Diencephalic syndrome in child with NF-1 and hypothalamic tumour
2 Children’s Brain Tumour Research Centre of Nottingham, University of Nottingham, Nottingham, UK, Email: Pilotto.chiara@spes.uniud.it
3 Department of Neuroradiology, Nottingham University Hospitals NHS Trust, Nottingham, UK
4 Department of Neurosurgery, Nottingham University Hospitals NHS Trust, Nottingham, UK
5 Nottingham University Hospitals NHS Trust, Nottingham, UK
6 Department of Ophthalmology, Nottingham University Hospitals NHS Trust, Nottingham, UK
Received: 06-Jul-2018 Accepted Date: Jul 23, 2018; Published: 30-Jul-2018
Citation: Pilotto C, Thomas A, Simon PSH, et al. Diencephalic syndrome in child with NF-1 and hypothalamic tumour. J Neurol Clin Neurosci. 2018;2(3)::01-03.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
We describe a 20 months old boy with neuro-fibromatosis type 1 (NF-1) who presented with diencephalic syndrome due to a large hypothalamic tumour and developed massive necrosis after chemotherapy associated with severe encephalopathy.
We report this case because of rapid progression of presenting symptoms, the rare association with diencephalic syndrome in NF-1, chemotherapy induction of “tumour lysis” associated with encephalopathy, reduced toxicity and sustained improvement with vinblastine, the therapeutic benefit of tumour drainage signs of resolution of diencephalic syndrome and then restoration of visual movements and function associated with developmental recovery. The presentation of tumour in this case highlights the importance for parents and doctor to known and recognize the precocious symptoms, and justifies sharing these features as an indicator with parents and GP’s to justify early / urgent specialist review, particularly in the first two years of life. Early recognition could offer a reduced risk of brain injury.
Keywords
NF-1; Diencephalic syndrome; Encephalopathy; Therapeutical approach
Introduction
NF-1 as one of the most common genetic disorders, is associated with an increased risk of benign and malignant tumours, the commonest in childhood being visual pathway glioma (VPG) [1,2]. Surveillance with clinical, ophthalmological examination or imaging in early life is performed to monitor for VPG, which can affect up to 20% of children with NF-1. The diencephalic syndrome (DS) is a rare disorder characterized by progressive emaciation and failure to thrive, with a normal caloric intake, due to a hypothalamic lesion. Its association with NF-1 has been reported rarely in literature [3]. DS, whilst well described with hypothalamic low grade astrocytoma, is extremely rare in children with NF-1, and there is one case reported with DS presenting as disease progression in a child with NF-1 and VPG [4].
Case Report
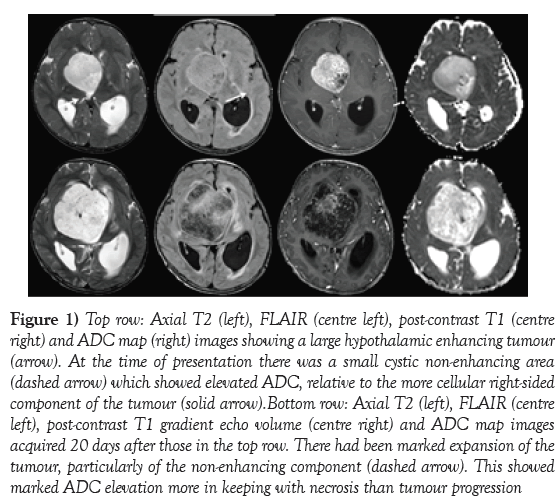
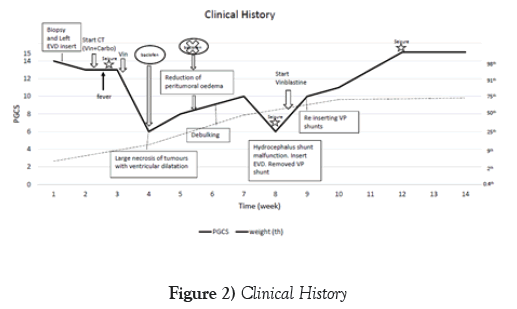
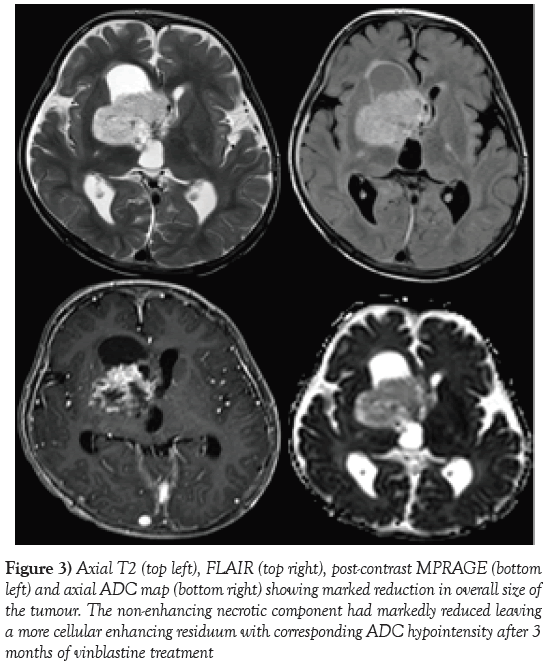
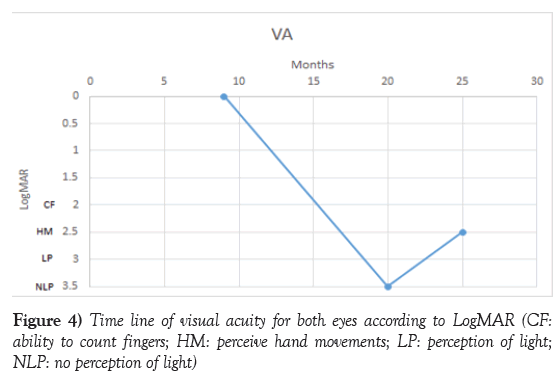
A 9-months old boy with a diagnosis of NF-1 (maternal history of NF-1 and presence of more than 6 café au lait macules) had been under surveillance with regular clinical and ophthalmic reviews. Normal growth, development and vision had been recorded. He was admitted at 20 months of age with a 5 months history of weight loss (50nd-2nd Centile), vomiting, poor feeding, rapid head growth (75th-91st centile) and developmental regression. He had right eye ptosis with pale optic discs. Cranial MRI showed a large hypothalamic lesion (4.9 x 4.9 x 4.5 cm) (Figure 1) which when biopsied showed a pilocytic astrocytoma with pilomyxoid areas, diffuse expression of GFAP, and no expression of IDH-1. ATRX was intact. BRAF V600 mutation was negative. Chemotherapy was commenced with carboplatin vincristine regimen. After the first treatment he presented acutely with fever, quadriparesis, and unequal pupils. Cranial MRI showed an increased tumour volume and hydrocephalus (Figure 1), which subsequently reduced. His neurological status fluctuated over a 7 week period (Figure 2). Surgical tumour debulking and re-establishment of ventricular drainage stabilised his condition and chemotherapy was restarted with vinblastine 6 mg/m2 weekly, decreasing to 5 mg/m2 to reduce the chemorelated pancytopenia. The last MRI showed a further reduction of tumour volume (Figure 3). Developmental progress and weight gain was re-established. There was a mild residual left hemi paresis. Despite persistent bilateral optic atrophy the child had light perception bilaterally (Figure 4).
Figure 1) Top row: Axial T2 (left), FLAIR (centre left), post-contrast T1 (centre right) and ADC map (right) images showing a large hypothalamic enhancing tumour (arrow). At the time of presentation there was a small cystic non-enhancing area (dashed arrow) which showed elevated ADC, relative to the more cellular right-sided component of the tumour (solid arrow).Bottom row: Axial T2 (left), FLAIR (centre left), post-contrast T1 gradient echo volume (centre right) and ADC map images acquired 20 days after those in the top row. There had been marked expansion of the tumour, particularly of the non-enhancing component (dashed arrow). This showed marked ADC elevation more in keeping with necrosis than tumour progression
Figure 3) Axial T2 (top left), FLAIR (top right), post-contrast MPRAGE (bottom left) and axial ADC map (bottom right) showing marked reduction in overall size of the tumour. The non-enhancing necrotic component had markedly reduced leaving a more cellular enhancing residuum with corresponding ADC hypointensity after 3 months of vinblastine treatment
Discussion
The delayed presentation with advanced diencephalic syndrome, blindness and developmental regression highlights the importance of parents and doctors in Primary care understanding the increased risk of tumour development in NF-1 patients. Early recognition of symptoms should reduce the risk of brain injury. Biopsy was undertaken to ascertain both histology prior to treatment but also to obtain tumour samples for bio-characterisation as new molecular markers may become the focus for new targeted therapy in trials and clinical practice during this child’s childhood. Primary treatment with chemotherapy is the current recommended approach [5] . Chemotherapy can be expected to offer improvement in DS (>70%) and >80% tumour non-progression rates in children with NF-1. However the effects on vision are not well reported [6-10]. Vincristine and Carboplatin, whilst the commonest combination for all VPGs, vinblastine monotherapy also has a growing evidence base for NF-1 associated VPG. The exaggerated tumour necrosis seen here has not be previously reported in either NF-1 associated VPG or in sporadic hypothalamic low grade glioma. This precipitates the suggestion that hypothalamic tumour associated with NF- 1, may justify a cautious approach to drug selection, perhaps justifying the use of vinblastine monotherapy in order to reduce intensity, risk of bone marrow and neurotoxicity whilst seeking equivalent or enhanced tumour control [11,12].
The imaging showed rapid expansion of the non-enhancing component of the tumour with associated increased diffusivity, in keeping with tumour necrosis rather than progression. This radiological response is frequently encountered following radiotherapy treatment but is extremely unusual after the use of chemotherapeutic agents. In distinction to the usual slow resolution of such findings after radiotherapy, in this case the radiological improvement occurred much more rapidly. In case of clinical doubt, further evaluation using MR perfusion (expected low relative cerebral blood volume) and MR spectroscopy (presence of lipid/macromolecular peak rather than choline elevation) would also be confirmatory.
The clinical improvement that occurred after tumour debulking and shunt revision was characterised by weight gain (from the 5th to above the 50th centile), resolution of encephalopathy and progressive recovery of vision. Recovery of weight after treatment of DS is reported within 6 months [13- 15]. The neurological recovery with improving of spasticity and recovery of vision was unexpected yet gratifying, fully justifying the attention of the multi-disciplinary brain injury rehabilitation team [16]. Seizures were controlled with levitiracetam. Symptoms of spasticity and associated pain were controlled with baclofen infusions, botox injections and neuropathic pain drugs. Hormonal deficiencies were replaced by the endocrine team and rehabilitation specialists sustained programmes of movement and stimulation to maximise attention and daily routines. Nutritional management required a combination of supplementary feeding using oral NG and IV feeding according to requirements.
Visual recovery came last with recovery of ophthalmoplegia and total blindness on initial examination. This is in contrast to studies which suggest that the visual loss is irreversible [17,18]. There are studies where less severe vision loss is demonstrated to improve or at least remain stable in 72% of subjects after treatment [19].
Conclusion
This child’s serious condition at referral represented progressive brain injury which with early warning advice could have been avoided. At the time of diagnosis of NF-1, and at subsequent annual reviews, there should be a balanced discussion with parents, explaining that complications are rare, but that unusual symptoms warrant early review by a Doctor with knowledge of NF-1. Information on the Headsmart website [20] should be given to parents and sent to GP, although developmental regression has not been recognised as a feature for the under 5 year old group. The association between DS and NF-1 is rare and may be an indication of enhanced chemosensitivity suggesting a cautious approach to chemotherapy. Where tumour necrosis after chemotherapy had occurred, surgical debulking and shunt revision were helpful interventions. Sustaining an optimistic approach with full rehabilitation and detailed clinical management has allowed recovery with hopeful prognosis, including early signs of partial vision recovery, despite complete blindness at presentation.
Acknowledgements
East Midlands Children and Young People’s Integrated Cancer Service (EMCYPICS).
REFERENCES
- Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handb Clin Neurol. 2013;115: 939-55.
- Bizzarri C, Bottaro G. Endocrine implications of neurofibromatosis 1 in childhood. Horm Res Paediatr. 2015;83(4): 232-41.
- Yazici N, Varan A, Akalan N, et al. Diencephalic tumors in children: a 30-year experience of a single institution. Childs Nerv Syst 2011;27(8): 1251-6.
- Cavicchiolo ME, Opocher E, Daverio M, et al. Diencephalic syndrome as sign of tumor progression in a child with neurofibromatosis type 1 and optic pathway glioma: a case report. Childs Nerv Syst. 2013;29(10):1941-45.
- Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Onco. 2013;15(4): 462-8.
- Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children's Oncology Group. Cancer. 2016;122(12):1928-36.
- Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747-54.
- Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265-84.
- Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(21):2641-7
- Gropman AL, Packer RJ, Nicholson HS, et al. Treatment of diencephalic syndrome with chemotherapy: growth, tumor response, and long term control. Cancer. 1998;83(1):166-72.
- Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358-63.
- Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II Weekly Vinblastine for Chemotherapy-Naive Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2016;34(29):3537-43.
- Kilday JP, Bartels U, Huang A, et al. Favorable survival and metabolic outcome for children with diencephalic syndrome using a radiation-sparing approach. J Neurooncol. 2014;116(1):195-204.
- Sardi I, Bresci C, Schiavello E, et al. Successful treatment with a low-dose cisplatin--etoposide regimen for patients with diencephalic syndrome. J Neurooncol. 2012;109(2): 375-83.
- Arends J, Bodoky G, Bozzetti F, et al. Espen (ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr. 2006;25(2):245-59.
- Fountain DM, Burke GA. Multidisciplinary rehabilitation for children with brain tumors: A systematic review. Dev Neurorehabil. 2017;20(2):68-75.
- Moreno L, Bautista F, Ashley S, et al. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46(12):2253-9.
- Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Onco. 2007;19(4):430-7
- Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6);790-7
- www.headsmart.org.uk