Haloperidol in lieu of conventional therapy in the treatment of diabetic related gastroparesis in the emergency department
2 Northwell Health Department of Emergency Medicine, New York, USA
3 Feinstein Institute for Medical Research, Manhasset, New York, USA
Received: 25-Jan-2000 Accepted Date: Apr 18, 2000; Published: 25-Apr-2000
Citation: Heuser W, Carp S, Fuehrer J, et al. Haloperidol in lieu of conventional therapy in the treatment of diabetic related gastroparesis in the emergency department. Clin Pharmacol Toxicol Res. 2019;2(1):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Diabetic associated gastroparesis (GP), manifested by intractable vomiting, nausea, and abdominal pain, and is a common presentation to the emergency department (ED). Conventional treatment is focused on symptomatic management and has historically focused on the use of prokinetic agents, and antiemetics, with or without an opioid agent for intractable pain; however, no algorithm currently exists. Given its novel mechanism of action as a dopamine-2 (D2) receptor antagonist, haloperidol may be an effective alternative agent, due to its analgesic and antiemetic properties. This study seeks to determine if using haloperidol in lieu of conventional agents reduces overall ED length of stay and reduces hospital admissions.
Keywords
Ha loperidol; Diabetic gastroparesis; Prokinetic; Emergency medicine
Introduction
Gastroparesis is a disorder affecting patients with both type I and type II diabetes, wherein the stomach takes too long to empty its contents, due to delayed gastric emptying. Stimulation of the vagus nerve controls the movement of the formulated food bolus through the digestive tract. If this nerve is damaged or has impaired function, the muscles of the stomach and intestines do not work properly, resulting in the movement of food being stopped or slowed down. Diabetes can damage the vagus nerve if blood glucose levels remain chronically elevated; the pathophysiology surrounding this has been well described in the literature and is centered around advanced glycation end products (AGE’s) [1]. Other potential mechanisms have been proposed, including autonomic neuropathy [2] dysfunction of the enteric system [3], and dysfunction of hormonal and neurotransmitter control mechanisms [2-4]. Patients with diabetic gastroparesis may experience symptoms such as intractable vomiting, nausea, and abdominal pain.
Diabetic associated gastroparesis is a common presentation in the emergency department. Conventional treatment is focused on symptomatic management, limited to prokinetic agents, and antiemetics, with or without an opioid agent for intractable pain, however no treatment algorithm currently exists. First line pharmacologic treatment is generally a combination of an antiemetic agent and a promotility drug, however, data surrounding these therapeutic options are limited, and therefore these drugs are used empirically. The American Journal of Gastroenterology recommends metoclopramide as the first line prokinetic therapy (domperidone can be prescribed if available given its comparable efficacy), erythromycin to improve gastric emptying and symptoms from delayed gastric emptying, antiemetics for associated nausea and vomiting, and lastly tricyclic antidepressants (TCA) can be considered for refractory nausea and vomiting in gastroparesis [5]. It is important to note that the aforementioned treatments (antiemetics and TCA’s) will not result in improved gastric emptying and are considered conditional recommendations with moderate to low evidence, respectively [6-9]. Despite these conditional recommendations, antiemetics remain a common stay as a treatment modality for gastroparesis given the debilitating effects of nausea and vomiting on an patient’s quality of life.
Antiemetics are helpful in relieving symptoms of nausea and vomiting, which is often considered the most unbearable and disabling for patients. Common classes of antiemetics used are phenothiazines, with prochlorperazine being the most commonly administered medication in its class. Phenothiazines are dopamine-receptor antagonists that work to relieve nausea and vomiting mechanistically through a reduction in neuronal signaling to the emetic center of the brain. Serotonin (5HT-3) receptor antagonists, such as ondansetron, are used as well, however, there is no clear data that supports their use in this setting; nevertheless, they may be helpful when all other medications have failed to provide symptom relief or for those patients considered “refractory” to first line treatment options [10].
As previously discussed, prokinetic agents remain the cornerstone of therapy in diabetic gastroparesis and are utilized to enhance GI motility, and thereby relieve symptoms of nausea and vomiting. Currently available agents include motilin receptor agonists (erythromycin) and dopamine D2 receptor antagonists (metoclopramide being the prototypical agent). 5HT-4 receptor agonists (cisapride and tegaserode) have prokinetic properties and have been used historically with success, however both of these agents have been removed from the market by the FDA, as a result of reports of cardiac arrhythmias and higher risks of ischemic events, respectively [11,12]. Erythromycin, a macrolide antibiotic structurally similar to the innate prokinetic hormone motilin, increases gastric emptying by accelerating antroduodenal contractions via motilin receptor agonism thereby improving the symptomatology associated with delayed gastric emptying [13-18]. Despite numerous studies demonstrating its superior efficacy, erythromycin is not recommended as the prokinetic of choice because of problems arising with tachyphylaxis and its inherent concerns regarding the potential for bacterial resistance with chronic administration [19,20]. On the other hand, dopamine D2-receptor antagonists such as metoclopramide and domperidone (limited availability in the United States) have been used with variable degrees of success in the treatment of gastroparesis [21,22]. Metoclopramide is a central and peripheral D2-receptor antagonist that also acts as a 5-hydroxytryptamine (5HT4) agonist and 5-HT3 antagonist; secondarily, metoclopramide blocks dopaminergic inhibition of motor activity, thereby decreasing receptive relaxation and increasing antral contractions [23,24]. Domperidone, which works similarly to metoclopramide as a peripheral D2-receptor antagonist, is efficacious with the potential for less side effects, however the intravenous form was removed in the 1980s by the FDA out of concern for serious cardiac dysrhythmias.
Given domperidone’s demonstrated efficacy in clinical trials, it is no surprise that agents with similar mechanisms at the D2 receptor will improve symptomatology in diabetic gastroparesis. Haloperidol, a typical butyrophenone, is a first-generation antipsychotic conventionally used for agitation, schizophrenia, and other psychiatric conditions [25]. Haloperidol exerts its effects by non-selectively blocking the dopamine-2 receptor in the brain including the area postrema which houses the chemoreceptor trigger zone (CTZ). Due to its dopamine antagonism, haloperidol may be an effective alternative agent due to both its analgesic and antiemetic properties. The mechanism surrounding haloperidol’s antiemetic is a direct extension of its activity at the dopamine receptor, however, the analgesic mechanisms attributed to haloperidol is not fully understood given its lack of direct activity at the opioid receptors or lack of involvement with neuronal transmission of substance P; some literature report that NMDA receptor modulation and isomeric similarity to meperidine may make it active at opiate receptors, however, further studies are needed to confirm correlation [26-29].
There is a paucity of data for the use of haloperidol in the treatment of diabetic gastroparesis and much of its use is an extrapolation of metoclopramide and domperidone’s pharmacology at the dopamine receptor. There have only been 2 studies that have looked at the use of haloperidol for diabetic gastroparesis: a randomized controlled double-blind trial comparing haloperidol combined with conventional therapy to conventional therapy alone in patients with symptomatic gastroparesis, along with a retrospective analysis involving 52 patients in a California emergency department [30,31]. Both of these studies enrolled a small sample size (n=33, and n=52 respectively), and were limited to a single center. Despite these limitations to external validity, both studies had a unique study design with the prospective trial adding haloperidol to conventional therapy and the HUGS trial utilizing haloperidol as an alternative to traditional analgesia and antiemetic therapy. The results of both studies were positive, indicating that haloperidol is a potentially viable, safe, and effective alternative or addition to conventional therapy. Our retrospective analysis is similar to the HUGS trial, with respect to its retrospective design and utilizing haloperidol as an alternative therapy rather than in addition to standard therapy, however, our study offers a greater sample size and more streamlined outcomes. We hope to validate the positivity of the results displayed in the trial by Ramirez et al. and offer a new addition in the armamentarium for the treatment and management of diabetic associated gastroparesis.
Methods
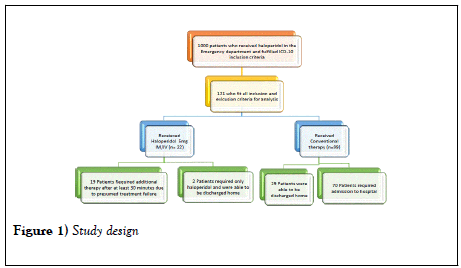
This retrospective chart review was submitted and approved by the Northwell Health Institutional review board. This study looked at a sample of 121 patients from 2014 to 2016, who met the inclusion criteria of the study; 22 patients who received haloperidol and 99 who received conventional therapy without haloperidol. A pictorial representation of the study design is depicted in Figure 1. Patients were excluded if they were pregnant, less than 18 years of age, given haloperidol for any other reason (i.e. psychiatric emergency) besides diabetic gastroparesis, history of QT prolongation recorded on a 12-lead electrocardiogram, or who had an allergy to haloperidol. Patients who were considered eligible were patients with an International Classification of Diseases (ICD10) code for Gastroparesis, Cyclic Vomiting, Abdominal pain, and Nausea/Vomiting. Patients were stratified to an algorithm with a treatment arm and a conventional therapy arm.
Primary outcomes included ED length of stay and rate of hospital admission. Secondary outcomes included the need for additional antiemetics and/or prokinetics in those patients considered “refractory” to conventional therapy (or haloperidol monotherapy) as determined by the treating emergency physician. The research objective was to compare the demographic and clinical factors between patients who received haloperidol versus no haloperidol. Demographic and clinical factors included age, sex, ethnicity, length of stay in the ED (in hours), and patient disposition (admission to hospital or discharge home).
Statistical analysis
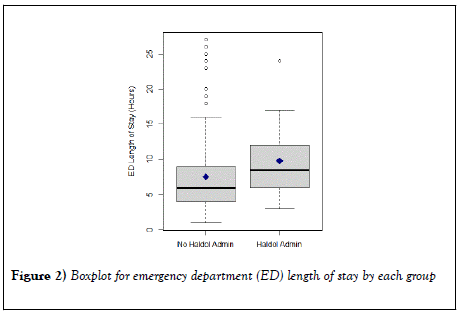
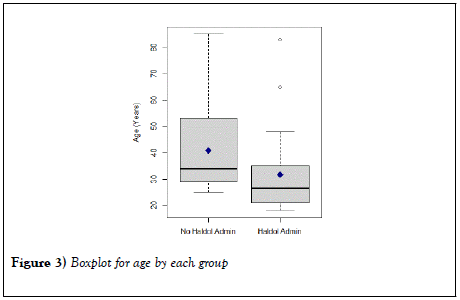
Results were presented as median, lower quartile (Q1), and upper quartile (Q3) for continuous variables and frequencies with percentages for categorical variables. The Mann-Whitney test was utilized for comparing continuous variables between groups (haloperidol versus no haloperidol). The Fisher’s exact test or chi-square test, as appropriate, was used to compare categorical variables between groups. Boxplots were presented to visualize the distributions of the ED length of stay and age according to group (Figures 2 and 3, respectively). Results were considered statistically significant if p <0.05. Analysis was conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 shows data comparing patients with and without haloperidol. Among 99 patients who didn’t receive haloperidol, there were 9 (9.1%) Caucasian, 71 (71.7%) African-American, 7 (7.1%) Hispanic, 8 (8.1%) Asians, and 4 (4.0%) other ethnicity. Among 22 patients who received haloperidol, there were 3 (13.6%) Caucasian, 15 (68.2%) African-American, 2 (9.1%) Asians, and 2 (9.1%) other ethnicity. There were no Hispanic patients receiving haloperidol. There was no statistically significant association between ethnicity and group (p <0.532). There were 19 (19.2%) male and 80 (80.8%) female who didn’t receive haloperidol. There were 8 (36.4%) male and 14 (63.6%) female who received haloperidol. There was no statistically significant association between sex and group (p <0.094).
| Variable | Haldol | No Haldol | p-value |
|---|---|---|---|
| (n=22) | (n=99) | ||
| Age (years) | 26.5 (21.0-35.0) | 34.0 (29.0-54.0) | 0.0007* |
| Length of Stay (hours) | 8.5 (6.0-12.0) | 6.0 (4.0-9.0) | 0.011* |
| Sex, n (%) | - | - | 0.094 |
| Female | 14 (63.6%) | 80 (80.8%) | - |
| Male | 8 (35.4%) | 19 (19.2%) | - |
| Disposition, n (%) | - | - | 0.024* |
| Admitted to Hospital | 10 (45.5%) | 70 (70.7%) | - |
| Discharged Home | 12 (54.6%) | 29 (29.3%) | - |
| Ethnicity, n (%) | - | - | 0.532 |
| African American | 15 (68.2%) | 71 (71.7%) | - |
| Asian | 2 (9.1%) | 8 (8.1%) | - |
| Caucasian | 3 (13.6%) | 9 (9.1%) | - |
| Hispanic | 0 (0%) | 7 (7.1%) | - |
| Other | 2 (9.1%) | 4 (4.0%) | - |
Data were presented as median (lower quartile – upper quartile) for continuous variables, and number (%) for categorical variables. p-values were computed using Mann-Whitney test, Chi-square test, or Fisher’s exact test, as appropriate. *Statistically significant results (p<0.05).
Table: Results of the univariable analysis of Haldol vs. No Haldol
Patients who didn’t receive haloperidol were significantly older, compared to the patients who received haloperidol (Median (Q1, Q3)=34.0 (29.0, 54.0) vs. 26.5 (21.0, 35.0); p <0.0007). Patients who didn’t receive haloperidol had significantly shorter ED length of stay, compared to the patients who received haloperidol (Median (Q1, Q3)=6.0 (4.0, 9.0) vs. 8.5 (6.0, 12.0); p <0.011). However, patients who didn’t receive haloperidol had a significantly higher rate of hospital admission, compared to patients who received haloperidol (70.7% vs. 45.5%, p <0.024).
Categorical variables
P-values were computed using Mann-Whitney test, chi-square test, or Fisher’s exact test, as appropriate.
* Statistically significant results (p <0.05).
Maximum and minimum values excluding outliers are represented by the top and bottom vertical lines (i.e. whiskers), outside the box, respectively. The horizontal line in the box indicates the median. The lower and upper quartiles are represented by the horizontal line, drawn in the top and bottom of the box. The diamond symbol represents the mean value. Dots represent the outliers.
Discussion
This study, as mentioned previously, sought to emulate, and validate the findings of previous studies that noted a potential role for haloperidol in the treatment of diabetic gastroparesis. The design of our study was retrospective in nature and sought to isolate the individual role of haloperidol vs. conventional therapy in terms of ED length of stay (LOS), and patient disposition. The rationale for our study stems from the fact that haloperidol ameliorates many, if not all, of the symptoms associated with diabetic gastroparesis (nausea, vomiting, reduced GI motility, etc.) while providing a unique sedating effect. Although our study did not achieve our primary objective of reducing ED length of stay, it did, however, reduce hospital admissions secondary to improved symptomatology, which is a more patient centered outcome and reduces cost to the health care system. In terms of ED length of stay and ultimate disposition, we found a statistically significant difference in both length of stay and disposition between groups. Those receiving haloperidol were more likely to be discharged home, but had a longer length of stay in the ED. Again, lack of data limits our study to elucidate the reason for longer length of stay. Possible hypotheses include initial administration of conventional therapies followed by haloperidol, lethargy from haloperidol administration resulting in longer observation times, or requirement for lengthier ongoing reassessment periods for patients who received haloperidol (pre or post administration). However, patients who received haloperidol for their pain were less likely to require hospital admission for pain control.
Haloperidol is fairly safe when given acutely in the management of diabetic gastroparesis, however, safety remains a concern for those individuals with potential for QT-interval prolongation; This subset of patients were excluded from this retrospective analysis. Other side effects have been extensively described in the literature and include hypotension, extrapyramidal symptoms, akathisia, and potentially neuroleptic malignant syndrome. Although these side effects are mostly overlooked in the setting of “one time” doses in the emergency department, it is important to consider these side effects prior to administration. The question arises of whether one should obtain an electrocardiogram (ECG) for every patient that is to be given haloperidol for the treatment of diabetic gastroparesis. In our opinion and given the results of many studies regarding the use of haloperidol in the ED, we find it impractical to perform an ECG prior to each administration, however, in those patients that are at higher risk (long QT syndrome, multiple QTc prolongation agents, etc.) caution should be advised or not used at all.
Few studies have been conducted to evaluate the use of haloperidol for the treatment of chronic abdominal pain attributable to gastroparesis. In this retrospective study, our ultimate goal was to assess the ED length of stay and disposition (discharge vs. admission) among groups receiving haloperidol and those not receiving haloperidol to determine if there was statistical significance that could potentially be attributed to haloperidol administration. The two groups were evaluated by demographic characteristics, specifically age, sex, and ethnicity. There was no statistically significant difference in sex or ethnicity between groups. However, a statistically significant difference in age between the groups, with younger patients being more likely to receive haloperidol. The reasons for this difference are unclear based on the documentation we had available for review, though we hypothesize providers may have been trying to avoid polypharmacy in older patients. Further studies may help elucidate the reasoning for this finding [30-31].
Limitations
Our study has several limitations. As a retrospective chart review-based study, we were limited in sample size, particularly since haloperidol is not yet widely used for gastroparesis pain. In order to expand our external and internal validity, specifically in the ED setting, we included patients with not only gastroparesis, but also cyclic vomiting, nausea, and generalized abdominal pain; this seemed to have limited the previous studies in regard to enrollment and overall external validity and applicability to a greater subset of patients in the ED. Another potential limitation and confounder is the significant difference in age between those receiving haloperidol and those not receiving haloperidol. Older patients were more likely to require hospital admission for pain control compared to younger patients, and older patients were less likely to receive haloperidol. It is unknown if the high admission rate is directly related to hesitation to administer haloperidol, as older patients tend to have more medical comorbidities or raise more clinical concern for decompensation at home. Additional limitations include the nature of our study design where patients and providers were not blinded to the administration of haloperidol, potentially introducing bias. Also, as a critique of the HUGS trial which displayed similarity to our limitations, it is unknown if there is a dose dependent effect of haloperidol in this disease state or if different routes of haloperidol administration would result in differing clinical efficacy.
Our major limitations are the retrospective nature of our study and subsequent small sample size. Given data collection through chart review, many patients had to be excluded due to unclear reason for medication administration, unclear diagnosis, or charting that did not definitively indicate haloperidol given for patient presenting with pain from gastroparesis. Though our results do have statistical significance, further prospective studies are needed to determine if haloperidol does, in fact, play a role in reduction of pain, admissions, and healthcare spending on gastroparesis. If future studies can show a statistically significant contribution of haloperidol to treatment of pain from gastroparesis, these results could potentially support change of clinical practice to include earlier and mainstream use of haloperidol, better pain control, fewer hospital admissions, and lower healthcare costs for these patients when they presented in the ED.
Conclusion
A statistically significant reduction in the rate of hospital admission, was found in patients with diabetic gastroparesis who received haloperidol as compared to conventional therapy; however, the overall mean ED length of stay was longer in patients who received haloperidol monotherapy. Haloperidol may represent a suitable, effective, and safe alternative to conventional therapy in the ED management of gastroparesis-associated nausea, vomiting, and abdominal pain. Further prospective studies are warranted to determine haloperidol’s true place in therapy for alleviating symptomatology associated with diabetic gastroparesis.
Conflicts of Interest
The author (s) declare (s) that there are no conflicts of interest regarding the publication of this manuscript.
Funding
The author (s) of this manuscript have no conflicts of interests to disclose (i.e. no industry funding received or other commercial relationships). The manuscript was performed as part of the employment of the author (s) (Employer: Northwell Health).
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to legal or ethical reasons.
REFERENCES
- Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953-61.
- Koch KL. Diabetic gastropathy: Gastric neuromuscular dysfunction in diabetes mellitus: A review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061-75.
- Smith DS, Ferris CD. Current concepts in diabetic gastroparesis. Drugs. 2003;63:1339-58.
- De Block CE, De Leeuw IH, Pelckmans PA et al. Current concepts in gastric motility in diabetes mellitus. Curr Diabetes Rev. 2006;2:113-30.
- Camilleri M, Parkman H, Shafi M et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
- Prakash C, Lustman PJ, Freedland KE et al. Tricyclic antidepressants for functional nausea and vomiting: Clinical outcome in 37 patients. Dig Dis Sci. 1998;43:1951-6.
- Sawhney MS, Prakash C, Lustman PJ et al. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418-24.
- Lustman PJ, Freedland KE, Griffith LS et al. Fluoxetine for depression in diabetes: A randomizeddouble-blind placebo-controlled trial. Diabetes Care. 2000;23:618-23.
- Lustman PJ, Griffith LS, Clouse RE et al. Effects of nortriptyline on depression and glycemic control in diabetes: Results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59:241-50.
- Barrett TW, DiPersio DM, Jenkins CA et al. A randomized, placebo- controlled trial of ondansetron, metoclopramide, and promethazine in adults. Am J Emerg Med. 2011;29:247-55.
- Smith DS, Ferris CD. Current concepts in diabetic gastroparesis. Drugs. 2003;63:1339-58.
- Fayyaz M, Lackner JM. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther Clin Risk Manag. 2008;4:41-8.
- Samsom M, Vermeijden JR, Smout AJ et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: A prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116-22.
- Janssens J, Peeters TL, Vantrappen G et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin preliminary studies. NEJM. 1990;322:1028-31.
- Kendall BJ, Chakravarti A, Kendall E et al. The effect of intravenous erythromycin on solid meal gastric emptying in patients with chronic symptomatic post-vagotomyantrectomy gastroparesis. Aliment Pharmacol Ther. 1997;11:381-85.
- Tack J, Janssens J, Vantrappen G et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology. 1992;103:72-79.
- Samsom M, Jebbink RJ, Akkermans LM et al. Effects of oral erythromycin on fasting and postprandial antroduodenal motility in patients with type I diabetes, measured with an ambulatory manometric technique. Diabetes Care. 1997;20:129-34.
- Richards RD, Davenport K, McCallum RW, et al. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol. 1993;88:203-7.
- Smith DS, Ferris CD. Current concepts in diabetic gastroparesis. Drugs. 2003;63:1339-58.
- Hawkyard CV, Koerner RJ. The use of erythromycin as a gastrointestinal prokinetic agent in adult critical care: Benefits versus risks. J Antimicrob Chemother. 2007;59:347-8.
- Abell TL, Bernstein RK, Cutts T et al. Treatment of gastroparesis: A multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263-83.
- Sturm A, Holtmann G, Goebell H et al. Prokinetics in patients with gastroparesis: A systematic analysis. Digestion. 1999;60:422-27.
- Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: A review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061-75.
- Smith DS, Ferris CD. Current concepts in diabetic gastroparesis. Drugs. 2003;63:1339-58.
- https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug- Shortage-Detail.aspx?type=rss&source=current&id=459
- Maltbie AA, Cavenar JO Jr, Sullivan JL et al. Analgesia and haloperidol: A hypothesis. Clin Psychiatry. 1979;40:323-6.
- Cendan CM, Pujalte JM, Portillo-Salido E et al. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology. 2005;182:485-93.
- Colclough G, McLarney JT, Sloan PA et al. Epidural haloperidol enhances epidural morphine analgesia: three case reports. J Opioid Manag. 2008;4:163-66.
- Seidel S, Aigner M, Ossege M, et al. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2013;8:CD004844.
- Roldan C, Chambers K, Paniagua L, et al. Randomized controlled double-blind trial comparing haloperidol combined with conventional therapy to conventional therapy alone in patients with symptomatic gastroparesis. Acad Emerg Med. 2017;24:1307-14.
- Ramirez R, Stalcup P, Croft B et al. Haloperidol undermining gastroparesis symptoms. Am J Emerg Med. 2016;68:80.