. This gene plays a major role in brain development, specifically neurogenesis, neural plasticity and cellular death [3]. It is already known that protein kinases are key enzymes for the regulation of basic cellular processes in all eukaryotes [4]. It encodes a highly conserved protein that plays an essential role in the development of the central nervous system.
Haploinsufficiency of DYRK1A is responsible for a syndrome characterized by intellectual disability (ID), microcephaly and dysmorphic features. This disorder also includes impaired speech development, autism spectrum disorder including anxious and/or stereotypic behaviour problems and microcephaly. Affected individual often have clinically recognizable phenotypes including a typical facial gestalt, feeding problems, seizures, hypertonia, gait disturbances and foot abnormalities [5-7]. Individuals with this condition were initially reported with partial monosomies of chromosome 21 as detected on routine karyotype that encompassed the DYRK1A gene (21q22.13) [8-10].
Voltage-gated sodium channel SCN1A are associated with a growing number of disorders including Generalized Epilepsy with Febrile Seizures Plus (GEFS+), Severe Myoclonic Epilepsy of Infancy (SMEI) and Familial Hemiplegic Migraine (FHM) [11].
Here, we present one patient with DYRK1A mutations and SCN1A mutation with details of the phenotype and genotype of this emerging syndrome.
Case Description
A 7 year 10 month old girl, presented with inattention, hyperactivity, impaired adaptive behaviour and cognitive delay to a private chamber. On query she had sleep disturbances in the form of decreased night time sleep. Mother also complained of self-abusing habit with biting herself causing trauma of her hands along with some abnormal sexual behaviour in the form of masturbation. In addition to that she had history of febrile seizure since her 10 month of age. Till date she had 7 attacks, all with fever, generalized tonic in nature, persisting for about 2-3 minute with postictal drowsiness. She did not have any history of afebrile seizure, fever with rash, jaundice, visual or hearing impairment.
She is the only issue of her non-consanguineous parents. None of her family members had similar type of illness or history of febrile or afebrile seizure, congenital anomaly or intellectual disability. She was born by lower uterine caesarean section at term. Her birth weight was 2 kg (small for gestational age). She had history of delayed cry and neonatal jaundice for which she was given phototherapy for 4 days. She was developmentally delayed in motor, cognition and speech; she started to sit at 1 year and started to walk at 2 year. Her speech was delayed, at 7 year she could only speak few meaningful words. She could not read or write nor did she go to school. Her vision and hearing was intact. She could feed herself but could not perform her chores. Her social and communication skills were altered; she could not follow the social customs and could not mix and play with peers.
On examination, the girl was conscious, cooperative, not fully oriented to surrounding. Her vitals were within normal limit. Her height was 114 cm (-2.2 SD), weight 15 kg (-3.3SD), BMI: 11.5 (-3.5 SD). OFC 42 cm (<3SD) suggestive of microcephaly. She had bitemporal narrowing, a prominent occiput and deep set eyes (Figure 1). She was not pale, anicteric, skin survey was normal. Nervous system was normal except higher psychic function. Her speech was incoherent, not clear, memory and orientation was altered. She walked with broad-based clumsy gait. She had some stereotypes like hand flapping. Psychological assessment by Bailey scale of infant development III: suggestive that the cognition was at 24 months.
Investigation
EEG: generalized slowing with epileptic discharges in the frontal region of both hemispheres. CT scan of brain showed mild cerebral atrophy without any structural abnormality. Karyotyping was normal, next generation sequencing was done, here only related genes were sequenced. It showed there was pathogenic variant of DYRK1A gene causative of phenotype and as an additional finding there was variant of uncertain significance in SCN1A gene.
Both the variations were detected in heterozygous condition by Sanger sequencing (Figures 2 and 3). The detail of the report was as follows:
1. Gene: DYRK1A+, Location: Intron 8; Variant: C 1239+1 G>A; Zygosity: heterozygous; Disease: Mental Retardation -7; Inheritance: Autosomal dominant; Classification: Pathogenic. 2. Additional finding: Gene: SCN1A (-); Location: Exon 11; Variant: C18 11 G>A; Zygosity: heterozygous; Disease: Generalized Epilepsy with Febrile Seizure Plus type 2; Early Infantile Epileptic Encephalopathy-6 (Dravet Syndrome); Inheritance: Autosomal Dominant; Classification: Uncertain significance.
We treated the girl with speech therapy, behavioural management and physical therapy. As she had sleep problems with treated her with melatonin. Due to financial constrain gene analysis of parents could not be done. So it could not be determined whether it is de novo or not.
Discussion
DYRK1A gene has 13 exons and spans~147.8 Kb of genomic DNA. Pathogenic sequence mutations in this gene result in loss of protein function and account for around 0.5% of syndromic intellectual disability. Patients typically have global developmental delay and microcephaly with a number of other common phenotypes including delayed speech and language, growth restriction, dysmorphic facial features, eye malformations and seizures [12]. It has also been studied extensively in the last decade because of its association with neuronal deficits, dendritic atrophy, spinal dysgenesis, precocious Alzheimer-like neurodegeneration, and cognitive deficits in Down syndrome individuals [13].
Our case was diagnosed late, at the age of 7 year 10 month. In literature, the age range of diagnosis showed a wide range from 17 months to 59 years [14,15]. Onset of clinical features was early in most of the published cases. Prenatal finding were present in 77% cases in one case series published by Loco et al. The antenatal findings were oligohydroamnios, polyhydroaminios, and low birth weight. 15 There was no evidence of antenatal sonography in our case. However, our case was a small for gestational age which matches with the literature and also our patient had history of delayed cry. Like other cases in literature this girl also was born with low birth weight (78% in case series of Jiangi et al.) [13].
Our patient mainly presented with intellectual disability and behavioural issues. Loco et al. and Jiangi Ji et al. also found ID in almost all of their patients in their case series. Other features such as speech delay, global developmental delay is also consistent with the previous studies [13-15]. Our patient was born from a noncosanguineous parents with no similar positive family history. Our case has some severe behavioural issues like presented with inattention, hyperactivity, self-abusing habit and masturbation. Behavioural issues were also common in other related case series (88%) and hyperactivity in 26%. Autism spectrum disorder was present in 45% cases in one case series. Febrile seizures were reported in 67 % cases and epilepsy or non-febrile seizures were observed in 60 % in case one series [15]. This girl only had febrile seizure although her age was beyond the age of simple febrile seizure.
In our case the important physical findings were microcephaly which is a common finding of other cases (86% and 92% in similar studies) [13,15,16]. She had dysmorphic face like bitemporal narrowing, prominent occiput, and deep set eyes. Dysmorphism was also found in case series by Luco et al in 98% of the cases. Our case had broad-based clumsy gait. Majority of the described cases had a broad based ataxic gate. A stiff gate has also been described [13,15,17]. Apart from this she had stereotypes like hand flapping. In this case, CT scan revealed mild cerebral atrophy. In related cases, 76% of patients had abnormal findings namely cortical atrophy, thin brain stem, thin corpus calosum, pituitary stalk hypoplasia, neuronal migration defect, enlarged cisternal megna etc. Among them cortical atrophy was the most common finding which matches with our case [15].
The final diagnosis is done by molecular genetic approaches including single gene testing, use of multi-gene panel and more comprehensive genetic testing [17,18]. We initially did karyotyping which was normal and then did next generation sequencing and sanger sequencing which came out abnormal as DYRKIA gene mutation but the location was in intron 8 which is a rare site.
DYRK1 related intellectual disability syndrome is inherited as an autosomal dominant manner. It usually represents as simplex case: a single occurrence in a family resulting from a de novo DYRK1A pathogenic variant [17]. The majority of mutations occurred within the kinase domain or the N-terminal domain in a previous case series. The mutations were of nonsense, frame shift and 5 splice site mutations [15]. In our case, the mutation occurred in 5 splice site. There does not seem to be a clear genotype-phenotype correlation with the mutations and phenotype reported previously. This gene was initially identified for its role in intellectual disability which is clinically defines as childhood onset of significant cognitive and adaptive impairment. Here it is to mention that recurrent disruption of this gene have been found in as many as 0.5% cases of ASD [14]. In our case autistic features and intellectual disability are present.
In addition to DYRK1A gene mutation, this case also had SCN1A gene mutation, which is heterozygous in nature. The SCN1A gene belongs to a family of genes that provide instructions for making sodium channels. These channels, which transport positively charged sodium atoms (sodium ions) into cells, play a key role in a cell's ability to generate and transmit electrical signals. It provides instructions for making one part (the alpha subunit) of a sodium channel called NaV1.1. These channels are primarily found in the brain, where they control the flow of sodium ions into cells. NaV1.1 channels are involved in transmitting signals from one nerve cell (neuron) to another. Communication between neurons depends on chemicals called neurotransmitters, which are released from one neuron and taken up by neighbouring neurons. The flow of sodium ions through NaV1.1 channels helps determine when neurotransmitters will be released [19].
This mutation has been described as uncertain significance. SCNIA mutation is related to early infantile epileptic encephalopathy 6 (EIEE 6, Dravet syndrome), Generalized Epilepsy With Febrile Seizures Plus, Type 2( GEFSP2), familial hemiplegic migraine 3 (FHM3) and autism.19 Our patient has repeated attacks of febrile generalized epilepsy and autistic features which may be correlated with the mutation although the mutation was suggested as variance of unknown significance.
Outpatient had features of intellectual disability, dimorphism which coincides with DYRK1A mutation while she had features of autism and epilepsy which coincides with SCN1A mutation. But in DYRKIA mutation autistic features may occur, so it is uncertain that the child’s clinical features reflect only one or both gene mutation.
There is no reported case of mutation of both DYRK1A and SCN1A in same patient till date.
Conclusion
Any child with syndromic features, intellectual disability, and behavioural abnormality should be addressed meticulously and DYRK1A mutation should be considered. Our case is a rare case with DYRK1A mutation in intron 8 associated with SCN1A gene mutation.
Conflict of Interest
There is no conflict of interest.
Funding
No funding has been taken for this paper.
Ethical Implications
Written informed consent has been taken from the parent of the patient. Ethical clearance has been taken from the ethical review committee of the institute.
References
- Guimera J, Casas C, Pucharcos C, et al. A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum Mol Genet. 1996;5(9):1305–10.
- Iossifov I, O'Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–21.
- Bronicki LM, Redin C, Drunat S, et al. Ten new cases further delineate the syndromic intellectual disability phenotype caused by mutations in DYRK1A. Eur J Hum Genet. 2015;23(11):1482-7.
- Aranda S, Laguna A, de la Luna S. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011;25(2): 449–62.
- Hämmerle B, Vera-Samper E, Speicher S, et al. Mnb/Dyrk1A is transiently expressed and asymmetrically segregated in neural progenitor cells at the transition to neurogenic divisions. Dev Biol. 2002;246(2):259–73.
- Lagran MMD, Benavides-Piccione R, Ballesteros-Yanez I, et al. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. Cereb Cortex. 2012;22(12):2867–77.
- Soppa U, Schumacher J, Florencio Ortiz V, et al. The Down syndrome-related protein kinase DYRK1A phosphorylates p27Kip1 and Cyclin D1 and induces cell cycle exit and neuronal differentiation. Cell Cycle. 2014;13(13):2084–100.
- Chettouh Z, Croquette MF, Delobel B, et al. Molecular mapping of 21 features associated with partial monosomy 21: involvement of the APP-SOD1 region. Am J Hum Genet. 1995;57(1):62–71.
- Matsumoto N, Ohashi H, Tsukahara M, et al. Possible narrowed assignment of the loci of monosomy 21-associated microcephaly and intrauterine growth retardation to a 1.2-Mb segment at 21q22.2. Am J Hum Genet. 1997;60(4):997–9.
- Bartsch O, Hinkel GK, Petersen MB, et al. A large family with subtelomeric translocation t (18; 21)(q23; q22. 1) and molecular breakpoint in the Down syndrome critical region. Hum Genet. 1997;100(5–6):669–75.
- Melinda S. Martin, Karoni Dutt, Ligia A. Papale, et al. Altered function of the SCN1A voltage-gated sodium channel leads to GABAergic interneuron abnormalities. J Biol Chem. 2010;285(13):9823–34.
- Jochem M.G. Evers. Structural analysis of pathogenic mutations in the DYRK1A gene in patients with developmental disorders. Hum Mol Genet. 2017;26(3):519-526.
- Ji J, Lee H, Argiropoulos B, et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. Eur J Hum Genet. 2015;23(11): 1473–81.
- Earl RK, Turner TN, Mefford HC, et al. Clinical phenotype of ASD-associated DYRK1A haploinsufficiency. Mol Autism. 2017;8:54.
- Luco SM, Pohl D, Sell E, et al. Case report of novel DYRK1A mutations in 2 individuals with syndromic intellectual disability and a review of the literature. BMC Med Genet. 2016;17:15.
- Courcet JB, Faivre L, Malzac P, et al. The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. J Med Genet. 2012;49(12):731-6.
- Van Bon BWM, Coe BP, de Vries BBA, et al. DYRK1A-Related Intellectual Disability Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® 2015. Seattle (WA): University of Washington, Seattle; 1993-2019.
- Møller RS, Kübart S, Hoeltzenbein M, et al. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am J Hum Genet. 2008;82(5):1165-70.
- Hamosh A, Scoot AF, Amberger J, et al. Mendelian Inheritance in Man, OMIM. Hum Mutat. 2000;15(1):57-61.