Ovoderm® an effective treatment to improve skin condition in patients with altered skin barrier function
Received: 06-Feb-2018 Accepted Date: Mar 06, 2018; Published: 13-Mar-2018
Citation: Aguirre A, Gil-Quintana E, Nuez M. Ovoderm® an effective treatment to improve skin condition in patients with altered skin barrier function. J Skin 2018;2(1):11-14.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Alterations in the stratum corneum and therefore in the skin barrier function are produced by diverse causes. The changes in the stratum corneum imply increases in water loss, reduction of the protective effect of the skin and also modifications in its mechanical functions. The aim of the present study was to evaluate the effectiveness of Ovoderm®, a dietary supplement consisting of eggshell membrane, to improve the skin condition of people with an altered barrier function.
Sixteen volunteers with a decreased skin barrier function were randomised to daily intake 300mg of Ovoderm® or 300mg of placebo during 60 days. Transepidermal waterloss (Tewameter®), firmness (Cutometer® R0), elasticity (Cutometer® R6) and fatigue (Cutometer® R9) of the skin were measured. At the end of the study there was a significant 43% of decline in the transepidermal waterloss in the volunteers intaking Ovoderm® that was not observed in the placebo group. Participants started the study with normal-affected skin and finished it with healthy-very healthy skin values. A similar tendency was observed in the skin elasticity that was increased by 13% in Ovoderm® group while the control group showed a decrease of 11%. The skin firmness improved significantly by 66% and the fatigue declined by 36% in Ovoderm® group while no significant changes were measured in the placebo group. These results showed that oral supplementation with Ovoderm® restores the skin barrier function in people with cutaneous alterations. Ovoderm® re-establishes the transepidermal waterloss values to those observed in people with healthy skin and it increases the functionality of the skin as evidenced by the improvements in firmness and elasticity and by the decrease in fatigue. Ovoderm® is an effective treatment that could prevent and manage more effectively the alteration of the skin barrier function restoring the skins’ health and its biomechanical properties
Keywords
Ovoderm®; Eggshell membrane; Altered skin barrier function; Firmness; Elasticity
The skin is the largest organ in the human body and takes part in vital functions. The skin consists of three layers: epidermis, dermis and hypodermis. The epidermis is the top layer of the skin, an avascular tissue that plays the barrier function role of the skin protecting it against external aggression agents. It is considered as a flat epithelium in a constant process of cornification called stratum corneum (SC). Depending on physiological conditions, the renewal of the epidermis covers a period of approximately 30 days, since the cell division occurs until the detachment of totally cornified cells. The epidermis is avascular, its care and maintenance are done by nutrient diffusion from the dermis [1]. The dermis is a vascularized connective tissue composed mainly by a structure of fibroblasts criss-crossed by a network of fibers of collagen, elastin and glycosaminoglycans (GAGs) such as hyaluronic acid (AH). Collagen represents about 60-80% of its dry weight and is mainly type I, whose main function is to provide resistance to the skin. Collagen and elastin are synthesized by fibroblasts and both give flexibility and elasticity to the skin [1]. The hypodermis is the deepest layer of skin and is made up of loose connective tissue, due to the presence of adipose tissue in this layer of the skin, its main function is the isolation and storage [2].
The skin is a physical, biochemical and immunological barrier that protects the body from mechanical aggressions, environmental pollutants, sunlight and penetration of microorganisms and other antigens. Any alteration of the skin barrier function will cause an increase in the sensitivity to UV radiation and an exponential increase in the risk of suffering infections.
Some treatments such as antineoplastic therapies cause alterations in the SC and as a result, an alteration in the skin barrier function [2,3]. The routinely employed and validated tool to measure the skin barrier function is the transepidermal water loss (TEWL) which is the normal permeation of water through the SC into the atmosphere [4]. The first result of the decreased skin barrier function is the increase of TEWL that involves the dehydration of the skin, resulting in alterations in its visco-elastic properties like firmness and elasticity. TEWL appears therefore as a method to know the real healthy state of the SC and the skin.
Currently, there is a growing interest in the development of food supplements that may be effective in modifying the structure, physiology and in consequence, the outward appearance and the functions of the skin [5]. The ingredients that have attracted more interest in this area are collagen and AH since they are bricks for the construction of the skin [6-8]. In vitro studies have shown the capacity of certain collagen peptides as potent antioxidants [9], also several experimental studies have shown the effectiveness of collagen in improving the properties of the skin [10-13]. Regarding the AH, it has been described also its role in the proliferation and differentiation processes of keratinocytes [14] as well as in the maintenance of cellular structure due to its capacity of water retention and its viscosity [15].
The eggshell membrane is a natural product containing collagen, glucosamine, GAGs and keratin among others. It has been demonstrated the ability of eggshell membrane to accelerate the cellular division of fibroblasts and to increase their metabolic activity by improving their collagen production. Eggshell membrane is also effective reducing the harmful effects of UV light and the inflammatory processes in skin [16-19]. In people without altered skin, the significant increase of skin elasticity after the intake of Ovoderm®, composed of eggshell membrane, was demonstrated [20].
The objective of this study was to assess the skin benefits of the oral intake of 300 mg of Ovoderm®, eggshell membrane, on people with normal-affected skin due to the alteration of their skin barrier function, as measured by TEWL.
Material and Methods
Food supplement under investigation
The dietary supplement used in this study was Ovoderm® (Eggnovo, Spain), consisting of eggshell membrane separated from eggshells by a patented process (Patents: ES 2 181580 B1 and ES 2 327087 B2). Compositional analysis of eggshell membranes has identified a high content of protein (collagen types I-V-X, elastin, keratin) [21,22] and moderate quantities of glucosamine and GAGs (chondroitin sulfate, HA) [23].
Study design
A randomized, double-blind and unicentric clinic-nutritional study was performed to evaluate the efficacy of the daily intake of an encapsulated food supplement containing 300 mg of Ovoderm® (eggshell membrane).
Participants had to intake one capsule a day of the food supplement with Ovoderm® or with placebo (microcrystalline cellulose) during 60 consecutive days.
Assessments were performed at the beginning (day 0) and at the end of the study (day 60). The study was approved by the Research Ethic Committee of the Quirón hospital and it was conducted by IDERMA - Instituto Quirón Dexeus.
Subjects
A total of 16 healthy volunteers, men and women, between 45 and 75 years old were enrolled in the study. The volunteers were randomized to each of the two treatment groups, 7 to Ovoderm® group and 9 to placebo group. Prior to the beginning of the oral treatment and data acquisition, a washout period of two weeks was stablished. Throughout the study volunteers could not use any cosmetic product on the test area. The subjects fulfilling all the inclusion criteria were enrolled in the study.
Inclusion criteria
The inclusion criteria were as follows: general good health, no hypersensitivity to any of the components, no digestive pathologies and not undergoing pharmacological treatment; healthy and balanced living and dietary habits; ability to understand the clinical study, personal informed consent to participate in the study and willingness and capability to follow the study rules and a fixed schedule. All the participants should have TEWL score higher than 16.
Exclusion criteria
The exclusion criteria were as follows: allergy to eggs; eating disorders; gastrointestinal disorders or digestive surgeries the previous two years; systemic illnesses altering intestinal motility or changes in the intestinal motility by stress; diabetes, hypo or hyper thyroidism; medication or drugs changing the intestinal motility; changes in the diet habits in the previous 2 months; pharmacological treatment and/or food supplements that change the body weight or the appetite; alcohol, drugs, drug products or alcohol abuse; stop smoking the last 6 months or planning to stop during the study; pregnancy or period of breast feeding; intake of nutritional supplements; changes in the usual skin care routine; lack of compliance and intellectual or mental inability to follow the study instructions; TEWL score equal or less to 15 measured according to the procedure explained subsequently.
Measurements
Transepidermal waterloss: TEWL was measured using the Tewameter® (Software Tewameter® MPA 580) and by applying a constant negative pressure. Prior to the measurement there was a 10 minute of acclimation. The scale is as follows: 0-10 (very healthy condition), 10-15 (healthy condition), 15-25 (normal condition), 25-30 (affected skin) and superior to 30 (critical condition) (Technical Guide).
Skin elasticity, firmness and fatigue or tiring effect: Skin elasticity was measured with the Cutometer® (Software Cutometer® MPA 580) by applying a constant negative pressure. Prior to the measurement there was a 10 minute of acclimation. The resistance of the skin to be sucked up by negative pressure (firmness) and its ability to return into its original position (elasticity) are displayed as curves. To measure the firmness R0 value was recorded. This parameter looks at the maximum amplitude and represents the passive behaviour of the skin to force (firmness). To analyse skin elasticity R6 value was recorded (Portion of the visco-elasticity on the elastic part of the curve), which decreases with increasing skin elasticity. R9 parameter represents tiring or fatigue effects of the skin after repeated suction and release of the skin. The lower the R9 value the smaller the fatigue or tiring effect (Technical Guide).
Statistical analysis
Statistical analysis of skin parameters was performed. Differences between baseline (day 0) and final data (day 60) were analysed by paired t-test. Statistical significance was considered when P ≤ 0.05. Results are shown as mean ± standard error.
Results
A total of 16 healthy volunteers, men and women, completed the full course of treatment and follow-up. There were no discomfort or adverse reactions reported. The mean age of participants was 51.06 ± 8.17 years old; results divided by group were: for control group 49.33 ± 9.03 years old and for Ovoderm® group 53.29 ± 6.09 years old.
Transepidermal waterloss
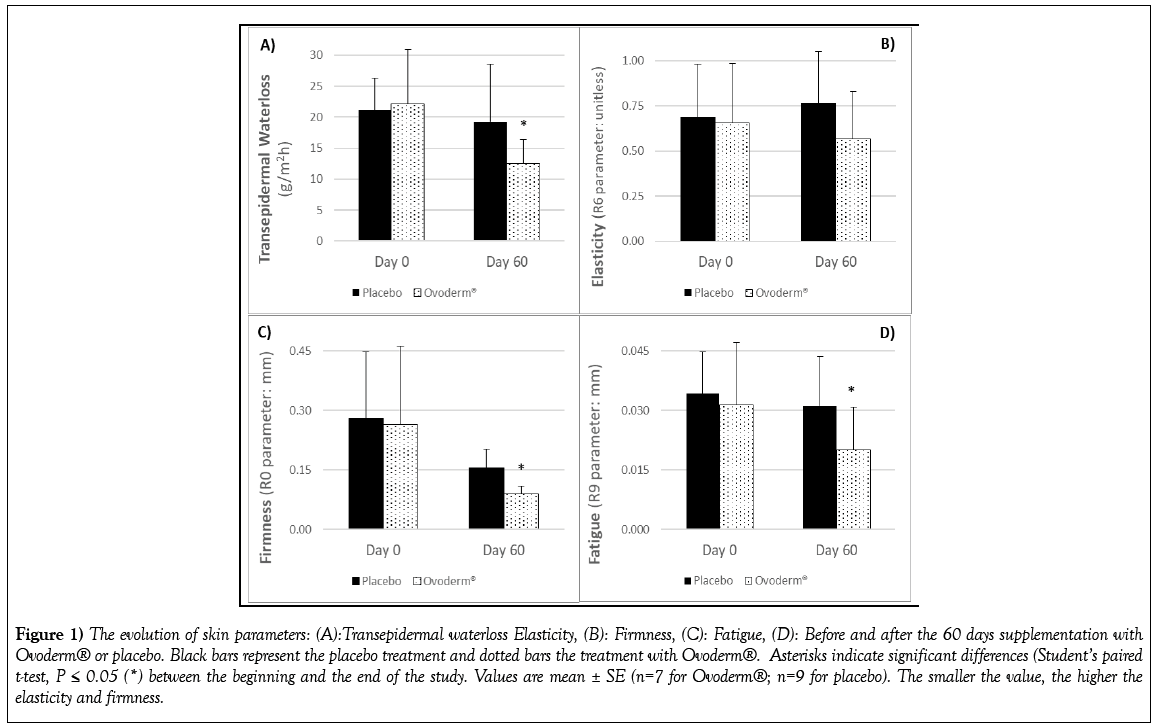
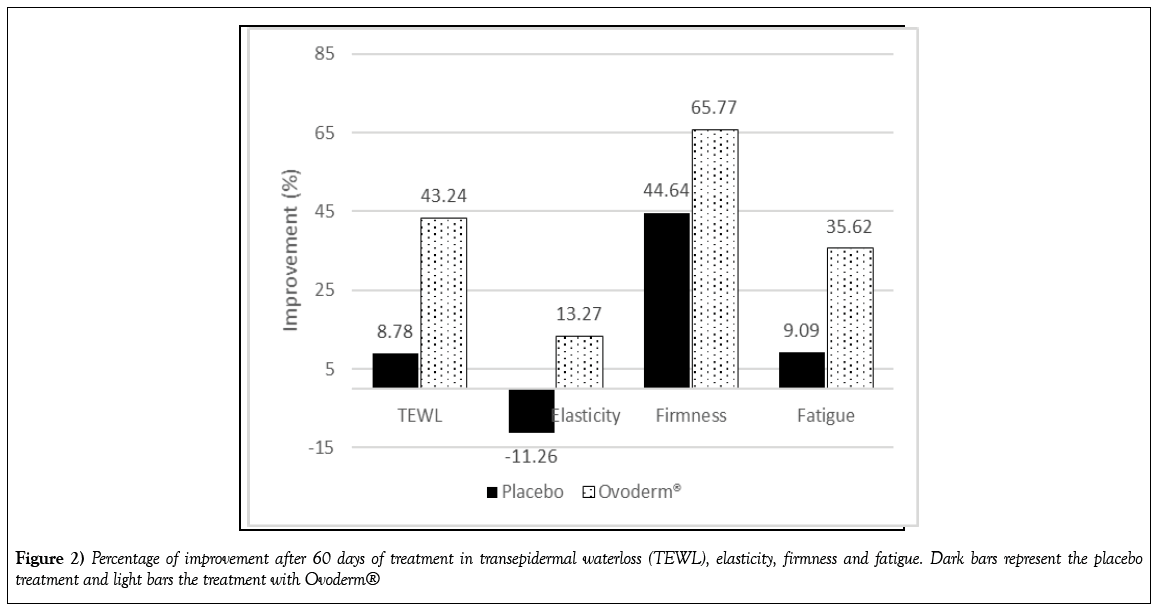
TEWL, known as the skin’s barrier function, was categorised as “normalaltered condition” in both groups (values higher than 16), the mean TEWL score at the beginning of the study was 21.55 ± 6.75 g/m2h (Figure 1A). Some subjects had TEWL score higher than 30 and none of them had “healthy” or “very healthy” skin. After 60 days of treatment Ovoderm® group showed a statistically significant reduction in the TEWL score from 22.10 ± 8.81 g/m2h the day 0 until 12.54 ± 3.83 g/m2h the day 60 while no significant changes were measured in the control group (from 21.12 ± 5.18 g/m2h at day 0 to 19.29 ± 9.29 g/m2h at day 60) (Figure 1A). The decline in Ovoderm® group was of 43.2% while in the placebo group the change was of 8.8% (Figure 2).
Figure 1: The evolution of skin parameters: (A):Transepidermal waterloss Elasticity, (B): Firmness, (C): Fatigue, (D): Before and after the 60 days supplementation with Ovoderm® or placebo. Black bars represent the placebo treatment and dotted bars the treatment with Ovoderm®. Asterisks indicate significant differences (Student’s paired t-test, P ≤ 0.05 (*) between the beginning and the end of the study. Values are mean ± SE (n=7 for Ovoderm®; n=9 for placebo). The smaller the value, the higher the elasticity and firmness.
Regarding TEWL categories, 71,4% of the participants in Ovoderm® group showed final TEWL scores categorised as healthy skin condition (< 15). Only two people in this group showed values higher than 15 but close to it (16.7 one and 15.8 the other) and 14% of the volunteers improved until very healthy skin condition. In the control group 44% of participants had healthy skin at the end of the study and 56% of the subjects showed normal-affected skin condition (TEWL values higher than 15; three of them reached values higher than 20 being two of them upper 30). At the end of the study the skin categories were maintained as “Normal-affected skin” in placebo group and healthy-very healthy in Ovoderm® group (data not shown).
Skin elasticity
After 60 days of treatment R6 values (unitless) declined in Ovoderm® group from 0.656 ± 0.330 (baseline) to 0.569 ± 0.263 the day 60 whereas and increased in the placebo group was measure from 0.689 ± 0.293 to 0.766 ± 0.287 (Figure 1B). This means an improvement of the skin elasticity of 13.3% in Ovoderm® group and a reduction of 11.3% in the placebo group after 60 days of treatment (Figure 2).
Skin firmness
The intake of Ovoderm® showed a statistically significant change from 0.264 ± 0.198 mm the day 0 to 0.090 ± 0.019 mm the day 60 while no significant differences were assessed in the placebo group (R0 score at day 0: 0.280 ± 0.169 mm and R0 score at day 60: 0.155 ± 0.048 mm) (Figure 1C). Ovoderm® improved significantly skin firmness a 65.8% whereas the placebo group showed a change of 44.6% (Figure 2).
Skin fatigue
There was a statistically significant reduction in the skin fatigue (R9 value) after 60 days intaking Ovoderm® from 0.031 ± 0.016 mm the day 0 to 0.021 ± 0.011 mm the day 60 (Figure 1D) what meant a decrease in skin fatigue of 35.62% (Figure 2). In the control group no, statistical differences were shown in the R9 values from 0.034 ± 0.011 mm at day 0 to 0.031 ± 0.012 mm after 60 days which was a reduction of 9.1% (Figure 2).
Discussion
The SC together with collagen, elastic fibers and viscosity of the interstitial liquid are closely related to the biomechanical properties and the health of the skin [24]. Alterations in the SC, and therefore in the skin barrier function, are produced by diverse causes; external agents: pollution and UV rays [19,25], diseased conditions and other situations such as antineoplastic treatments [2,3]. The antineoplastic treatments due to their lack of specificity attack both, malignant cells and rapid proliferation cells as skin cells, causing the alteration of the SC and therefore of the skin barrier function [2,3]. The caused damaged implies the change or the suspension of the oncologic treatment, which interferes in the expected treatment success [26,27]. The changes in the SC imply increases in water loss, reduction of the protective effect of the skin and also modifications in its mechanical functions. All together they cause the loss of elasticity and the appearance of wrinkles and sagging mainly due to the progressive degeneration of the collagen fibers in the dermis.
The present study was focused on participants with altered skin barrier function as shown by TEWL values higher than 16. There was a significant decline in TEWL values in Ovoderm® group that was not observed in the placebo group. Volunteers’ intaking placebo ended the study in normalaffected skin condition whereas the participants in taking Ovoderm® were categorized as healthy-very healthy skin condition. So, this dietary supplement is able to transform a normal-altered skin in a healthy-very healthy skin. There are few studies using Ovoderm® as an oral treatment to improve the skin physiology in parameters such as elasticity, hydration and pigmentation [20] but this is the first study that demonstrates its effectiveness as a treatment to repair the SC and the skin barrier function in people with such parameters altered. Moreover, parameters related to visco-elastic properties and physiology of the skin was also measured. Significant improvements were observed in skin firmness and fatigue after 60 days of Ovoderm® intake. Similarly, an increase in elasticity was also viewed. Our results are in agreement with previous investigations in which oral administration of collagen-derived products shown improvement in skin elasticity [8,28]. The improvements observed in skin elasticity, firmness and TEWL suggest a long-lasting positive physiological effect, in contrast to effects of topical skin-care products, which increase skin elasticity predominantly by enhancing epidermal hydration [29] or acting as humectants or by providing an artificial barrier to trans-epidermal water loss [30].
Conclusion
A variety of different conditions can cause the alteration of SC and the barrier function of the skin. Ovoderm® appears as an effective treatment to repair the SC and the skin barrier function in people with altered parameters. The daily intake of the dietary supplement containing Ovoderm® is able to transform a normal-altered skin in a healthy-very healthy skin, together with a significant improvement in the visco-elastic properties of the skin; for instance, elasticity, firmness and fatigue. Summarizing, the present study demonstrates for the first time that the oral intake of Ovoderm® strengthens and recovers the skin barrier function and the visco-elastic properties of the skin. More research needs to be done in order to further demonstrate the beneficial effects of Ovoderm® in subjects with altered skin barrier function.
REFERENCES
- Champion RH, Burton JL, Ebling FJG. Textbook of Dermatology (Rook, Wilkinson, Ebling eds), 5th ed. Oxford, UK: Blackwell Scientific Publications 1992.
- Bensadoun RJ, Humbert P, Krutman J, et al. Daily baseline skin care in the prevention, treatment and supportive care of skin toxicity in oncology patiens: recommendations from a multinational expert panel. Cancer Manag Res 2013;5:401-8.
- Dreno B, Bensadoun RJ, Humbert P, et al. Algorithm for dermocosmetic use in the management of cutaneous side-effects associated with targeted therapy in oncology. J Eur Acad Dermatol Venereol 2013;27:1071-80.
- Fluhr JW, Feingold KR, Elias PM. Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp Dermatol 2006;15:483-92.
- Proksch E, Schunck M, Zague V, et al. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol Physiol 2014;27:113-9.
- Schwartz SR, Park J. Ingestion of BioCell Collagen (®), a novel hydrolysed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging 2012;7:267-73.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol 2015;14:291-301.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol 2014;27:47-55.
- Wang B, Wang YM, Chi CF, et al. Isolation and characterization of collagen and antioxidant collagen peptides from scales of Croceine Croaker (Pseudosciaena crocea). Mar drugs 2013;11: 4641-61.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin Pharmacol Physiol 2014;27: 47-55.
- Borumand M, Sibilla S. Effects of a nutritional supplement containing collagen peptides on skin elasticity, hydration and wrinkles. J Med Nutrition and Nutraceuticals 2015;4:47-53.
- Liang J, Pei X, Zhang Z, et al. The protective effects of long-term oral administration of marine collagen hydrolysate from Chum Salmon on collagen matrix homeostasis in the chronological aged skin of Sprague-Dawley male rats. J Food Sci 2010;75: H230-H238.
- Haratake A, Watase D, Fujita T, et al. Effects of oral administration of collagen peptides on skin collagen content and its underlying mechanism using a newly developed low collagen skin mice model. J Functional Foods 2015;16:174-182.
- Jee HC, Jin HJ, Ji HK, et al. Synergistic effect of interleukin-6 and hyaluronic acid on cell migration and ERK activation in human keratinocytes. J Korean Med Sci 2014;29: S210-S216.
- Stern R, Maibach HI. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin Dermatol 2008;26:106-22.
- Candilish JK, Scougall RK. L-5-hydroxylysine as a constituent of the shell membrane of the hen's egg. Int J Protein Res 1969;1:299-306.
- Yoo J, Park K, Yoo Y, et al. Effects of egg shell membrane hydrolysates on anti-inflammatory, anti-wrinkle, anti-microbial activity and moisture-protection. Korean J Food Sci Anim Resour 2014;34:26-32.
- Yoo JH, Kim JK, Yang HJ, et al. Effects of egg shell membrane hydrolysates on UVB-radiation-induced wrinkle formation in SKH-1 hairless mice. Korean J Food Sci Anim Resour 2015;35:58-70.
- Park K, Yoo J, Shin Y et al. Effects of egg shell membrane hydrolysates on skin whitening, wound healing and UV-protection. Korean J Food Sci Ani Resour 2012;32:308-15.
- Aguirre A, Gil-Quintana E, Fenaux M, et al. Beneficial effects of oral supplementation with ovoderm on human skin physiology: Two pilot studies. J Diet Suppl 2017;14:706-14.
- Wong M, Hendrix MJC, Von der Mark K, et al. Collagen in the egg shell membranes of the hen. Devel Biol 1984;104:28-36.
- Arias JL, Fernandez MS, Dennis JE, et al Collagens of the chicken eggshell membranes. Connect Tissue Res 1991;26:37-45.
- Jingwen Du. Proteomic analysis provides new insight into the chicken eggshell cuticle. J of Proteomics 2012;75:2697-706.
- Langton AK, Graham HK, McConnell JC, et al. Organization of the dermal matrix impacts the biomechanical properties of skin. Br J Dermatology 2017;177:818-27.
- Imokawa G, Koichi I. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J Mol Sci 2015;16:7753-75.
- Byun HJ, Lee HJ, Yang JI, et al. Daily skin care habits and the risk of skin eruptions and symptoms in cáncer patients. Ann Oncol 2012;23:1992-8
- De Tursi M, Zilli M, Carella C, et al. Skin toxicity evaluation in patients treated with cetuximab for metastatic colateral colorectal cancer. A new tool for more accurate comprehension of quality of life impacts. Onco Targets Ther 2017;10:3007-15.
- Asserin J, Lati E, Shihoya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosm Dermatol 2015;4:291-301.
- Liu D, Nikoo M, Boran G, et al. Regenstein JM collagen and gelatin. Annu Rev Food Sci Technol 2015;6:527-57
- Buraczewska I, Broström U, Lodén M. Artificial reduction in transepidermal water loss improves skin barrier function. Br J Dermatol 2007;157:82-86.