Potential therapeutic applications of WNT/β-catenin interventions in treating human disease
Received: 01-Aug-2018 Accepted Date: Aug 30, 2018; Published: 06-Sep-2018
Citation: Richards T, Goggolidou P. Potential therapeutic applications of WNT/β-catenin interventions in treating human disease. J Clin Gen Genomics. 2018;1(2):17-20.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Introduction
The WNT/β-catenin signaling pathway (conventionally called the canonical WNT pathway) plays an essential role in embryonic development, tissue homeostasis, tissue injury repair and stem cell maintenance [1]. In recent years, due to the pathway’s notable upregulation in many different cancers [1], targets that modify its signalling responses have been sought. The significant role of WNT signalling in human disease has also been demonstrated by its hyperactivation in other hyperproliferative diseases [2,3].
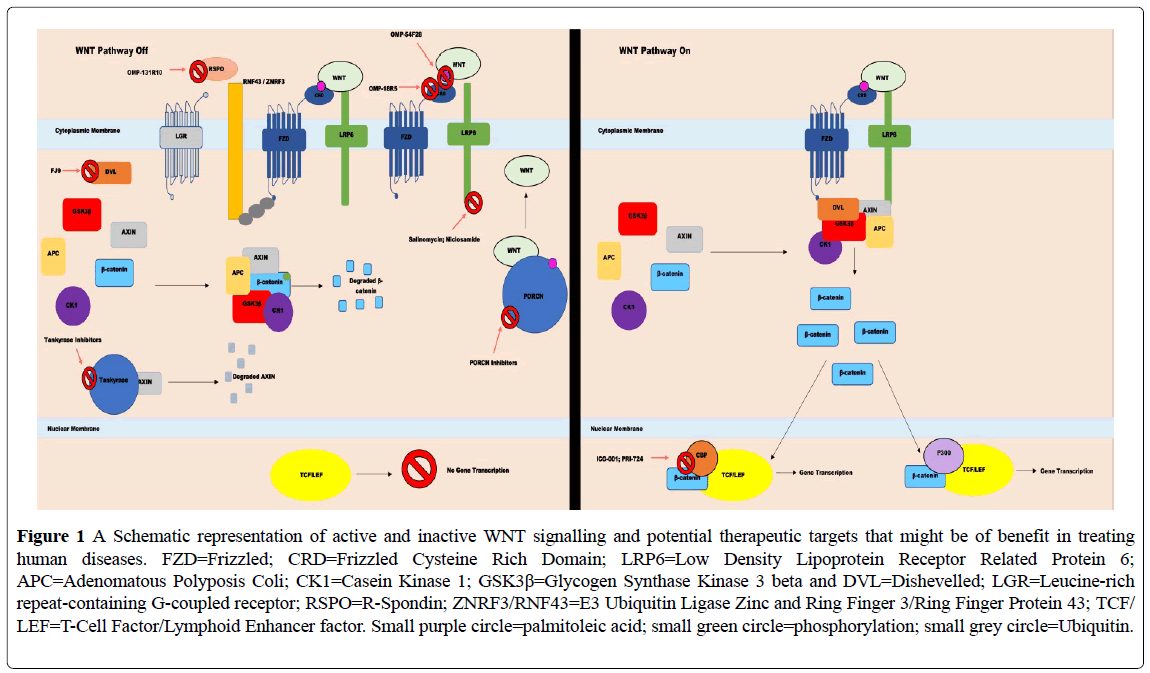
The canonical WNT pathway upregulates gene expression by rescuing β- catenin from its destruction complex [1]. In an environment lacking WNTs, β-catenin is phosphorylated by a combination of proteins; Adenomatous Polyposis Coli (APC), the scaffold protein AXIN and the kinases Casein Kinase 1(CK1) and Glycogen Synthase Kinase 3 beta (GSK3β) [4]. This phosphorylation results in the ubiquitination of β- catenin and its degradation by the proteasome (Figures 1 and 2) [4]. In the upstream component of the pathway, WNT ligands bind to the cysteine rich domain (CRD) of the Frizzled receptor (FZD), along with its coreceptor Low Density Lipoprotein Receptor Related Protein 5 (LRP5) or (LRP6) [1]. Dishevelled (DVL) binds to AXIN via its DIX domain and is then recruited to the Frizzled receptor by interactions between Frizzled and its own PDZ domain [1]. The introduction of DVL to the FZD-LRP6 complex allows for WNT ligands to promote DVL-dependent LRP6 phosphorylation, in turn switching off the destruction complex [1]. β- catenin stabilization allows for its accumulation in the cytoplasm and translocation to the nucleus, where it binds with T-Cell Factor/Lymphoid Enhancer factor (TCF/LEF) and co-activators, resulting in downstream activation of gene expression [1].
Figure 1: A Schematic representation of active and inactive WNT signalling and potential therapeutic targets that might be of benefit in treating human diseases. FZD=Frizzled; CRD=Frizzled Cysteine Rich Domain; LRP6=Low Density Lipoprotein Receptor Related Protein 6; APC=Adenomatous Polyposis Coli; CK1=Casein Kinase 1; GSK3β=Glycogen Synthase Kinase 3 beta and DVL=Dishevelled; LGR=Leucine-rich repeat-containing G-coupled receptor; RSPO=R-Spondin; ZNRF3/RNF43=E3 Ubiquitin Ligase Zinc and Ring Finger 3/Ring Finger Protein 43; TCF/ LEF=T-Cell Factor/Lymphoid Enhancer factor. Small purple circle=palmitoleic acid; small green circle=phosphorylation; small grey circle=Ubiquitin.
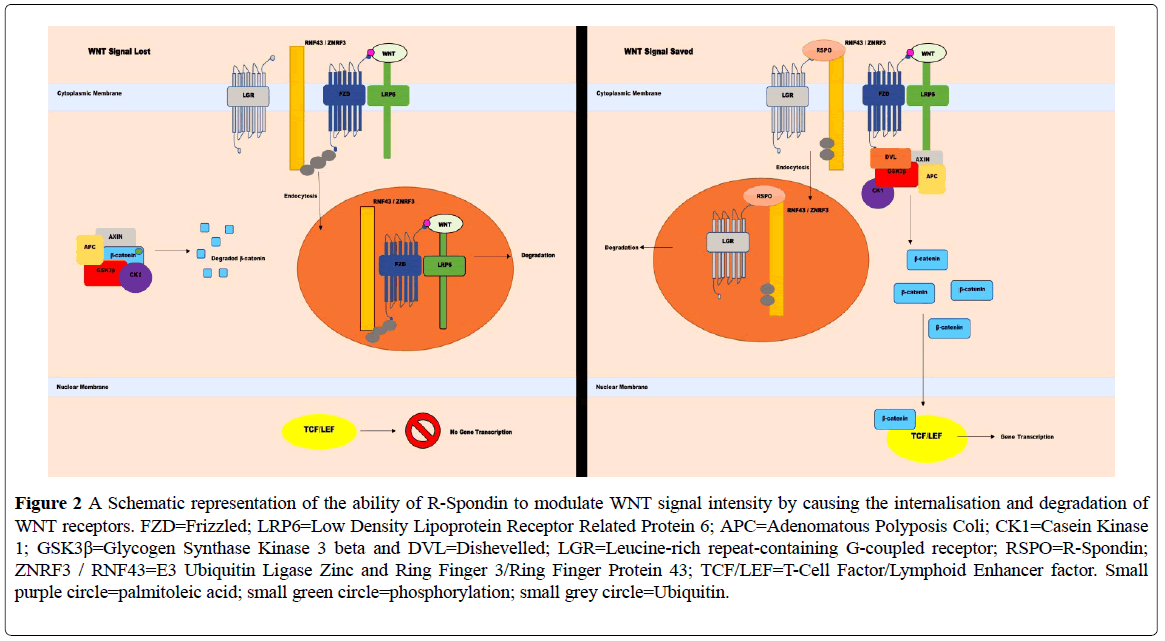
Figure 2: A Schematic representation of the ability of R-Spondin to modulate WNT signal intensity by causing the internalisation and degradation of WNT receptors. FZD=Frizzled; LRP6=Low Density Lipoprotein Receptor Related Protein 6; APC=Adenomatous Polyposis Coli; CK1=Casein Kinase 1; GSK3β=Glycogen Synthase Kinase 3 beta and DVL=Dishevelled; LGR=Leucine-rich repeat-containing G-coupled receptor; RSPO=R-Spondin; ZNRF3 / RNF43=E3 Ubiquitin Ligase Zinc and Ring Finger 3/Ring Finger Protein 43; TCF/LEF=T-Cell Factor/Lymphoid Enhancer factor. Small purple circle=palmitoleic acid; small green circle=phosphorylation; small grey circle=Ubiquitin.
A negative feedback loop is generated by two β-catenin target genes, which produce the trans membrane proteins E3 Ubiquitin Ligase Zinc and Ring Finger 3 (ZNRF3) and Ring Finger Protein 43 (RNF43) [5,6]. Their expression allows binding to Frizzled, promoting both Frizzled and LRP6 ubiquitination, internalisation and degradation and reducing the total number of active WNT receptors, in turn decreasing WNT signal potential [5,6]. This system is countered by the R-spondin proteins (RSPO 1-4) that bind to the Leucine-rich repeat-containing G-coupled receptor (LGR) 4/5 and 6 [5,6]. The binding of these R-spondins to LGR results in the formation of a complex between LGR and ZNRF3/RNF43, causing their internalization and destruction and removing them from the membrane, while leaving Frizzled intact and increasing WNT signalling potential [5,6]. Overexpression of R-spondins or mutations in either RNF43 or ZNRF3 give rise to the potential of up-regulating WNT signal activity [5]. It has been reported that inactivating mutations of both E3 ligases have occurred in cancer, along with both R-spondin 2 and R-spondin 3 gene fusions, in a rare form of APC wild-type colon cancer [6].
Many of the above proteins have been investigated in cancer to determine whether they are valid targets for treatment. Antibodies have been developed to target the ability of WNT ligands to bind to FZD. Vantictumab, also known as OMP-18R5, is one of these monoclonal antibodies. This antibody can bind to multiple FZD receptors by targeting an epitope shared between them [7]. Direct binding of the antibody effectively blocks the ability of WNT ligands from binding to their FZD receptors and stopping the activation of the signalling cascade [7]. OMP-54F28 is a recombinant fusion protein made from the Fc fragment of an IgG antibody combined with the FZD8 receptor and its CRD domain [8]. Its method of action differs from that of Vantictumab, in that instead of targeting FZD receptors directly, it sequesters WNT ligands away from FZD, prevents them from binding to FZD and subsequently activating the pathway [8]. An alternative antibody target is the RSPO3 ligand. OMP-131R10 is an IgG1 antibody that binds to RSPO3 and prevents it from binding to its LGR target receptors [9], blocking the action of RNF43 and Zrnf3 on Frizzled and LRP6 [5,6].
Other interventions upstream of β-catenin that have been employed in treatment strategies include targeting the interactions between Frizzled and Dishevelled or disabling LRP6 phosphorylation. In terms of the former, FJ9 inhibits interactions between Dishevelled and FZD7 [10]. The protein Frizzled binds to Dishevelled by interactions between its carboxy-terminal region and Dishevelled’s PDZ domain [11]; this interaction is disrupted by small molecule inhibitors like FJ9, which inhibit the formation of the FZDDVL complex and in turn stop further signalling, allowing for β-catenin destruction[10]. Alternatively, LRP6 phosphorylation can be stopped by Salinomycin [12] and Niclosamide [13] which are both able to promote the degradation of LRP6, removing a major component required for destruction complex inhibition and in turn promoting β-catenin degradation.
Targeting β-catenin’s interaction with its co-activators and thus modifying β-catenin-controlled gene expression and TCF/LEF has also been investigated. ICG-001 [11,14] and PRI-724 [15] are small molecule inhibitors that block CBP co-activator binding to β-catenin, promoting p300 binding instead. CBP induces the expression of genes that favour proliferation, whereas p300 promotes the expression of genes favouring differentiation [11]. The absence of CBP binding has been noted to increase caspase activity by downregulating survivin gene expression [14], allowing for cancer cell elimination. Cyclin D1 expression is also reported to be downregulated [14].
The proteins Tankyrase and Porcupine (PORCN) also play important roles in WNT signalling. Tankyrase inhibitors play a role in stopping AXIN degradation by inhibiting the activity of Tankyrase enzymes, increasing the concentration of AXIN and allowing for more effective destruction of β- catenin[16]. Alternatively, PORCN inhibitors, such as LGK974 [17] and C59 [18] seek to disrupt the catalytic activity of the enzyme Porcupine [6]. This stops the addition of the acyl group palmitoleic acid to WNT, preventing WNT secretion and removing its Frizzled binding capability [6].
Short interfering (si) RNA is another possible method for modification of the WNT pathway in cancer. Two notable examples are the siRNAs developed to target FZD7 [19] and β-catenin [20] that lead to a reduction in FZD7 and β-catenin expression in colorectal cancer cell lines. FZD7 si- RNA was shown to decrease TCF transcriptional activity and reduce the expression of prominent downstream WNT targets, such as myc and Survivin [19]. These cells also showed a decrease in invasion potential and cell viability [19]. β-catenin si-RNA was found to increase the number of cells undergoing apoptosis and to decrease invasion potential [20], the former event not being noted in colorectal cancer cells treated with FZD7 si-RNA[20].
WNT inhibitors have, in recent years, entered clinical trials. Vantictumab and OMP-54F28 have completed Phase 1 clinical trials, as well as Phase 1 clinical trials in combination therapies (Table 1). PRI-724 has completed Phase 1 clinical trials in solid tumours and acute myeloid leukaemia and it has also completed Phase 2 clinical trials in chronic myeloid leukaemia (Table 1). LGK974 is currently undergoing Phase 1 clinical trials (Table 1). Furthermore, Tankyrase and PORCN inhibitors have been successful in treating ADPKD in mice [21]; ICG-001 has generated positive findings in mouse models of pulmonary fibrosis [22] and PRI-724 has recently completed phase 1 clinical trials in Hepatitis C Virus-related Cirrhosis (ClinicalTrials.gov Identifier: NCT02195440) [23]. This provides hope on the potential therapeutic successes of other WNT inhibitors in the treatment of many hyperproliferative disorders.
| Treatment | Trial Number | Disease | Phase | Completion | Report |
|---|---|---|---|---|---|
| Vantictumab | NCT01345201 | Cancer | 1 | 05/2014 | Grade 1/2: fatigue; vomiting abdominal pain; constipation; diarrhoea and nausea [24]. Grade 3: diarrhoea and vomiting [24]. In the trial one patient had a bone fracture at day 110[24]. |
| NCT01973309 | Cancer | 1 | 12/2017 | Fatigue; constipation; neutropenia; diarrhoea; nausea; abdominal pain [25]. With grade 3 neutropenia; leukopenia and pelvic pain [25]. Early in the study: 2 cases of grade 2 bone fragility fractures [25]. | |
| NCT02005315 | Cancer | 1 | 11/2017 | Nausea; fatigue; dysgeusia; rash and constipation [26]. Grade 3: fatigue and nausea [26]. 2 instances of grade 2 bone fragility fractures early in the trial [26]. | |
| NCT01957007 | Cancer | 1 | 06/2017 | No data reported. | |
| OMP-54F28 | NCT01608867 | Cancer | 1 | 06/2017. | Grade 1/2: anorexia; fatigue; hypocalcaemia; nausea; hypertension; peripheral oedema and vomiting [8]. Grade 3: anaemia[8]. |
| NCT02092363 | Cancer | 1 | 12/2017 | Nausea; fatigue; neutropenia; alopecia; anorexia and vomiting [8]. Grade 3: neutropenia and hypophosphatemia [7]. | |
| NCT02069145 | Cancer | 1 | 07/2017 | No data reported | |
| NCT02050178 | Cancer | 1 | 06/2017 | No data reported | |
| PRI-724 | NCT01302405 | Cancer | 1 | Terminated - 10/15: Low Enrolment. | Grade 2: diarrhoea; elevated bilirubin; hypophosphatemia; nausea; fatigue; anorexia; thrombocytopenia; alkaline phosphatase elevation[15]. Grade 3: Hyperbilirubinemia [15]. |
| NCT01764477 | Cancer | 1 | 10/2015 | Grade 3/4: abdominal pain; neutropenia; anemia; fatigue and elevated alkaline phosphatase [28]. | |
| NCT01606579 | Cancer | 1/2 | 12/2016 | No data reported | |
| NCT02195440 | Hepatitis C Virus-infected Cirrhosis | 1 | 03/2017 | Grade 1/2: Fatigue; Nausea; Vomiting; Headache; pruritus; Constipation; Diarrhoea; Insomnia; Rash; Vertigo; Fever; Thrombocytopenia; Anaemia; leukopenia; hyperglycaemia; hyperbilirubinemia; Alanine and Aspartate Aminotransferase [23]. Grade 3: Nausea; Fever; Bleeding and Alanine and Aspartate Aminotransferase [24]. | |
| LGK974 | NCT01351103 | Cancer | 1 | ongoing | Dysgeusia; decreased appetite; nausea; fatigue; diarrhoea; vomiting; hypercalcemia; alopecia; asthenia; hypomagnesemia [29]. Grade 3: asthenia; fatigue; decreased appetite; enteritis [29]. |
| OMP-131R10 | NCT02482441 | Cancer | 1 | 03/2018 | Nausea; decreased appetite; diarrhoea; vomiting and weight loss [9]. |
Table 1: A table showing clinical trial data for a number of WNT pathway inhibitors.
In a review by Kahn [11], the potential challenges that could arise from modifying the WNT pathway were discussed. As WNT is a key modulator of cellular signalling in many tissues, effects on organ systems such as the intestines, skin and hematopoietic systems, as well as bone loss or breakage may be observed upon WNT signalling inhibition [11]. The observations from WNT signalling treatments typically consisted of grade 1 and 2 adverse effects, including nausea, vomiting, diarrhoea and fatigue (Table 1) [8-9,11,15,23-27,29]. Treatment with Vantictumab has resulted in cases of grade 3 vomiting [24], diarrhea [24], fatigue [26], nausea [26], neutropenia [25], leukopenia [25] and pelvic pain [25]. During trial NCT01345201 there was one instance with a participant who received a bone fracture on day 110 [24]. This bone fracture was accompanied by a significant increase in β- C-terminal telopeptide (β-CTX), acting as a marker of bone degradation [24]. During this trial, two other participants also showed signs of doubling in β-CTX, but this returned to baseline levels after treatment with zoledronic acid [24]. This bone fragility wasn’t unique to this trial, as both NCT01973309 and NCT02005315 trials resulted in participants with grade 2 bone fragility fractures early within their trials, but were able to revise their safety plans eliminating the appearance of further fractures [25,26]. Raised levels of β-CTX were also seen after treatment with another inhibitor, OMP-54F28, which resulted in six participants showing elevated levels of β-CTX, five of which managed to reverse this increase with zoledronic acid [8]. This resulted in another trial for OMP-54F28, NCT02092363, adopting zoledronic acid as a baseline for those with postmenopausal status [27]. OMP-54F28 also showed grade 3 anemia [8] in one trial and grade 3 hypophosphatemia and neutropenia in another [27]. Pri-724 showed one case of dose-limiting reversible grade 3 hyperbilirubinemia, but was mostly met with grade 1 and 2 side effects [15]. In another trial, NCT01764477, showed more grade 3 and 4 adverse effects such as abdominal pain, neutropenia and anemia [28]. In its treatment of Hepatitis C virus-infected cirrhosis, it has been reported to cause grade 3 nausea, fever and bleeding [23]. LGK974 has been reported to cause grade 3 asthenia; fatigue; decreased appetite and enteritis [29]. Outside of clinical trials, Tankyrase inhibitors have been reported to cause intestinal epithelium degeneration and a reduction in intestinal crypt cell proliferation, attributed to their effects in silencing the WNT pathway [16].
Conclusion
Although modifying the WNT signalling pathway shows great promise, it is important to note that studies are still in their early days. It remains unclear whether these therapies will be successful in permanently treating hyper proliferative diseases without causing significant adverse effects, due to WNT signallings diverse role in tissue homeostasis, stem cell maintenance and embryogenesis. Nevertheless, the future is bright and WNT signalling targets may prove of great importance in personalized interventions in diseases of variable manifestation and age ranges.
REFERENCES
- Yang K, Wang X, Zhang H, et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis:Implications in targeted cancer therapies. Laboratory Investigation; a Journal of Technical Methods and Pathology 2016;96:116-36.
- Tan RJ, Zhou D, Zhou L, et al. Wnt/β-catenin signaling and kidney fibrosis. Kidney International Supplements 2014;4:84-90.
- Ten Dam, Bank RA, Werker PMN, et al. Further evidence of the involvement of the WNT signaling pathway in Dupuytren’s disease. Journal of Cell Communication and Signaling 2016;10:33-40.
- Stamos JL, Weis WI. The β-Catenin Destruction Complex. Cold Spring Harbor Perspectives in Biology 2013;5.
- Hao HX, Jiang X, Cong F. Control of WNT Receptor Turnover by R-spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers 2016;8:54.
- Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017;169: 985-99.
- Gurney A, Axelrod F, Bond CJ, et al. WNT pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proceedings of the National Academy of Sciences of the United States of America 2012;109:11717-22.
- Tai D, Wells K, Arcaroli J, et al. Targeting the WNT Signaling Pathway in Cancer Therapeutics. The Oncologist 2015;20:1189-98.
- Bendell J, Strickler J, Kapoun A, et al. Initial results from a phase 1a/b study of OMP-131R10, a first-in-class anti-RSPO3 antibody, in advanced solid tumors and previously treated metastatic colorectal cancer (CRC). European Journal of Cancer 2016;69:S29-S30.
- Fujii N, You L, Xu Z, et al. An Antagonist of Dishevelled Protein-Protein Interaction Suppresses β-Catenin-Dependent Tumor Cell Growth. Cancer Res. 2007;67:573-9.
- Kahn M. Can we safely target the WNT pathway? Nature Reviews. Drug Discovery 2014;13: 513-32.
- Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both WNT/β-catenin and mTORC1 signaling in breast and prostate cancer cells. Journal of Cellular Biochemistry, 2014;115:1799-1807.
- Arend RC, Londoño-Joshi, AI, Samant RS, et al. Inhibition of WNT/β-catenin pathway by niclosamide:a therapeutic target for ovarian cancer. Gynecol Oncol. 2014;134:112-20.
- Emami KH, Nguyen C, Kim DH, et al. A small molecule inhibitor of β-catenin/CREB-binding protein transcription. Proceedings of the National Academy of Sciences of the United States of America 2004;101:12682-7.
- Ning Y, Yang D, Cole S, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31:2501-2501.
- Lau T, Chan E, Callow M, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132-44.
- Liu J, Pan S, Hsieh MH, et al. Targeting WNT-driven cancer through the inhibition of Porcupine by LGK974. Proceedings of the National Academy of Sciences of the United States of America 2013;110:20224-9.
- Proffitt KD, Pendharkar V, Virshup DM, et al. Pharmacological Inhibition of the WNT Acyltransferase PORCN Prevents Growth of WNT-Driven Mammary Cancer. Cancer Res. 2013;73:502-7.
- Ueno K, Hiura M, Suehiro Y, et al. Frizzled-7 as a Potential Therapeutic Target in Colorectal Cancer. Neoplasia 2008;10:697-705.
- Li K, Zhou ZY, Luo HS, et al. Knockdown of β-catenin by siRNA influences proliferation, apoptosis and invasion of the colon cancer cell line SW480. Oncology Letters 2016;11:3896-3900.
- Li A, Xu Y, Fan S, et al. Canonical WNT inhibitors ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI Insight 2018;3.
- Henderson WR, Chi EY, Ye X, et al. Inhibition of WNT/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proceedings of the National Academy of Sciences of the United States of America 2010;107:14309-14.
- Kimura K, Ikoma A, Shibakawa M, et al. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/β-Catenin Inhibitor, in Patients with Hepatitis C Virus-related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMedicine 2017;23:79-87.
- Smith DC, Rosen LS, Chugh R, et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. J Clin Oncol. 2013;31:2540.
- Mita MM, Becerra C, Richards DA, et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with paclitaxel (P) in patients (pts) with 1st to 3rd-line metastatic HER2-negative breast cancer (BC). Journal of Clinical Oncology 2016;34:2516.
- Messersmith W, Cohen S, Shahda S, et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with nab-paclitaxel (Nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (PC). Annals of Oncology 2016;27:677.
- O’Cearbhaill RE, McMeekin DS, Mantia-Smaldone G, et al. Phase 1b of WNT inhibitor ipafricept (IPA, decoy receptor for WNT ligands) with carboplatin (C) and paclitaxel (P) in recurrent platinum-sensitive ovarian cancer (OC). Journal of Clinical Oncology 2016;34:2515.
- Ko AH, Chiorean EG, Kwak EL, et al. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol. 2016;34:e15721.
- Janku F, Connolly R, LoRusso P, et al. Abstract C45: Phase I study of WNT974, a first-in-class Porcupine inhibitor, in advanced solid tumors. Molecular Cancer Therapeutics 2015;14.