Disorders of Ca2+ handling and cell-to-cell coupling are implicated in the development of acute heart failure in intact animal hearts: An ultrastructural study
- *Corresponding Author:
- Dr Narcis Tribulova
Institute for Heart Research, Slovak Academy of Sciences, 840 05 Bratislava, Dúbravská cesta 9, PO Box 104.
Telephone: 004212 5466405
fax: 004212 54776637
E-mail: narcisa.tribulova@savba.sk
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
Ca2+ overload; Guinea pig; Malignant arrhythmias; Pig; Rat; Ultrastructure
General classification of cardiac arrhythmias assumes that all disturbances of rhythm result from one of two primary abnormalities in electrical activity. The first is an abnormality in impulse initiation and the second is an abnormality in impulse propagation, although both may coexist. The former is particularly associated with triggered activity and/or abnormal automaticity, whereas the latter with conduction block and re-entry [1,2]. In both clinical and experimental settings, enhanced triggered activity may result from early and delayed after-depolarization (EAD and DAD, respectively) [3]. These abnormalities in cardiac electrical activity are attributed primarily to disturbed Ca2+ handling [3,4]. Dysregulated, spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) occurs in the form of waves of self-propagating Ca2+-induced Ca2+ release [5]. Altered Ca2+ handling in chronic pathophysiological conditions results from permanent upregulation/downregulation of Ca2+ transport systems, while in acute events from transient activation/inhibition of those systems. Ca2+ leaks, suppression of SR Ca2+-ATPase or activation of the Na+/Ca2+ exchanger result in Ca2+overload, which has been implicated in both arrhythmias and contractile dysfunction associated with either chronic or acute heart failure [6-9].
Importantly, a high free concentration of Ca2+ impairs electrical coupling at the gap junctions via inhibition of Cx43 channels that can affect conduction velocity [10-12]. Consequently, cardiac cell-to-cell uncoupling may result in slowing and blocking of conduction, thereby facilitating development of re-entrant arrhythmias such as ventricular fibrillation (VF) and atrial fibrillation (AF) [2,3,13-19]. In both clinical and experimental settings, VF is often initiated by triggered activity (due to EAD or DAD) and occurs in the presence of defects of myocardial intercellular coupling. Cell-to-cell uncoupling also promotes an increase in the dispersion of refractoriness [20], another factor that favours the development of re-entrant arrhythmias [3]. Evidence suggests that focal areas of uncoupling in the myocardium increase the likelihood of arrhythmic triggers and more widespread uncoupling is required to support sustained arrhythmias [21,22]. The mechanisms underlying AF are complex, involving increased spontaneous ectopic firing of atrial cells and impulse re-entry through atrial tissue. Triggered activity as well as impairment of Ca2+ handling and cell-to-cell coupling that may lead to altered conduction properties and multiple reentrant circuits is likely implicated in the development of AF [15,23,24].
Most life-threatening arrhythmias occur in patients or animals with structural heart disease, in whom arrhythmogenic substrates play an important role. Individuals without structural heart disease may also suffer from arrhythmias [25,26] due to genetically aberrant ionic currents (eg, Ik, RyR), or acute conditions that modify both Ca2+ handling and ion channel function (eg, drugs, oxidative stress).
AF is the most common arrhythmia that increases mortality, risk for stroke and morbidity. Prolonged duration of AF leads to difficulties in conversion into sinus rhythm and maintainance of sinus rhythm after successful conversion. It is due to abnormal Ca2+ handling as well as pronounced structural and electrophysiological remodelling in chronic AF [23-25,27].
VF is the main cause of sudden cardiac death. To prevent its occurrence in high-risk patients, an implantable cardioverter defibrillator is used to terminate VF by electrical shock followed by sinus rhythm restoration. However, there are common problems such as failure of electrical cardioversion and postshock re-initiation of VF, which, consequently, increase the number of shocks needed [28,29].
Despite progressive current therapies based on implantable cardioverter defibrillator and catheter ablation, both VF and AF remain a major health problem. Further understanding of the mechanisms and factors responsible for the onset and maintenance of these arrhythmias is essential. There is also a need to explore possible factors that may affect outcome of electrical or drug-induced cardioversion.
We have previously shown that acute interventions such as low K+ perfusion, burst atrial pacing or intramyocardial noradrenaline (NA) infusion facilitate occurrence of severe cardiac arrhythmias, whereby alterations in Ca2+ and gap junctions are most likely involved [15,16,18,30,31]. The purpose of the present study was to comprehensively determine the impact of Ca2+ disorders on the ultrastructure of cardiomyocytes before occurrence and during sustaining of these arrhythmias in intact hearts.
Methods
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, Publication No 85-23, revised 1996. All experiments were approved by the respective institutional animal care and use committees.
Taking into consideration the differences in cardiac Ca2+ handling between rats and guinea pigs [32], atria and ventricles [33], and small and large mammals [27], ultrastructural alterations of the cardiomyocytes in the hearts of rats, guinea pigs and Landrace pigs in previously reported acute conditions [15,16,30,31] to induce Ca2+ disturbances and arrhythmias were explored.
The following experimental protocols were used: low K+ perfusion of isolated rat heart to induce sustained VF; low K+ perfusion of isolated guinea pig heart to induce sustained VF; burst atrial pacing of isolated guinea pig heart to induce prolonged AF; and focal intramyocardial NA infusion in open-chest pig to induce ventricular tachycardia (VT).
Low K+ perfusion of isolated rat heart
Adult Wistar Kyoto rats were anesthetized and the hearts were quickly excised and perfused via cannulated aorta at constant pressure with oxygenated Krebs-Henseleit solution containing (in mmol/L) 118 NaCl, 25 NaHCO3, 2.9 KCl, 1.2 MgSO4, 1.8 CaCl2, 1.3 KH2PO4 and 11.5 glucose. Bipolar electrocardiogram was continuously recorded. After 20 min of equilibration period in standard Krebs- Henseleit solution, the rat hearts were perfused with a low K+ (1.2 mmol/L) solution for a period of 60 min, unless sustained VF lasting 2 min occurred earlier (as reported previously [30]). The hearts were harvested at the end of the equilibration period (n=6), at 15 min of low K+ perfusion (n=6), and at 2 min of VF (n=6) for electron microscopy examination.
Low K+ perfusion of isolated guinea pig heart
Adult guinea pigs were euthanized by stunning and the aorta of the excised heart was immediately cannulated for perfusion at constant pressure with oxygenated Tyrode solution (in mmol/L: 136.9 NaCl, 2.8 KCl, 1.8 CaCl2, 1.0 MgCl2, 11.9 NaHCO3, 0.4 NaH2PO4 and 11.5 glucose). Bipolar epicardial electrocardiograms were continuously monitored. After 15 min of stabilization with standard solution, the hearts were perfused with a low K+ solution (1.4 mmol/L) until sustained VF occurred (as previously reported [16]). The hearts were harvested during stabilization (n=6), at 15 min of low K+ perfusion (n=6) and during VF (n=6) for electron microscopy examination.
Burst atrial pacing of isolated guinea pig heart
Aged guinea pigs were euthanized by stunning and the aorta of the excised heart was immediately cannulated for the perfusion at constant pressure with oxygenated modified Tyrode solution (in mmol/L: 126 NaCl, 2.8 KCl, 1.8 CaCl2, 1.0 MgCl2, 24 NaHCO3, 0.4 NaH2PO4 and 5.5 glucose). After 20 min of equilibration, the left atrium was subjected to programmed stimulation by a 1 s burst of electrical rectangular pulses, 50 pulses/s to 70 pulses/s, 1 ms in duration and 1.5 times the threshold voltage. The right atrial electrocardiogram was recorded for incidence of arrhythmias (as previously reported [15]). For the examination, atrial tissue was taken during basic conditions (n=6), during pacing (n=6) and during AF (n=6).
Focal intramyocardial NA infusion in open-chest pig
Adult domestic Landrace pigs were anesthetized, ventilated and subjected to sternotomy. Electrocardiogram and aortic blood pressure were recorded. Left ventricular intramyocardial infusion of 10 μL/min of NA (100 μmol/L) in the presence of Ca2+ (2.5 mmol/L) was used to induce VT. The drill biopsies were randomly obtained from the left ventricle during basal conditions (n=6), upon 50 s of infusion (n=6) and during VT (n=6) from infusion area (as previously reported [31,34]).
For transmission electron microscopic examinations, small, approximately 1 mm3 tissue blocks of the left ventricle were fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate, postfixed in 1% osmium tetroxide, dehydrated in ethanol, infiltrated by propylene oxide and embedded in resin (Epon 812, Serva Heidelberg, Germany). The ultrathin sections were stained with uranyl acetate and lead phosphate, and examined using an electron microscope (Tesla BS 500, Tesla Brno, Czech Republic).
Results
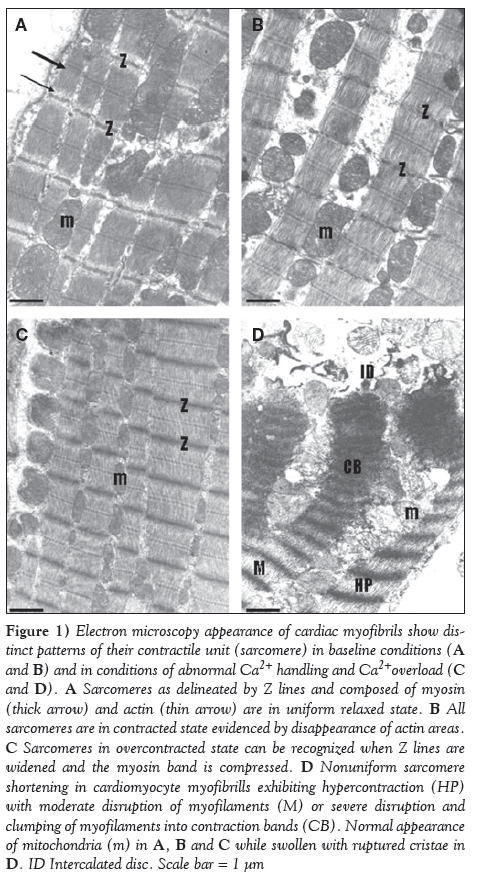
Representative electron microscopic images of cardiomyocyte myofbrils and their contractile units, ie, sarcomeres, in four distinct patterns, are shown in Figure 1. In the heart during normal conditions, regular contraction is followed by relaxation and these processes are visible according to relaxed and contracted state of sarcomeres (Figures 1A and 1B). Pathophysiological conditions, particularly those accompanied by disturbances in cytosolic [Ca2+]i, result in disturbances in contraction-relaxation processes that is recognized by overcontracted or hypercontracted cardiomyocytes (Figure 1C) and even by the presence of contracture with contraction bands (Figure 1D). The latter is considered to be an irreversible change, jeopardizing the viability of the cardiomyocyte.
Figure 1: Electron microscopy appearance of cardiac myofibrils show distinct patterns of their contractile unit (sarcomere) in baseline conditions (A and B) and in conditions of abnormal Ca2+ handling and Ca2+overload (C and D). A Sarcomeres as delineated by Z lines and composed of myosin (thick arrow) and actin (thin arrow) are in uniform relaxed state. B All sarcomeres are in contracted state evidenced by disappearance of actin areas. C Sarcomeres in overcontracted state can be recognized when Z lines are widened and the myosin band is compressed. D Nonuniform sarcomere shortening in cardiomyocyte myofibrills exhibiting hypercontraction (HP) with moderate disruption of myofilaments (M) or severe disruption and clumping of myofilaments into contraction bands (CB). Normal appearance of mitochondria (m) in A, B and C while swollen with ruptured cristae in D. ID Intercalated disc. Scale bar = 1 μm
Low K+ perfusion of isolated rat and guinea pig hearts and sustained VF
Within 10 min to 20 min of perfusion of rat heart with a K+-deficient Krebs-Henseleit solution, the incidence of premature beats, bigeminy, and transient VT and/or VF were registered and these preceded the development of sustained VF. This lethal arrhythmia occurred during 20 min to 40 min of low K+ perfusion (as previously reported [30]).
The continual recordings of ventricular bipolar electrocardiograms during low K+ perfusion of guinea pig heart revealed changes in R and T configuration, changes in the R vector, incidence of premature beats, bigeminy and sudden VT, which usually degenerated into VF. Sustained VF appeared within 15 min to 30 min of low K+ perfusion (as previously reported [16]).
Myocardial ultrastructure alterations: Ultrastructural examination revealed that in comparison with the normal subcellular architecture of the cardiomyocytes and preserved intermyocyte junctions, the myocardial tissue from both rat and guinea pig heart subjected to K+-deficient perfusion was characterized by nonuniformly altered cardiomyocytes. Accordingly, the majority of cardiomyocytes were reversibly altered, exhibiting subcellular changes of various degrees. Sporadic irreversibly injured cardiomyocytes were present. Nonuniformly affected cardiomyocytes were heterogeneously distributed throughout the myocardium. Severely damaged cardiomyocytes were edematous with apparently injured integrity of mitochondria and intermyocyte junctions. Less-affected cardiomyocytes exhibited mild edema and moderate mitochondrial alterations as well as a mild dissociation of fascia adherens junctions.
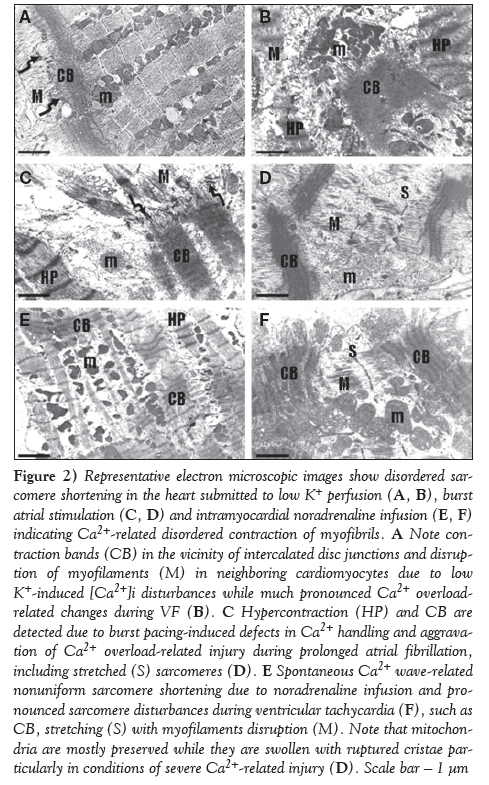
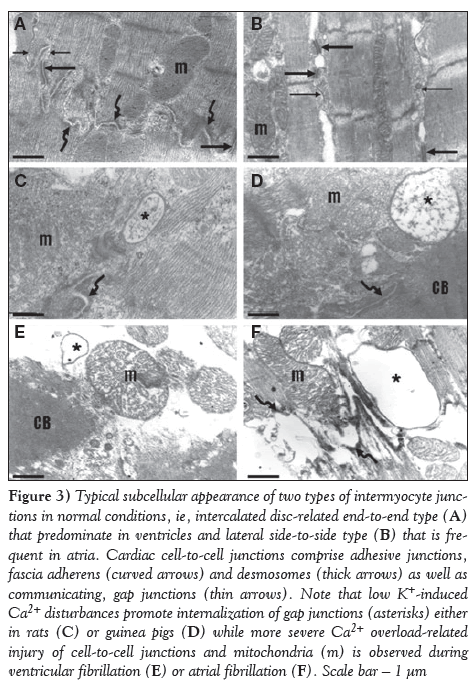
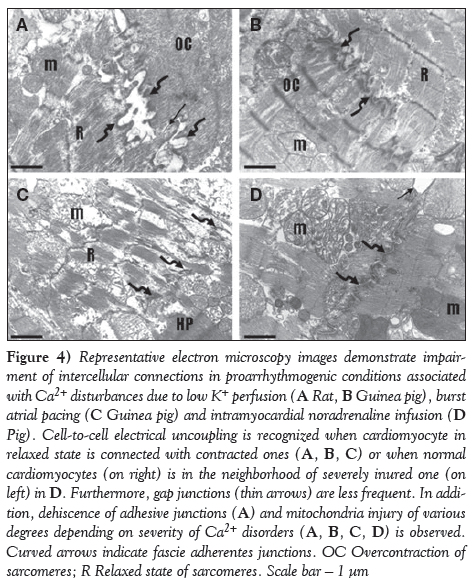
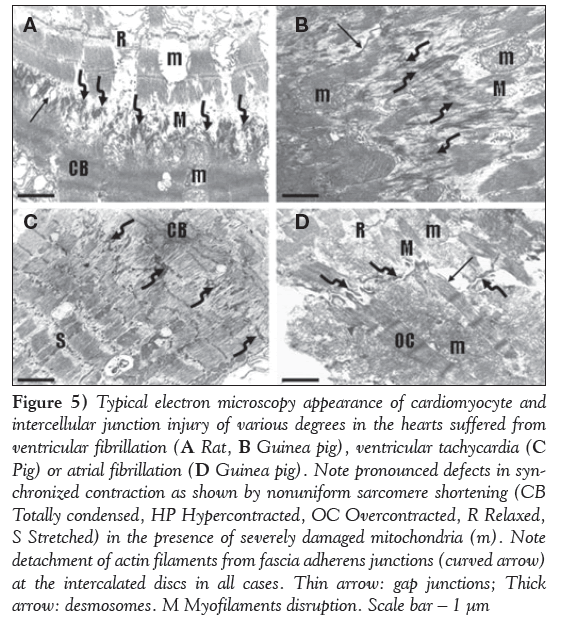
As demonstrated on representative electron microscopic images, perfusion of either rat or guinea pig hearts with K+-deficient solution caused irregular contraction of majority of cardiomyocytes, whereby overcontraction and hypercontraction of sarcomeres or, sporadically, contraction bands were observed (Figure 2A), as well as apparent alterations in intercellular junctions, ie, in addition to normal connections of neighbouring cardiomyocytes with adhesive junctions and gap junctions at the intercalated disc (Figure 3A), the internalization of gap junctions in the form of annular profiles was detected (Figures 3C and 3D). Furthermore, dehiscence of adhesive junctions was frequently found (Figure 4A). Impairment of gap junctionmediated cell-to-cell coupling was recognized when neighbouring cardiomyocytes differed in patterns of sarcomeres, ie, contracted in one and relaxed in the adjacent cardiomyocyte (Figures 4A and 4B). Overall, these changes were more pronounced due to transient arrhythmias and preceded occurrence of sustained VF. Marked deterioration of ultrastructure indicating cardiac cell-to-cell uncoupling and dysregulation of synchronized contraction was observed in fibrillating myocardium (Figures 2B, 3E, 5A and 5B).
Figure 2: Representative electron microscopic images show disordered sarcomere shortening in the heart submitted to low K+ perfusion (A, B), burst atrial stimulation (C, D) and intramyocardial noradrenaline infusion (E, F) indicating Ca2+-related disordered contraction of myofibrils. A Note contraction bands (CB) in the vicinity of intercalated disc junctions and disruption of myofilaments (M) in neighboring cardiomyocytes due to low K+-induced [Ca2+]i disturbances while much pronounced Ca2+ overloadrelated changes during VF (B). C Hypercontraction (HP) and CB are detected due to burst pacing-induced defects in Ca2+ handling and aggravation of Ca2+ overload-related injury during prolonged atrial fibrillation, including stretched (S) sarcomeres (D). E Spontaneous Ca2+ wave-related nonuniform sarcomere shortening due to noradrenaline infusion and pronounced sarcomere disturbances during ventricular tachycardia (F), such as CB, stretching (S) with myofilaments disruption (M). Note that mitochondria are mostly preserved while they are swollen with ruptured cristae particularly in conditions of severe Ca2+-related injury (D). Scale bar ? 1 μm
Figure 3: Typical subcellular appearance of two types of intermyocyte junctions in normal conditions, ie, intercalated disc-related end-to-end type (A) that predominate in ventricles and lateral side-to-side type (B) that is frequent in atria. Cardiac cell-to-cell junctions comprise adhesive junctions, fascia adherens (curved arrows) and desmosomes (thick arrows) as well as communicating, gap junctions (thin arrows). Note that low K+-induced Ca2+ disturbances promote internalization of gap junctions (asterisks) either in rats (C) or guinea pigs (D) while more severe Ca2+ overload-related injury of cell-to-cell junctions and mitochondria (m) is observed during ventricular fibrillation (E) or atrial fibrillation (F). Scale bar ? 1 μm
Figure 4: Representative electron microscopy images demonstrate impairment of intercellular connections in proarrhythmogenic conditions associated with Ca2+ disturbances due to low K+ perfusion (A Rat, B Guinea pig), burst atrial pacing (C Guinea pig) and intramyocardial noradrenaline infusion (D Pig). Cell-to-cell electrical uncoupling is recognized when cardiomyocyte in relaxed state is connected with contracted ones (A, B, C) or when normal cardiomyocytes (on right) is in the neighborhood of severely inured one (on left) in D. Furthermore, gap junctions (thin arrows) are less frequent. In addition, dehiscence of adhesive junctions (A) and mitochondria injury of various degrees depending on severity of Ca2+ disorders (A, B, C, D) is observed. Curved arrows indicate fascie adherentes junctions. OC Overcontraction of sarcomeres; R Relaxed state of sarcomeres. Scale bar ? 1 μm
Figure 5: Typical electron microscopy appearance of cardiomyocyte and intercellular junction injury of various degrees in the hearts suffered from ventricular fibrillation (A Rat, B Guinea pig), ventricular tachycardia (C Pig) or atrial fibrillation (D Guinea pig). Note pronounced defects in synchronized contraction as shown by nonuniform sarcomere shortening (CB Totally condensed, HP Hypercontracted, OC Overcontracted, R Relaxed, S Stretched) in the presence of severely damaged mitochondria (m). Note detachment of actin filaments from fascia adherens junctions (curved arrow) at the intercalated discs in all cases. Thin arrow: gap junctions; Thick arrow: desmosomes. M Myofilaments disruption. Scale bar ? 1 μm
Burst atrial pacing of isolated guinea pig heart and AF
During the first 5 min of repetitive stimulation, a few brief poststimulus arrhythmias, atrial tachycardias and fibrillo-flutters were recorded. Within the 5 min to 30 min period of burst pacing, prolonged poststimulus AF lasting 0.5 min to 15 min was detected; however, no arrhythmias were registered in the left ventricle (details are reported in [15]).
Myocardial ultrastructure alterations: Normal appearance of atrial subcellular structure was observed in basal conditions. Cardiomyocytes exhibited uniform pattern of sarcomeres either in contracted or relaxed forms (Figure 3B). Both types of gap junctions were frequently observed (ie, intercalated disc related end-to-end form, similarly in the ventricle [Figure 3A], and lateral side-to side gap junctions [Figure 3B]). Prolonged burst pacing that was accompanied by transient posstimulus arrhythmias resulted in cardiomyocyte alterations that were heterogenosly distributed throughout the myocardium and consisted of mitochondria injury, nonuniform sarcomere shortening and impairment of cardiac cell-to-cell coupling (Figures 2C and 4C). These changes preceded the occurrence of prolonged AF, which aggravated further cardiomyocyte and cell-to-cell junctions injury (Figures 2D and 5D).
Intramyocardial NA infusion in open-chest pig and VT
Focal left ventricular intramyocardial infusion of NA in the presence of calcium in open-chest pigs consistently produced VT within 60 s. Before the development of VT, the triggered activity was recognized by focally enhanced contractility in the area of infusion. Premature beats initiated VT that could be maintained for 30 min (as previously reported [31,34]).
Myocardial ultrastructure alterations: NA infusion resulted in prominent Ca2+ overload-related changes consisting of nonuniform pattern of myofibrils in individual cadiomyocytes, ie, exhibiting dramatic shortening of the sarcomere in the vicinity of relaxing sarcomeres (Figures 2E and 2F). Impairment of electrical coupling between two neighbouring cardiomyocytes was recognized when one was in a contracted state while the other was in a relaxed state. This was observed particularly during VT (Figures 4D and 5D). Overall, features were characterized by nonuniform alterations of the cardiomyocytes and their junctions throughout myocardium in the area of infusion.
Discussion
In the present study, we characterized subcellular changes in rat, guinea pig and Landrace pig hearts subjected to various acute proarrhythmogenic conditions. Comparative findings suggest that there is a common feature of ultrastructural alterations that precede the occurrence of life-threatening cardiac arrhythmias regardless of species and cardiac atria/ventricular-related differences in Ca2+ handling and intercellular coupling. Most importantly, the findings demonstrate the impact of disturbances in [Ca2+]i and cell-to-cell coupling, key factors implicated in the development of AF and VF, on the ultrastructure of the cardiomyocytes and their junctions.
To strengthen this approach, we placed representative electron microscopy images from four types of experiments together in one panel for clear and comparative demonstration. To avoid interference resulting from heart disease, we used healthy animals with intact hearts for the investigation. The heart was exposed to acute conditions, such as hypokalemia, sudden increase of catecholamines or fast-beating rates that in some way mimic clinical proarrhythmogenic events. K+-deficient perfusion resulted in a decrease of heart rate, prolonged repolarization, lengthened action potential duration and increased [Ca2+]i [18,30]. In parallel with these changes, there was an increase in the incidence of triggered electrical activity manifested by EAD that was linked with the occurrence of ventricular premature beats [18,30]. Focal intramyocardial infusion of NA resulted in regional positive chrono- and inotropic effects due an increase of cyclic AMP and shortening of action potential duration, promoting DAD and VT induction [34]. Cardiac burst pacing ex vivo or chronic fast pacing in vivo are current models to induce and investigate the mechanisms of AF [17]. After prolonged episodes of rapid electrical activity, the atrial action potential was shortened because of a reduction in the IKs-type calcium current [3]. Moreover, AF is initiated by triggered activity most likely due to Ca2+ disturbances.
The hallmarks of the normal heart function are both rapid contraction/ relaxation of the ‘working’ cardiomyocytes and rapid electrical impulse propagation between them. In such conditions, the contractile units (sarcomeres) are in synchrony, as shown in Figures 1A and 1B. Calcium is crucial in normal cardiac contraction, while disturbances of excitation-contraction coupling result in both contractile dysfunction and arrhythmogenesis, particularly in cardiac disease but also in acute heart failure (35). The latter is supported by our study demonstrating disturbances in synchronized contraction (Figures 1D, 2A, 2C and 2E) and cardiac cell-to-cell communication (Figures 3 and 4) in the setting of K+ deficiency, increased levels of catecholamines or abnormally fast beating rates. Such conditions are accompanied by alterations in Ca2+ transport systems and cytosolic free calcium that affect not only synchronous heart function, but even facilitate occurrence of malignant arrhythmias. Both AF and VF aggravate further subcellular injury of the cadiomyocytes (Figure 5) and, thereby, their function. Growing lines of evidence suggest that enhancement of Ca2+influx, spontaneous Ca2+ oscillations, Ca2+ sparks (ie, Ca2+ leaks from sarcoplasmic reticulum) and Ca2+ overload jeopardize systole and diastole and can trigger arrhythmias [6,36-38].
Calcium changes and triggered activity have been investigated mostly in multicellular preparations (eg, cultured cardiac cells, isolated cardiac muscle) and rarely in the whole heart, either ventricles [39] or atria [25]. Our approach, based on ultrastructure investigation of samples from the whole heart, allowed detection of overall features of myocardial alterations as well as alterations on the level of individual cardiomyocytes. Pacing-induced nonuniform Ca2+ dynamics in rat atria has been revealed by rapid-scanning confocal microscope (25), while our examination revealed consequences of such changes by detecting nonuniform sarcomere shortening (Figures 2C and 4C). The irregular contraction and hypercontraction of sarcomeres strongly indicate intracellular Ca2+ disorders. Moreover, we hypothesize that spontaneous Ca2+ waves initiate observed hypercontractions of sarcomeres while severe Ca2+ overload results in extreme sarcomere shortening and contraction bands. Consequently, there was a pronounced asynchrony of sarcomeres within individual cardiomyocytes and between cardiomyocytes. It should be noted that myocardial heterogeneity of ultrastructural alterations was apparent in all investigated acute models, ie, there were more and less affected cardiomyocytes. This heterogeneity may be attributed, in part, to the degree of defects in cardiac cell-to-cell coupling.
Activated cardiac muscle exhibits a typical sarcomere length dependence of force development. Results of mechanical measurements on single cardiac myofibrils implied that high stretching is accompanied by irreversible structural alterations within cardiac sarcomeres, most likely thick filaments disarray and disruption binding sites between myosin and titin due to changes in tertiary structure. Loss of a regular thick filament organization may impair active force generation (40). We have demonstrated that thick myosin filaments can be disrupted in conditions of acute calcium disorders (Figures 1D, 2A, 2B, 2C, 2D, 2F, 5B and 5D), thereby contributing to contractile dysfunction. Furthermore, we have shown that pronounced Ca2+ disturbances resulting in marked sarcomere length shortening in individual cardiomyocytes are often in the vicinity of intercellular junctions (Figure 2).
The cycle of Ca2+ fluxes during the normal heart beat, which underlies the coupling between excitation and contraction, permitting a highly synchronized action of cardiac sarcomeres. However, the rapid force changes in nonuniform cardiac muscle (due to chronic or acute pathophysiological events) may cause arrhythmogenic Ca2+ waves to propagate by the activation of neighbouring sarcoplasmic reticulum via diffusing Ca2+ ions and contribute to inhomogeneous sarcomere length [6]. Such Ca2+ wave propagation in individual cardiomyocytes perhaps induces sarcomere alterations, as demonstrated in Figure 2C, in conditions of intramyocardial NA infusion. Of note, arrhythmogenesis in the failing heart has been associated with excessive, spontaneous SR Ca2+ release in the form of waves of Ca2+-induced Ca2+ release causing oscillations in myocyte membrane potential known as EAD and DAD [4-6]. The extent of SR Ca2+ leak is important because it can lead to reduction of SR Ca2+ available for release, causing systolic dysfunction; elevation diastolic [Ca2+]i, contributing to diastolic dysfunction; and triggered arrhythmias [36]. It is likely that Ca2+ waves may also propagate to neighbouring cardiomyocytes through functional gap junction channels and aggravate contraction disturbances. On the other hand, uncoupling at gap junction channels may inhibit both spreading of ‘injury’ current and spreading of arrhythmogenic Ca2+ waves (Figures 2A and 4).
Importantly, ultrastructure examination revealed that conditions accompanied by Ca2+ overload facilitated occurrence of annular gap junctions (Figure 3). Gap junction plaques, consisting of a cluster of connexin channels, exhibit dynamic characteristics resulting in formation and removal from cardiac cell membranes. Annular gap junctions (double-membrane intracellular structures) are, in fact, internalized gap junctions, and this unique process provides a mechanism for downregulating several hundreds of connexin channels at one time [41]. Once internalized, connexins are degraded by lysosomes and proteasomes [41]. Our findings of enhanced occurrence of annular gap junctions profile in acute proarrhythmogenic conditions suggest that increase in [Ca2+]i may facilitate internalization of gap junctions, ie, to participate in downregulation of intercellular electrical and metabolic communication between adjacent cardiomyocytes.
Finally, it has been reported that mitochondria can also be implicated in cardiomyocyte Ca2+ cycling (42). Mitochondrial Ca2+ signalling contributes to the regulation of cellular energy metabolism, and participates in cardiac excitation-contraction coupling through their ability to store Ca2+, shape the cytosolic Ca2+ signals and generate ATP required for contraction. However, controversy remains whether the fast cytosolic Ca2+ transients underlying cardiac excitationcontraction coupling in the beating heart are transmitted rapidly into the matrix compartment or slowly integrated by the mitochondrial Ca2+ transport machinery. Many more questions arise regarding how mitochondria affect contraction/relaxation in diseased or acutelly injured hearts. In this context, it is worthwhile to emphasize that in the condition of Ca2+ disturbances and Ca2+ overload that preceded malignant arrhythmia occurrence, we observed various degree of mitochondria injury, ie, mild, moderate (potentially reversible) and severe (irreversible) alterations.
Conclusion
There were some limitations to the present study because, despite the demonstration of close association between abnormal Ca2+ handling and ultrastructural alterations, we have not proven a direct causality between these variables. Nevertheless, our findings suggest that myocardial heterogeneity of high [Ca2+]i-related subcellular injury of cardiomyocytes is a common feature that increases the propensity of the heart to malignant arrhythmias. Nonuniform sarcomere shortening most likely reflects cytosolic Ca2+ oscillations, disturbances in Ca2+ wave propagation and Ca2+ overload. These disturbances are associated with impairment of cardiac-cell-to-cell coupling that facilitates occurrence of VF or AF and with disruption of myofilaments that impairs contractile function. These results provide a novel paradigm linking arrhythmogenesis and contractile disorders that may contribute to acute heart failure.
Acknowledgements
This study was supported by APVV 0241- 11, 0348-12, SK-CZ-2013-0256 and VEGA 2/0046/12, 2/0167/15 grants. The authors thank Mrs Adela Kniezova for excellent technical assistance.
References
- Hoffman BF, Rosen MR. Cellular mechanisms for cardiac arrhythmias. Circ Res 1981;49:1-15.
- Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 2004;84:431-88.
- Janse MJ. Electrophysiology of arrhythmias. Arch Mal Coeur Vaiss 1999;92:9-16.
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med 2004;14:61-6.
- Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol 2009;297:997-1002.
- Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev 2007;87:457-506.
- Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol 2008;44:31-43.
- Lakatta EG. Functional implication of sponatenous sarcoplasmic resticulum Ca2+ release in the heart. Cardiovasc Res 1992;26:193-2014.
- Hasenfuss G. Pieske B. Calcium cycling in congestive heart failure. J Mol Cel Cardiol 2002;34:951-69.
- De Mello WC. Cell coupling and impulse propagation in the failing heart. J Cardiovasc Electrophysiol 1999;10:1409-20.
- Kléber G. The potential role of Ca2+ for electrical cell-to-cell uncoupling and conduction block in myocardial tissue. Basic Res Cardiol 1992;87:131-43.
- Shaw RM, Rudy Y. Ionoc mechanisms of propagation in cardiac tissue. Circ Res 1997; 81:727-41.
- Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin-43 gap junction distribution correlates with location of re-entrant circuits in the epicardial border zone of healing infarcts that cause ventricular tachycardia. Circulation 1997;95:988-96.
- Spach MS. Mechanisms of the dynamics of reentry in a fibrillating myocardium. Developing a genes-to-rotors paradigm. Circ Res 2001;88:753-5.
- Tribulova N, Varon D, Polack-Charcon S, Buscemi P, Slezak J, Manoach M. Aged heart as a model for prolonged atrial fibrillo-flutter. Exp Clin Cardiol 1999;4:64-72.
- Tribulová N, Manoach M, Varon D, Okruhlicová L, Zinman T, Shainberg A. Dispersion of cell-to-cell uncoupling precedes low K+-induced ventricular fibrillation. Physiol Res 2001;50:247-59.
- Tribulova N, Knezl V, Okruhlicova L, Slezak J. Myocardial gap junctions: Targets for novel approaches in the prevention of life- threatening cardiac arrhythmias. Physiol Res 2008;57:1-13.
- Tribulova N, Seki S, Radosinska J, et al. Myocardial Ca2+ handling and cell-to-cell coupling, key factors in prevention of sudden cardiac death. Can J Physiol Pharmacol 2009;87:1120-9.
- Tribulova N, Knezl V, Shainberg A, Seki S, Soukup T. Thyroid hormones and cardiac arrhythmias. Vascul Pharmacol 2010;52:102-12.
- Liu F, Gustein DE. The cardiac gap junction: A potential therapeutic target in the treatment of heart disease. Mt Sinai J Med 2002;69:421-4.
- Gutstein DE, Morley GE, Tamaddon H, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin 43. Circ Res 2001;88:333-9.
- Gutstein DE, Danik SB, Lewitton S, et al. Focal gap junction uncoupling and spontaneous ventricular ectopy. Am J Physiol Heart Circ Physiol 2005;289:1091-8.
- Kostin S, Kleina G, Szalayb Z, Heinb S, Bauerb EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovascular Research 2002;54:361-79.
- Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011;121:2955-68.
- Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci USA 2007;104:18309-14.
- Priori SG, Barhanin J, Hauer RN, et al. Genetic and molecular basis of cardiac arrhythmias: Impact on clinical management. Study group on molecular basis of arrhythmias of the working group on arrhythmias of the european society of cardiology. Eur Heart J 1999;20:174-95
- Trafford AW, Clarke JD, Richards MA, Eisner DA, Dibb KM. Calcium signalling microdomains and the t-tubular system in atrial mycoytes: Potential roles in cardiac disease and arrhythmias. Cardiovasc Res 2013;98:192-203.
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 1998;98:2334-51.
- Zaugg CE, Wu ST, Lee RJ, Parmley WW, Buser PT, Wikman-Coffelt J. Importance of calcium for the vulnerability to ventricular fibrillation detected by premature ventricular stimulation: Single pulse versus sequential pulse methods. J Mol Cell Cardiol 1996;28:1059-72.
- Tribulova N, Okruhlicova L, Imanaga I, Hirosawa N, Ogawa K, Weismann P. Factors involved in the susceptibility of spontaneously hypertensive rats to low K+-induced arrhythmias. Gen Physiol Biophys 2003;22:369-82.
- Tribulova N, Kessler R, Goetzfried S, Thomas S, Podzuweit T, Manoach M. Cardiomyocyte alterations during Ca2+ overload linked with arrhythmias. Cardiovasc J S Afr 2004;15:9-10.
- Lewartowski B, Zdanowski K. Net Ca2+ influx and sarcoplasmic reticulum Ca2+ uptake in resting single myocytes of the rat heart: Comparison with guinea-pig. J Mol Cell Cardiol 1990;22:1221-9.
- Smyrnias I, Mair W, Harzheim D, Walker SA, Roderick HL, Bootman MD.Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation- contraction coupling and inotropic stimulation.Cell Calcium 2010;47:210-23.
- Podzuweit T. Catecholamine-cyclic-AMP-Ca+-induced ventricular tachycardia in the intact pig heart. Basic Res Cardiol 1980;75:772-9.
- Hohendanner F, Walther S, Maxwell JT, et al. Inositol-1,4,5- trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J Physiol 2015;593:1459-77.
- Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol 2014;76:107-27.
- Laflamme MA, Becker PL. Ca2+-induced current oscillations in rabbit ventricular myocytes. Circ Res 1996;78:707-16.
- Sarai N, Kihara Y, Izumi T, Mitsuiye T, Matsuoka S, Noma A. Nonuniformity of sarcomere shortenings in the isolated rat ventricular myocyte. Jpn J Physiol 2002;52:371-81.
- Minamikawa T, Cody SH, Williams DA. In situ visualization of spontaneous calcium waves within perfused whole rat heart by confocal imaging. Am J Physiol 1997;272:236-43.
- Weiwad WK, Linke WA, Wussling MH. Sarcomere length-tension relationship of rat cardiac myocytes at lengths greater than optimum. J Mol Cell Cardiol 2000;32:247-59.
- Jordan K, Chodock R, Hand AR, Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci 2001;114:763-73.
- Dedkova EN, Blatter LA. Calcium signaling in cardiac mitochondria. J Mol Cell Cardiol 2013;58:125-33.
- *Corresponding Author:
- Dr Narcis Tribulova
Institute for Heart Research, Slovak Academy of Sciences, 840 05 Bratislava, Dúbravská cesta 9, PO Box 104.
Telephone: 004212 5466405
fax: 004212 54776637
E-mail: narcisa.tribulova@savba.sk
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
BAckground: It has been previously reported that various acute interventions causing myocardial abnormalities in Ca2+ handling and defects in intercellular coupling facilitate the occurrence of malignant arrhythmias.
Objectives: To comprehensively determine the impact of such Ca2+- related disorders induced in intact animal hearts on the ultrastructure of the cardiomyocytes before occurrence and during sustaining of severe arrhythmias.
Methods: Three types of acute experiments known to be accompanied by disturbances in Ca2+ handling were performed. Langendorff-perfused rat and guinea pig hearts were subjected to K+-deficient perfusion to induce ventricular fibrillation; Langendorff-perfused guinea pig heart underwent burst atrial pacing to induce atrial fibrillation; and open chest pig heart was used for intramyocardial noradrenaline infusion to induce ventricular tachycardia. Tissue samples for electron microscopy examination were obtained during basal conditions, previous occurrence and during sustaining of malignant arrhythmias.
Results: The comparative findings suggest that myocardial heterogeneity of high [Ca2+]i-induced subcellular injury of the cardiomyocytes and their junctions is a common feature that precede occurrence ventricular tachycardia, ventricular fibrillation or atrial fibrillation regardless of the species and atria/ventricular-related differences in Ca2+ handling and intercellular coupling. The primary changes consisted of nonuniform sarcomere shortening, which most likely reflects cytosolic Ca2+ oscillations; disturbances in Ca2+ wave propagation; and Ca2+ overload. These disturbances were linked with defects in cardiac cell-to-cell coupling that increased the propensity of the heart to malignant arrhythmias. Moreover, Ca2+overload led to disruption of myofilaments, jeopardizing contractile function.
Conclusions: The results provide a novel paradigm linking Ca2+- related arrhythmogenesis and contractility disorders that may contribute to acute heart failure.
-Keywords
Ca2+ overload; Guinea pig; Malignant arrhythmias; Pig; Rat; Ultrastructure
General classification of cardiac arrhythmias assumes that all disturbances of rhythm result from one of two primary abnormalities in electrical activity. The first is an abnormality in impulse initiation and the second is an abnormality in impulse propagation, although both may coexist. The former is particularly associated with triggered activity and/or abnormal automaticity, whereas the latter with conduction block and re-entry [1,2]. In both clinical and experimental settings, enhanced triggered activity may result from early and delayed after-depolarization (EAD and DAD, respectively) [3]. These abnormalities in cardiac electrical activity are attributed primarily to disturbed Ca2+ handling [3,4]. Dysregulated, spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) occurs in the form of waves of self-propagating Ca2+-induced Ca2+ release [5]. Altered Ca2+ handling in chronic pathophysiological conditions results from permanent upregulation/downregulation of Ca2+ transport systems, while in acute events from transient activation/inhibition of those systems. Ca2+ leaks, suppression of SR Ca2+-ATPase or activation of the Na+/Ca2+ exchanger result in Ca2+overload, which has been implicated in both arrhythmias and contractile dysfunction associated with either chronic or acute heart failure [6-9].
Importantly, a high free concentration of Ca2+ impairs electrical coupling at the gap junctions via inhibition of Cx43 channels that can affect conduction velocity [10-12]. Consequently, cardiac cell-to-cell uncoupling may result in slowing and blocking of conduction, thereby facilitating development of re-entrant arrhythmias such as ventricular fibrillation (VF) and atrial fibrillation (AF) [2,3,13-19]. In both clinical and experimental settings, VF is often initiated by triggered activity (due to EAD or DAD) and occurs in the presence of defects of myocardial intercellular coupling. Cell-to-cell uncoupling also promotes an increase in the dispersion of refractoriness [20], another factor that favours the development of re-entrant arrhythmias [3]. Evidence suggests that focal areas of uncoupling in the myocardium increase the likelihood of arrhythmic triggers and more widespread uncoupling is required to support sustained arrhythmias [21,22]. The mechanisms underlying AF are complex, involving increased spontaneous ectopic firing of atrial cells and impulse re-entry through atrial tissue. Triggered activity as well as impairment of Ca2+ handling and cell-to-cell coupling that may lead to altered conduction properties and multiple reentrant circuits is likely implicated in the development of AF [15,23,24].
Most life-threatening arrhythmias occur in patients or animals with structural heart disease, in whom arrhythmogenic substrates play an important role. Individuals without structural heart disease may also suffer from arrhythmias [25,26] due to genetically aberrant ionic currents (eg, Ik, RyR), or acute conditions that modify both Ca2+ handling and ion channel function (eg, drugs, oxidative stress).
AF is the most common arrhythmia that increases mortality, risk for stroke and morbidity. Prolonged duration of AF leads to difficulties in conversion into sinus rhythm and maintainance of sinus rhythm after successful conversion. It is due to abnormal Ca2+ handling as well as pronounced structural and electrophysiological remodelling in chronic AF [23-25,27].
VF is the main cause of sudden cardiac death. To prevent its occurrence in high-risk patients, an implantable cardioverter defibrillator is used to terminate VF by electrical shock followed by sinus rhythm restoration. However, there are common problems such as failure of electrical cardioversion and postshock re-initiation of VF, which, consequently, increase the number of shocks needed [28,29].
Despite progressive current therapies based on implantable cardioverter defibrillator and catheter ablation, both VF and AF remain a major health problem. Further understanding of the mechanisms and factors responsible for the onset and maintenance of these arrhythmias is essential. There is also a need to explore possible factors that may affect outcome of electrical or drug-induced cardioversion.
We have previously shown that acute interventions such as low K+ perfusion, burst atrial pacing or intramyocardial noradrenaline (NA) infusion facilitate occurrence of severe cardiac arrhythmias, whereby alterations in Ca2+ and gap junctions are most likely involved [15,16,18,30,31]. The purpose of the present study was to comprehensively determine the impact of Ca2+ disorders on the ultrastructure of cardiomyocytes before occurrence and during sustaining of these arrhythmias in intact hearts.
Methods
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, Publication No 85-23, revised 1996. All experiments were approved by the respective institutional animal care and use committees.
Taking into consideration the differences in cardiac Ca2+ handling between rats and guinea pigs [32], atria and ventricles [33], and small and large mammals [27], ultrastructural alterations of the cardiomyocytes in the hearts of rats, guinea pigs and Landrace pigs in previously reported acute conditions [15,16,30,31] to induce Ca2+ disturbances and arrhythmias were explored.
The following experimental protocols were used: low K+ perfusion of isolated rat heart to induce sustained VF; low K+ perfusion of isolated guinea pig heart to induce sustained VF; burst atrial pacing of isolated guinea pig heart to induce prolonged AF; and focal intramyocardial NA infusion in open-chest pig to induce ventricular tachycardia (VT).
Low K+ perfusion of isolated rat heart
Adult Wistar Kyoto rats were anesthetized and the hearts were quickly excised and perfused via cannulated aorta at constant pressure with oxygenated Krebs-Henseleit solution containing (in mmol/L) 118 NaCl, 25 NaHCO3, 2.9 KCl, 1.2 MgSO4, 1.8 CaCl2, 1.3 KH2PO4 and 11.5 glucose. Bipolar electrocardiogram was continuously recorded. After 20 min of equilibration period in standard Krebs- Henseleit solution, the rat hearts were perfused with a low K+ (1.2 mmol/L) solution for a period of 60 min, unless sustained VF lasting 2 min occurred earlier (as reported previously [30]). The hearts were harvested at the end of the equilibration period (n=6), at 15 min of low K+ perfusion (n=6), and at 2 min of VF (n=6) for electron microscopy examination.
Low K+ perfusion of isolated guinea pig heart
Adult guinea pigs were euthanized by stunning and the aorta of the excised heart was immediately cannulated for perfusion at constant pressure with oxygenated Tyrode solution (in mmol/L: 136.9 NaCl, 2.8 KCl, 1.8 CaCl2, 1.0 MgCl2, 11.9 NaHCO3, 0.4 NaH2PO4 and 11.5 glucose). Bipolar epicardial electrocardiograms were continuously monitored. After 15 min of stabilization with standard solution, the hearts were perfused with a low K+ solution (1.4 mmol/L) until sustained VF occurred (as previously reported [16]). The hearts were harvested during stabilization (n=6), at 15 min of low K+ perfusion (n=6) and during VF (n=6) for electron microscopy examination.
Burst atrial pacing of isolated guinea pig heart
Aged guinea pigs were euthanized by stunning and the aorta of the excised heart was immediately cannulated for the perfusion at constant pressure with oxygenated modified Tyrode solution (in mmol/L: 126 NaCl, 2.8 KCl, 1.8 CaCl2, 1.0 MgCl2, 24 NaHCO3, 0.4 NaH2PO4 and 5.5 glucose). After 20 min of equilibration, the left atrium was subjected to programmed stimulation by a 1 s burst of electrical rectangular pulses, 50 pulses/s to 70 pulses/s, 1 ms in duration and 1.5 times the threshold voltage. The right atrial electrocardiogram was recorded for incidence of arrhythmias (as previously reported [15]). For the examination, atrial tissue was taken during basic conditions (n=6), during pacing (n=6) and during AF (n=6).
Focal intramyocardial NA infusion in open-chest pig
Adult domestic Landrace pigs were anesthetized, ventilated and subjected to sternotomy. Electrocardiogram and aortic blood pressure were recorded. Left ventricular intramyocardial infusion of 10 μL/min of NA (100 μmol/L) in the presence of Ca2+ (2.5 mmol/L) was used to induce VT. The drill biopsies were randomly obtained from the left ventricle during basal conditions (n=6), upon 50 s of infusion (n=6) and during VT (n=6) from infusion area (as previously reported [31,34]).
For transmission electron microscopic examinations, small, approximately 1 mm3 tissue blocks of the left ventricle were fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate, postfixed in 1% osmium tetroxide, dehydrated in ethanol, infiltrated by propylene oxide and embedded in resin (Epon 812, Serva Heidelberg, Germany). The ultrathin sections were stained with uranyl acetate and lead phosphate, and examined using an electron microscope (Tesla BS 500, Tesla Brno, Czech Republic).
Results
Representative electron microscopic images of cardiomyocyte myofbrils and their contractile units, ie, sarcomeres, in four distinct patterns, are shown in Figure 1. In the heart during normal conditions, regular contraction is followed by relaxation and these processes are visible according to relaxed and contracted state of sarcomeres (Figures 1A and 1B). Pathophysiological conditions, particularly those accompanied by disturbances in cytosolic [Ca2+]i, result in disturbances in contraction-relaxation processes that is recognized by overcontracted or hypercontracted cardiomyocytes (Figure 1C) and even by the presence of contracture with contraction bands (Figure 1D). The latter is considered to be an irreversible change, jeopardizing the viability of the cardiomyocyte.
Figure 1: Electron microscopy appearance of cardiac myofibrils show distinct patterns of their contractile unit (sarcomere) in baseline conditions (A and B) and in conditions of abnormal Ca2+ handling and Ca2+overload (C and D). A Sarcomeres as delineated by Z lines and composed of myosin (thick arrow) and actin (thin arrow) are in uniform relaxed state. B All sarcomeres are in contracted state evidenced by disappearance of actin areas. C Sarcomeres in overcontracted state can be recognized when Z lines are widened and the myosin band is compressed. D Nonuniform sarcomere shortening in cardiomyocyte myofibrills exhibiting hypercontraction (HP) with moderate disruption of myofilaments (M) or severe disruption and clumping of myofilaments into contraction bands (CB). Normal appearance of mitochondria (m) in A, B and C while swollen with ruptured cristae in D. ID Intercalated disc. Scale bar = 1 μm
Low K+ perfusion of isolated rat and guinea pig hearts and sustained VF
Within 10 min to 20 min of perfusion of rat heart with a K+-deficient Krebs-Henseleit solution, the incidence of premature beats, bigeminy, and transient VT and/or VF were registered and these preceded the development of sustained VF. This lethal arrhythmia occurred during 20 min to 40 min of low K+ perfusion (as previously reported [30]).
The continual recordings of ventricular bipolar electrocardiograms during low K+ perfusion of guinea pig heart revealed changes in R and T configuration, changes in the R vector, incidence of premature beats, bigeminy and sudden VT, which usually degenerated into VF. Sustained VF appeared within 15 min to 30 min of low K+ perfusion (as previously reported [16]).
Myocardial ultrastructure alterations: Ultrastructural examination revealed that in comparison with the normal subcellular architecture of the cardiomyocytes and preserved intermyocyte junctions, the myocardial tissue from both rat and guinea pig heart subjected to K+-deficient perfusion was characterized by nonuniformly altered cardiomyocytes. Accordingly, the majority of cardiomyocytes were reversibly altered, exhibiting subcellular changes of various degrees. Sporadic irreversibly injured cardiomyocytes were present. Nonuniformly affected cardiomyocytes were heterogeneously distributed throughout the myocardium. Severely damaged cardiomyocytes were edematous with apparently injured integrity of mitochondria and intermyocyte junctions. Less-affected cardiomyocytes exhibited mild edema and moderate mitochondrial alterations as well as a mild dissociation of fascia adherens junctions.
As demonstrated on representative electron microscopic images, perfusion of either rat or guinea pig hearts with K+-deficient solution caused irregular contraction of majority of cardiomyocytes, whereby overcontraction and hypercontraction of sarcomeres or, sporadically, contraction bands were observed (Figure 2A), as well as apparent alterations in intercellular junctions, ie, in addition to normal connections of neighbouring cardiomyocytes with adhesive junctions and gap junctions at the intercalated disc (Figure 3A), the internalization of gap junctions in the form of annular profiles was detected (Figures 3C and 3D). Furthermore, dehiscence of adhesive junctions was frequently found (Figure 4A). Impairment of gap junctionmediated cell-to-cell coupling was recognized when neighbouring cardiomyocytes differed in patterns of sarcomeres, ie, contracted in one and relaxed in the adjacent cardiomyocyte (Figures 4A and 4B). Overall, these changes were more pronounced due to transient arrhythmias and preceded occurrence of sustained VF. Marked deterioration of ultrastructure indicating cardiac cell-to-cell uncoupling and dysregulation of synchronized contraction was observed in fibrillating myocardium (Figures 2B, 3E, 5A and 5B).
Figure 2: Representative electron microscopic images show disordered sarcomere shortening in the heart submitted to low K+ perfusion (A, B), burst atrial stimulation (C, D) and intramyocardial noradrenaline infusion (E, F) indicating Ca2+-related disordered contraction of myofibrils. A Note contraction bands (CB) in the vicinity of intercalated disc junctions and disruption of myofilaments (M) in neighboring cardiomyocytes due to low K+-induced [Ca2+]i disturbances while much pronounced Ca2+ overloadrelated changes during VF (B). C Hypercontraction (HP) and CB are detected due to burst pacing-induced defects in Ca2+ handling and aggravation of Ca2+ overload-related injury during prolonged atrial fibrillation, including stretched (S) sarcomeres (D). E Spontaneous Ca2+ wave-related nonuniform sarcomere shortening due to noradrenaline infusion and pronounced sarcomere disturbances during ventricular tachycardia (F), such as CB, stretching (S) with myofilaments disruption (M). Note that mitochondria are mostly preserved while they are swollen with ruptured cristae particularly in conditions of severe Ca2+-related injury (D). Scale bar ? 1 μm
Figure 3: Typical subcellular appearance of two types of intermyocyte junctions in normal conditions, ie, intercalated disc-related end-to-end type (A) that predominate in ventricles and lateral side-to-side type (B) that is frequent in atria. Cardiac cell-to-cell junctions comprise adhesive junctions, fascia adherens (curved arrows) and desmosomes (thick arrows) as well as communicating, gap junctions (thin arrows). Note that low K+-induced Ca2+ disturbances promote internalization of gap junctions (asterisks) either in rats (C) or guinea pigs (D) while more severe Ca2+ overload-related injury of cell-to-cell junctions and mitochondria (m) is observed during ventricular fibrillation (E) or atrial fibrillation (F). Scale bar ? 1 μm
Figure 4: Representative electron microscopy images demonstrate impairment of intercellular connections in proarrhythmogenic conditions associated with Ca2+ disturbances due to low K+ perfusion (A Rat, B Guinea pig), burst atrial pacing (C Guinea pig) and intramyocardial noradrenaline infusion (D Pig). Cell-to-cell electrical uncoupling is recognized when cardiomyocyte in relaxed state is connected with contracted ones (A, B, C) or when normal cardiomyocytes (on right) is in the neighborhood of severely inured one (on left) in D. Furthermore, gap junctions (thin arrows) are less frequent. In addition, dehiscence of adhesive junctions (A) and mitochondria injury of various degrees depending on severity of Ca2+ disorders (A, B, C, D) is observed. Curved arrows indicate fascie adherentes junctions. OC Overcontraction of sarcomeres; R Relaxed state of sarcomeres. Scale bar ? 1 μm
Figure 5: Typical electron microscopy appearance of cardiomyocyte and intercellular junction injury of various degrees in the hearts suffered from ventricular fibrillation (A Rat, B Guinea pig), ventricular tachycardia (C Pig) or atrial fibrillation (D Guinea pig). Note pronounced defects in synchronized contraction as shown by nonuniform sarcomere shortening (CB Totally condensed, HP Hypercontracted, OC Overcontracted, R Relaxed, S Stretched) in the presence of severely damaged mitochondria (m). Note detachment of actin filaments from fascia adherens junctions (curved arrow) at the intercalated discs in all cases. Thin arrow: gap junctions; Thick arrow: desmosomes. M Myofilaments disruption. Scale bar ? 1 μm
Burst atrial pacing of isolated guinea pig heart and AF
During the first 5 min of repetitive stimulation, a few brief poststimulus arrhythmias, atrial tachycardias and fibrillo-flutters were recorded. Within the 5 min to 30 min period of burst pacing, prolonged poststimulus AF lasting 0.5 min to 15 min was detected; however, no arrhythmias were registered in the left ventricle (details are reported in [15]).
Myocardial ultrastructure alterations: Normal appearance of atrial subcellular structure was observed in basal conditions. Cardiomyocytes exhibited uniform pattern of sarcomeres either in contracted or relaxed forms (Figure 3B). Both types of gap junctions were frequently observed (ie, intercalated disc related end-to-end form, similarly in the ventricle [Figure 3A], and lateral side-to side gap junctions [Figure 3B]). Prolonged burst pacing that was accompanied by transient posstimulus arrhythmias resulted in cardiomyocyte alterations that were heterogenosly distributed throughout the myocardium and consisted of mitochondria injury, nonuniform sarcomere shortening and impairment of cardiac cell-to-cell coupling (Figures 2C and 4C). These changes preceded the occurrence of prolonged AF, which aggravated further cardiomyocyte and cell-to-cell junctions injury (Figures 2D and 5D).
Intramyocardial NA infusion in open-chest pig and VT
Focal left ventricular intramyocardial infusion of NA in the presence of calcium in open-chest pigs consistently produced VT within 60 s. Before the development of VT, the triggered activity was recognized by focally enhanced contractility in the area of infusion. Premature beats initiated VT that could be maintained for 30 min (as previously reported [31,34]).
Myocardial ultrastructure alterations: NA infusion resulted in prominent Ca2+ overload-related changes consisting of nonuniform pattern of myofibrils in individual cadiomyocytes, ie, exhibiting dramatic shortening of the sarcomere in the vicinity of relaxing sarcomeres (Figures 2E and 2F). Impairment of electrical coupling between two neighbouring cardiomyocytes was recognized when one was in a contracted state while the other was in a relaxed state. This was observed particularly during VT (Figures 4D and 5D). Overall, features were characterized by nonuniform alterations of the cardiomyocytes and their junctions throughout myocardium in the area of infusion.
Discussion
In the present study, we characterized subcellular changes in rat, guinea pig and Landrace pig hearts subjected to various acute proarrhythmogenic conditions. Comparative findings suggest that there is a common feature of ultrastructural alterations that precede the occurrence of life-threatening cardiac arrhythmias regardless of species and cardiac atria/ventricular-related differences in Ca2+ handling and intercellular coupling. Most importantly, the findings demonstrate the impact of disturbances in [Ca2+]i and cell-to-cell coupling, key factors implicated in the development of AF and VF, on the ultrastructure of the cardiomyocytes and their junctions.
To strengthen this approach, we placed representative electron microscopy images from four types of experiments together in one panel for clear and comparative demonstration. To avoid interference resulting from heart disease, we used healthy animals with intact hearts for the investigation. The heart was exposed to acute conditions, such as hypokalemia, sudden increase of catecholamines or fast-beating rates that in some way mimic clinical proarrhythmogenic events. K+-deficient perfusion resulted in a decrease of heart rate, prolonged repolarization, lengthened action potential duration and increased [Ca2+]i [18,30]. In parallel with these changes, there was an increase in the incidence of triggered electrical activity manifested by EAD that was linked with the occurrence of ventricular premature beats [18,30]. Focal intramyocardial infusion of NA resulted in regional positive chrono- and inotropic effects due an increase of cyclic AMP and shortening of action potential duration, promoting DAD and VT induction [34]. Cardiac burst pacing ex vivo or chronic fast pacing in vivo are current models to induce and investigate the mechanisms of AF [17]. After prolonged episodes of rapid electrical activity, the atrial action potential was shortened because of a reduction in the IKs-type calcium current [3]. Moreover, AF is initiated by triggered activity most likely due to Ca2+ disturbances.
The hallmarks of the normal heart function are both rapid contraction/ relaxation of the ‘working’ cardiomyocytes and rapid electrical impulse propagation between them. In such conditions, the contractile units (sarcomeres) are in synchrony, as shown in Figures 1A and 1B. Calcium is crucial in normal cardiac contraction, while disturbances of excitation-contraction coupling result in both contractile dysfunction and arrhythmogenesis, particularly in cardiac disease but also in acute heart failure (35). The latter is supported by our study demonstrating disturbances in synchronized contraction (Figures 1D, 2A, 2C and 2E) and cardiac cell-to-cell communication (Figures 3 and 4) in the setting of K+ deficiency, increased levels of catecholamines or abnormally fast beating rates. Such conditions are accompanied by alterations in Ca2+ transport systems and cytosolic free calcium that affect not only synchronous heart function, but even facilitate occurrence of malignant arrhythmias. Both AF and VF aggravate further subcellular injury of the cadiomyocytes (Figure 5) and, thereby, their function. Growing lines of evidence suggest that enhancement of Ca2+influx, spontaneous Ca2+ oscillations, Ca2+ sparks (ie, Ca2+ leaks from sarcoplasmic reticulum) and Ca2+ overload jeopardize systole and diastole and can trigger arrhythmias [6,36-38].
Calcium changes and triggered activity have been investigated mostly in multicellular preparations (eg, cultured cardiac cells, isolated cardiac muscle) and rarely in the whole heart, either ventricles [39] or atria [25]. Our approach, based on ultrastructure investigation of samples from the whole heart, allowed detection of overall features of myocardial alterations as well as alterations on the level of individual cardiomyocytes. Pacing-induced nonuniform Ca2+ dynamics in rat atria has been revealed by rapid-scanning confocal microscope (25), while our examination revealed consequences of such changes by detecting nonuniform sarcomere shortening (Figures 2C and 4C). The irregular contraction and hypercontraction of sarcomeres strongly indicate intracellular Ca2+ disorders. Moreover, we hypothesize that spontaneous Ca2+ waves initiate observed hypercontractions of sarcomeres while severe Ca2+ overload results in extreme sarcomere shortening and contraction bands. Consequently, there was a pronounced asynchrony of sarcomeres within individual cardiomyocytes and between cardiomyocytes. It should be noted that myocardial heterogeneity of ultrastructural alterations was apparent in all investigated acute models, ie, there were more and less affected cardiomyocytes. This heterogeneity may be attributed, in part, to the degree of defects in cardiac cell-to-cell coupling.
Activated cardiac muscle exhibits a typical sarcomere length dependence of force development. Results of mechanical measurements on single cardiac myofibrils implied that high stretching is accompanied by irreversible structural alterations within cardiac sarcomeres, most likely thick filaments disarray and disruption binding sites between myosin and titin due to changes in tertiary structure. Loss of a regular thick filament organization may impair active force generation (40). We have demonstrated that thick myosin filaments can be disrupted in conditions of acute calcium disorders (Figures 1D, 2A, 2B, 2C, 2D, 2F, 5B and 5D), thereby contributing to contractile dysfunction. Furthermore, we have shown that pronounced Ca2+ disturbances resulting in marked sarcomere length shortening in individual cardiomyocytes are often in the vicinity of intercellular junctions (Figure 2).
The cycle of Ca2+ fluxes during the normal heart beat, which underlies the coupling between excitation and contraction, permitting a highly synchronized action of cardiac sarcomeres. However, the rapid force changes in nonuniform cardiac muscle (due to chronic or acute pathophysiological events) may cause arrhythmogenic Ca2+ waves to propagate by the activation of neighbouring sarcoplasmic reticulum via diffusing Ca2+ ions and contribute to inhomogeneous sarcomere length [6]. Such Ca2+ wave propagation in individual cardiomyocytes perhaps induces sarcomere alterations, as demonstrated in Figure 2C, in conditions of intramyocardial NA infusion. Of note, arrhythmogenesis in the failing heart has been associated with excessive, spontaneous SR Ca2+ release in the form of waves of Ca2+-induced Ca2+ release causing oscillations in myocyte membrane potential known as EAD and DAD [4-6]. The extent of SR Ca2+ leak is important because it can lead to reduction of SR Ca2+ available for release, causing systolic dysfunction; elevation diastolic [Ca2+]i, contributing to diastolic dysfunction; and triggered arrhythmias [36]. It is likely that Ca2+ waves may also propagate to neighbouring cardiomyocytes through functional gap junction channels and aggravate contraction disturbances. On the other hand, uncoupling at gap junction channels may inhibit both spreading of ‘injury’ current and spreading of arrhythmogenic Ca2+ waves (Figures 2A and 4).
Importantly, ultrastructure examination revealed that conditions accompanied by Ca2+ overload facilitated occurrence of annular gap junctions (Figure 3). Gap junction plaques, consisting of a cluster of connexin channels, exhibit dynamic characteristics resulting in formation and removal from cardiac cell membranes. Annular gap junctions (double-membrane intracellular structures) are, in fact, internalized gap junctions, and this unique process provides a mechanism for downregulating several hundreds of connexin channels at one time [41]. Once internalized, connexins are degraded by lysosomes and proteasomes [41]. Our findings of enhanced occurrence of annular gap junctions profile in acute proarrhythmogenic conditions suggest that increase in [Ca2+]i may facilitate internalization of gap junctions, ie, to participate in downregulation of intercellular electrical and metabolic communication between adjacent cardiomyocytes.
Finally, it has been reported that mitochondria can also be implicated in cardiomyocyte Ca2+ cycling (42). Mitochondrial Ca2+ signalling contributes to the regulation of cellular energy metabolism, and participates in cardiac excitation-contraction coupling through their ability to store Ca2+, shape the cytosolic Ca2+ signals and generate ATP required for contraction. However, controversy remains whether the fast cytosolic Ca2+ transients underlying cardiac excitationcontraction coupling in the beating heart are transmitted rapidly into the matrix compartment or slowly integrated by the mitochondrial Ca2+ transport machinery. Many more questions arise regarding how mitochondria affect contraction/relaxation in diseased or acutelly injured hearts. In this context, it is worthwhile to emphasize that in the condition of Ca2+ disturbances and Ca2+ overload that preceded malignant arrhythmia occurrence, we observed various degree of mitochondria injury, ie, mild, moderate (potentially reversible) and severe (irreversible) alterations.
Conclusion
There were some limitations to the present study because, despite the demonstration of close association between abnormal Ca2+ handling and ultrastructural alterations, we have not proven a direct causality between these variables. Nevertheless, our findings suggest that myocardial heterogeneity of high [Ca2+]i-related subcellular injury of cardiomyocytes is a common feature that increases the propensity of the heart to malignant arrhythmias. Nonuniform sarcomere shortening most likely reflects cytosolic Ca2+ oscillations, disturbances in Ca2+ wave propagation and Ca2+ overload. These disturbances are associated with impairment of cardiac-cell-to-cell coupling that facilitates occurrence of VF or AF and with disruption of myofilaments that impairs contractile function. These results provide a novel paradigm linking arrhythmogenesis and contractile disorders that may contribute to acute heart failure.
Acknowledgements
This study was supported by APVV 0241- 11, 0348-12, SK-CZ-2013-0256 and VEGA 2/0046/12, 2/0167/15 grants. The authors thank Mrs Adela Kniezova for excellent technical assistance.
References
- Hoffman BF, Rosen MR. Cellular mechanisms for cardiac arrhythmias. Circ Res 1981;49:1-15.
- Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 2004;84:431-88.
- Janse MJ. Electrophysiology of arrhythmias. Arch Mal Coeur Vaiss 1999;92:9-16.
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med 2004;14:61-6.
- Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol 2009;297:997-1002.
- Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev 2007;87:457-506.
- Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol 2008;44:31-43.
- Lakatta EG. Functional implication of sponatenous sarcoplasmic resticulum Ca2+ release in the heart. Cardiovasc Res 1992;26:193-2014.
- Hasenfuss G. Pieske B. Calcium cycling in congestive heart failure. J Mol Cel Cardiol 2002;34:951-69.
- De Mello WC. Cell coupling and impulse propagation in the failing heart. J Cardiovasc Electrophysiol 1999;10:1409-20.
- Kléber G. The potential role of Ca2+ for electrical cell-to-cell uncoupling and conduction block in myocardial tissue. Basic Res Cardiol 1992;87:131-43.
- Shaw RM, Rudy Y. Ionoc mechanisms of propagation in cardiac tissue. Circ Res 1997; 81:727-41.
- Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin-43 gap junction distribution correlates with location of re-entrant circuits in the epicardial border zone of healing infarcts that cause ventricular tachycardia. Circulation 1997;95:988-96.
- Spach MS. Mechanisms of the dynamics of reentry in a fibrillating myocardium. Developing a genes-to-rotors paradigm. Circ Res 2001;88:753-5.
- Tribulova N, Varon D, Polack-Charcon S, Buscemi P, Slezak J, Manoach M. Aged heart as a model for prolonged atrial fibrillo-flutter. Exp Clin Cardiol 1999;4:64-72.
- Tribulová N, Manoach M, Varon D, Okruhlicová L, Zinman T, Shainberg A. Dispersion of cell-to-cell uncoupling precedes low K+-induced ventricular fibrillation. Physiol Res 2001;50:247-59.
- Tribulova N, Knezl V, Okruhlicova L, Slezak J. Myocardial gap junctions: Targets for novel approaches in the prevention of life- threatening cardiac arrhythmias. Physiol Res 2008;57:1-13.
- Tribulova N, Seki S, Radosinska J, et al. Myocardial Ca2+ handling and cell-to-cell coupling, key factors in prevention of sudden cardiac death. Can J Physiol Pharmacol 2009;87:1120-9.
- Tribulova N, Knezl V, Shainberg A, Seki S, Soukup T. Thyroid hormones and cardiac arrhythmias. Vascul Pharmacol 2010;52:102-12.
- Liu F, Gustein DE. The cardiac gap junction: A potential therapeutic target in the treatment of heart disease. Mt Sinai J Med 2002;69:421-4.
- Gutstein DE, Morley GE, Tamaddon H, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin 43. Circ Res 2001;88:333-9.
- Gutstein DE, Danik SB, Lewitton S, et al. Focal gap junction uncoupling and spontaneous ventricular ectopy. Am J Physiol Heart Circ Physiol 2005;289:1091-8.
- Kostin S, Kleina G, Szalayb Z, Heinb S, Bauerb EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovascular Research 2002;54:361-79.
- Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 2011;121:2955-68.
- Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci USA 2007;104:18309-14.
- Priori SG, Barhanin J, Hauer RN, et al. Genetic and molecular basis of cardiac arrhythmias: Impact on clinical management. Study group on molecular basis of arrhythmias of the working group on arrhythmias of the european society of cardiology. Eur Heart J 1999;20:174-95
- Trafford AW, Clarke JD, Richards MA, Eisner DA, Dibb KM. Calcium signalling microdomains and the t-tubular system in atrial mycoytes: Potential roles in cardiac disease and arrhythmias. Cardiovasc Res 2013;98:192-203.
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 1998;98:2334-51.
- Zaugg CE, Wu ST, Lee RJ, Parmley WW, Buser PT, Wikman-Coffelt J. Importance of calcium for the vulnerability to ventricular fibrillation detected by premature ventricular stimulation: Single pulse versus sequential pulse methods. J Mol Cell Cardiol 1996;28:1059-72.
- Tribulova N, Okruhlicova L, Imanaga I, Hirosawa N, Ogawa K, Weismann P. Factors involved in the susceptibility of spontaneously hypertensive rats to low K+-induced arrhythmias. Gen Physiol Biophys 2003;22:369-82.
- Tribulova N, Kessler R, Goetzfried S, Thomas S, Podzuweit T, Manoach M. Cardiomyocyte alterations during Ca2+ overload linked with arrhythmias. Cardiovasc J S Afr 2004;15:9-10.
- Lewartowski B, Zdanowski K. Net Ca2+ influx and sarcoplasmic reticulum Ca2+ uptake in resting single myocytes of the rat heart: Comparison with guinea-pig. J Mol Cell Cardiol 1990;22:1221-9.
- Smyrnias I, Mair W, Harzheim D, Walker SA, Roderick HL, Bootman MD.Comparison of the T-tubule system in adult rat ventricular and atrial myocytes, and its role in excitation- contraction coupling and inotropic stimulation.Cell Calcium 2010;47:210-23.
- Podzuweit T. Catecholamine-cyclic-AMP-Ca+-induced ventricular tachycardia in the intact pig heart. Basic Res Cardiol 1980;75:772-9.
- Hohendanner F, Walther S, Maxwell JT, et al. Inositol-1,4,5- trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J Physiol 2015;593:1459-77.
- Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol 2014;76:107-27.
- Laflamme MA, Becker PL. Ca2+-induced current oscillations in rabbit ventricular myocytes. Circ Res 1996;78:707-16.
- Sarai N, Kihara Y, Izumi T, Mitsuiye T, Matsuoka S, Noma A. Nonuniformity of sarcomere shortenings in the isolated rat ventricular myocyte. Jpn J Physiol 2002;52:371-81.
- Minamikawa T, Cody SH, Williams DA. In situ visualization of spontaneous calcium waves within perfused whole rat heart by confocal imaging. Am J Physiol 1997;272:236-43.
- Weiwad WK, Linke WA, Wussling MH. Sarcomere length-tension relationship of rat cardiac myocytes at lengths greater than optimum. J Mol Cell Cardiol 2000;32:247-59.
- Jordan K, Chodock R, Hand AR, Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci 2001;114:763-73.
- Dedkova EN, Blatter LA. Calcium signaling in cardiac mitochondria. J Mol Cell Cardiol 2013;58:125-33.