Primer of vein of galen malformation management
Received: 12-Apr-2022, Manuscript No. puljphcm-22-4752; Editor assigned: 18-Apr-2022, Pre QC No. puljphcm-22-4752 (PQ); Accepted Date: May 16, 2022; Reviewed: 27-Apr-2022 QC No. puljphcm-22-4752 (Q); Revised: 12-May-2022, Manuscript No. puljphcm-22-4752 (R); Published: 25-May-2022, DOI: 10.37532/puljphcm.22.5 (3).30-34
Citation: Reddy R, Lucke-Wold B. Primer of Vein of Galen Malformation Management. J Pedia Health Care Med. 2022; 5(3):30-34.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Vein of Galen Malformations offer a unique clinical challenge that can present as heart failure and hydrocephalus. The mainstay of treatment is embolization and management of systemic issues. Recently, radiation treatment has also offered some benefit. Herein we review the literature and present common findings, pathophysiology, and management strategies. This user-friendly guide can help clinicians and researchers tackle this important topic.
Keywords
Vein of Galen Malformation; Treatment; Heart failure; Management strategies
Introduction
Vein of Galen Malformations (VGAM) are pediatric congenital anomalies, identified by multiple arteriovenous shunts that drain into a dilated median cerebral venous collector, namely the median prosencephalic vein of Markowski (PVM). The PVM, whose misnomer is the vein of Galen, is a persistent embryonic channel that is usually absent at the adult stage, first recognized by Raybaud and Strother [1]. The first reported case of VGAM was published in 1937 [2]. While the true incidence is unknown, it is estimated to be 1 in every 10,000 to 25,000 births [3]. VGAMs form 1% of all pediatric congenital anomalies and 30% of pediatric congenital vascular malformations [4]. Thus far, VGAMs seem to occur equally likely within males and females [5].
It is important to understand the embryological origin of VGAMs for proper therapeutic intervention. In the choroidal stage of development, choroid plexuses form and supply blood to the brain, primarily via multiple choroidal arteries, while venous drainage is principally through the median PVM. As the cortical arterial network matures, the choroidal arteries slowly lose their role in vascularization while the internal cerebral veins take over venous drainage. The internal cerebral veins terminate in the posterior aspect of the vein of Markowski, which then typically involutes. Only the posterior aspect persists, which is in turn named the vein of Galen [6]. In a VGAM, the anterior portion of the vein of Markowski persists due to the high-pressure from the feeding choroidal arteries. Thus, involution is prevented and the PVM forms the aneurysmal component that is typical in a VGAM [6].
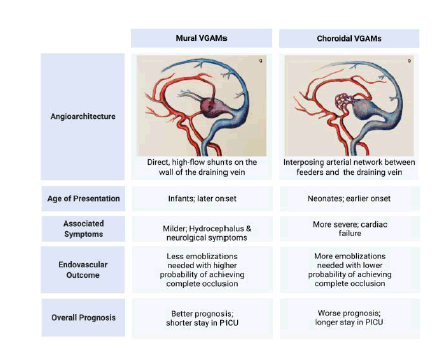
VGAMs are anatomically classified into mural or choroidal types, which is critical for determining clinical manifestation and prognosis (Figure 1) [7]. The mural type is characterized by direct, high-flow shunts on the wall of the draining PVM [8]. However, the choroidal type is characterized by multiple fistulas with interposing arterial networks between the feeding vessels and the draining vein [8]. In general, the choroidal type presents with high-output cardiac failure within a few days after birth while the mural type manifests later in childhood with hydrocephalus and neurologic symptoms [5]. The choroidal type also tends to be more difficult to treat, which will be elucidated in more detail later.
Since the choroidal arteries are the predominant artery suppliers in VGAMs, VGAMs are thought to develop within 6-11 weeks of gestation (aligning with the choroidal embryonic stage) [8-10].
However, VGAMs can be diagnosed anywhere from prenatal time points until later in childhood [5]. VGAMs are prenatally detected about 29% of the time, usually during late second or third trimester [9]. Other clinical presentations may include high-output congestive heart failure (which may lead to hydrops in severe cases), developmental delay, hydrocephalus, or seizures [11, 12]. One retrospective study of 21 cases showed cardiomegaly (64%), enlarged neck vessels (32%), ventriculomegaly (24%), and polyhydramnios (16%) [13, 14]. Cerebral ischemic changes may result in porencephaly, and intracranial hemorrhage can occur, although rarely {9}.
Prognosis is largely dependent on cardiopulmonary and neurological status. As will be explained in later sections, this is associated with age of presentation as well [15]. For example, if VGAM manifests with antenatal cardiac failure (with hydrops) or cerebral damage results in irreversible multiorgan failure, mortality rates are quite high [4, 11, 12]. Antenatal outcomes, based on a retrospective study looking at 21 cases, ranged from termination of pregnancy (42.9%) to neonatal death (28.6%). 28.6% of cases resulted in live births, with long-term outcomes of abnormal neurologic outcomes [16, 17]. While the advent of endovascular treatment has greatly improved prognosis (as will be elaborated later), long term outcomes still appear to be less favorable than short term follow-ups may describe, as patients with “good” outcomes based on angiographic parameters still show signs of minor neuropsychological disorders [18]. One rare occurrence is the spontaneous thrombosis of VGAMs. Case reports dictate that this is usually found upon serial imaging and results in better prognosis with uneventful recovery typically [19-21].
Medical management
A typical differential diagnosis associated with VGAM may include midline intracranial cystic masses (e.g.: arachnoid cysts, choroid plexus cysts, pineal tumors, choroid papilloma, etc.). Another common anatomic variant is cavum vergae, marked by posterior extension of the normal cave of the septum pellucidum. It would look like a posterior midline cyst on an axial section [22]. However, these diagnoses would not show any blood flow when using color Doppler, while VGAM will have blood flow associated with it [9]. Prenatal detection typically relies on prenatal ultrasonography, which shows a midline anechoic mass superior to thalami, often called a “comet tail” or “keyhole” sign [13, 14]. Color Doppler is the main way to distinguish from other differential diagnoses, which will show turbulent blood flow (with or without associated signs of cardiac failure). Fetal echocardiography is suggested to monitor cardiac function and blood flow as well [9]. While 3D ultrasound is comparable to MRI to see further details of position and the feeder vessels in the prenatal setting, MRI is recommended postnatally to confirm and rule out other anomalies [23]. MRI can provide details regarding the VGAM’s configuration, but also help see any secondary findings such as ventricular compression or cerebral atrophy [9, 24]. This information is vital for prognosis evaluation and therapeutic decision making [8]. CT imaging can also help identify secondary findings (ventricular enlargement, brain atrophy, etc.) while CT angiography can provide a more detailed view of vasculature compared to MRI [25].
Once the diagnosis has been confirmed, interventions can be categorized as medical management/non-surgical options and procedural options (which include endovascular treatment or surgical intervention). In general, surgery is avoided and reserved for situations, such as intracranial hemorrhage or hydrocephalus [8, 26]. Otherwise, multidisciplinary treatment/management is advised in conjunction with endovascular treatment.
Medical management largely depends on when the VGAM was detected/presented. If prenatally detected, monitoring is critical if there is any evidence of hemodynamic decompensation and/or hydrops [24]. Symptomatic presentation in neonates is associated with congestive heart failure, because of left to right shunting due to the low resistance vasculature of the VGAM [27-29]. Infants may present with cyanosis from shunting across the Patent Ductus Arteriosus (PDA) and atrial septum, due to volume and pressure overload on the right ventricle [8]. They may also present with encephalomalacia, seizures, and multiorgan dysfunction due to poor perfusion [8]. Thus, echocardiography is vital for monitoring cardiac function [28, 29]. Otherwise, the overall goal is to stabilize patients medically until endovascular intervention can be performed (see next section). For patients with acyanotic heart failure, medications can include diuretics and/or inotrope therapy. However, if patients also have pulmonary hypertension, therapies may include beta agonists, phosphodiesterase inhibitors, digoxin, and prostaglandin infusions [8]. Lastly, older infants and children may present with hydrocephalus, developmental delay, seizures, and/or dilated facial veins. When present, cardiac failure tends to be easier to treat and without associated pulmonary hypertension [8]. As mentioned previously, surgical options are only considered in certain cases, such as severe hydrocephalus or intracranial hemorrhage.
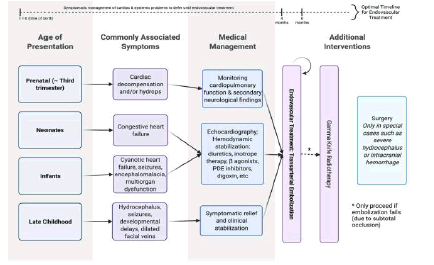
To summarize, intervention should include a combination of endovascular treatment (see next section) and interdisciplinary medical management as detailed above, with surgical intervention being the last resort for specialized cases (Figure 2). An interdisciplinary care team for VGAMs should include pediatric neurosurgery, cardiology, neonatology, and maternal-fetal medicine, with continual monitoring of cardiac function, hydrops, and/or hydrocephalus [9]. Parents of affected patients should be counseled and helped to secure access to a tertiary care facility with access to a neonatal intensive care unit. Moreover, while general genetic counseling should be offered to all patients, no specific chromosomal abnormality is associated with VGAM [9].
Figure 2: Outline of treatment algorithm for VGAMs. This figure outlines the general principle of treating VGAMS – namely, that embolization is the first line treatment. Since the optimal time frame is 4-6 months of age, medical management mostly centers around symptomatic management and/or stabilization of cardiac and systemic symptoms. Clinical manifestation is affected by age of presentation and anatomical categorization. Anatomical categorization (namely, a mural or choroidal VGAM) also greatly affects prognosis; please see Figure 1 for more information.
Endovascular treatment
The advent of endovascular treatment in the 1990s greatly improved the prognosis of VGAMs, which was responsible for moving away from surgical management of VGAMs [5]. Microsurgical clipping used to focus on flow reduction instead of total fistula occlusion, but this led to poor outcomes, hypothesized to be due to revascularization [5]. Thus, for VGAMs in specific, embolization is considered the first line of treatment, with the goal of achieving complete occlusion. Trans-arterial embolization is preferred over transvenous, where access is achieved preferably via the femoral puncture or the umbilical artery [8, 15, 30, 31]. Embolization is usually done between 4 and 6 months of age to optimally balance the efficacy of embolization vs the risk of cerebral impairment [32-33]. Thus, the goal of medical management is often to stabilize the patient’s cardiac and systemic symptoms to deter to this period for optimal embolization. Furthermore, it is important to keep in mind that embolization often requires multiple successive procedures, as complete occlusion may not be achieved initially [8]. In a retrospective study across 4 decades in one institution, mural VGAMs had an average of 1.4 interventions vs a choroidal average of 2.6 interventions [5].
Moreover, while only 12.5% of mural VGAMs presented within the first month of life, 70% of choroidal VGAMs presented within the first month. Complete embolization was also achieved more often in mural VGAMs (87.5%) vs choroidal VGAMs (11.1%) [5].Moreover, gamma knife radiotherapy has recently been shown to reduce VGAM size and many feeders when used as a second line therapy after ineffective embolization [5]. However, radiosurgery is not recommended as a first line treatment since it requires time for induced endothelial cell proliferation to lead to luminal closure. Thus, it cannot provide immediate hemodynamic relief [5].
Intraoperative concerns depend upon the age of presentation, which determines symptoms and prognosis as mentioned earlier. In general, intraoperative management tends to focus on preventing further deterioration of neurological status, which is primarily done by avoiding rises in Intracranial Pressure (ICP), maintaining normal Cerebral Perfusion Pressure (CPP), and cerebral oxygenation. These factors affect the titration of anesthetics [34].
To better understand why, it is important to consider other congenital conditions and/or symptoms. Many neonates present with cardiac related symptoms, while infants and older children may present with increased head circumference, neurological deficits, and seizures [34]. Examples of congenital heart pathologies associated with VGAM include ASD, VSD, and PDA. These patients are usually treated with sildenafil and milrinone to avoid increasing peripheral resistance while increasing cardiac output, but this may result in cardiac insult due to decreased diastolic pressure (arising from blood flow via the VGAM), leading to possible myocardial ischemia [34]. Furthermore, traditional ketamine induction for coronary heart disease patients is avoided due to the risk of increasing ICP. As such, titrated inhalation and opioid induction is recommended.
Other intraoperative considerations include the effects of macrocephaly. The presence of a larger head compared to normal subjects of similar age can result in an anticipated difficult airway. This makes intraprocedural positioning very important, especially to avoid kinking neck vessels (a common complication noted in the introduction section) [34]. Lastly, a higher PaO2 than normal may be required to ensure proper brain oxygenation, due to the propensity for malnourishment and anemia. This may result from a failure to thrive, compounded by poor feed tolerance (from cardiac failure) and hydrocephalus-related nausea and vomiting.
Post-operative complications can span a wide spectrum. Commonly expected complications include the development of new focal neurological deficits, worsening hydrocephalus, seizures, precipitation of congestive heart failure, and more [34]. Several of these symptoms occur due to the sudden shift in intracranial circulatory dynamics after embolization, potentially resulting in cerebral hyper perfusion, edema, venous infarcts, intracranial hemorrhage, cerebral venous thrombosis, and worsening hydrocephalus [34]. Technical complications can also arise, although they are rare in comparison to previously mentioned complications. For example, instances of separation of the distal catheter and the embolization of a single platinum coil to the lung have been noted (both did not result in symptoms during the 101 month follow up) [34]. Other operative complications can include severe effects resulting from glue progression into the superior sagittal sinus, cardiac failure, and/or intraventricular hemorrhage. Milder complications include glue granulomas (treated with steroids and antibiotics) and transverse sinus thrombosis (treated with anticoagulants). In summary, technical complications tend to be more common in neonates vs infants, most probably due to the smaller size and increased fragility of vasculature [15]. Moreover, treatment during the neonatal period generally results in poorer neurological outcomes, most probably correlating to the increased severity of disease and cardiological symptoms [15].
Post-operative management should include monitoring of hemodynamic functionality (can be done using fine Doppler analysis), cardiopulmonary symptoms, and any neurological deficits in a pediatric intensive care unit (with choroidal VGAMs generally requiring longer care than mural VGAMs) [5, 8]. Arteriography can also be done, typically 11 to 24 months post-embolization [34].
Conclusion
VGAMs form between 6-11 weeks of gestation, resulting from one or more arteriovenous fistulas that shunt blood from choroidal arteries to the PVM, an embryonic precursor of the vein of Galen that persists and prevents involution. VGAMs can be detected prenatally (usually during the third trimester) or present until late childhood, with varying symptoms associated with the age of manifestation. The most common symptoms/presentations include congestive heart failure or hemodynamic compensation, hydrops, hydrocephalus, neurological deficits, seizures, and/or dilated facial veins. The ideal management of VGAMs should be multidisciplinary management of cardiac and systemic symptoms in conjunction with endovascular treatment, particularly transarterial embolization, most optimally around 6 months of age. Other intervention options include gamma knife radiotherapy as a second line treatment or surgery, as a last resort and only in specialized cases. Although endovascular treatment has certainly improved the prognosis of VGAMs, there is more work to be done as long-term outcomes are not optimal. Even in patients deemed to have “good” outcomes from an angiographic perspective, minor neurocognitive impairments may be observed (e.g.: forgetfulness, poor memory and/or concentration, etc.). When thinking about long-term management of VGAMs, the focus should not only be an angiographic cure, but also on restoring/maintaining neurocognitive and clinical parameters. Further research and improvement are needed in this regard.
REFERENCES
- Raybaud CA, Strother CM. Persisting abnormal embryonic vessels in intracranial arteriovenous malformations. Acta Radiol Suppl. 1986; 369:136–8.
Google Scholar Cross Ref - Jaeger JR, Forbes RP, Dandy WE. Bilateral congenital cerebral arteriovenous communication aneurysm. Trans Am Neurol Assoc. 1937; 63:173–6.
Google Scholar Cross Ref - Pooh RK, Degani S. Fetal cerebral circulation. In: Timor-Tritsch I.E. Monteagudo A. Pilu G. Malinger G. Ultrasonography of the prenatal brain. 3rd ed. The McGraw-Hill Company, New York, NY2012: 428-46
Google Scholar Cross Ref - Recinos PF, Rahmathulla G, Pearl M, et al. Vein of Galen malformations: epidemiology, clinical presentations, management. Neurosurg Clin N Am. 2012; 23(1):165-77.
Google Scholar Cross Ref - Hosmann A, El-Garci A, Gatterbauer B, et al. Multimodality Management of Vein of Galen Malformations-An Institutional Experience. World Neurosurg. 2018; 112:e837-e847.
Google Scholar Cross Ref - Lasjaunias PL, Alvarez H, Rodesch G, Garcia-Monaco R, Ter Brugge K, Burrows P, et al. Aneurysmal malformations of the vein of Galen. Follow-up of 120 children treated between 1984 and 1994. Interv Neuroradiol. 1996; 2:15–26.
Google Scholar Cross Ref - Gailloud P, O'Riordan DP, Burger I, et al. Diagnosis and management of vein of galen aneurysmal malformations. J Perinatol. 2005; 25(8):542-51.
Google Scholar Cross Ref - Monteagudo A. Vein of Galen Aneurysmal Malformation. Am J Obstet Gynecol. 2020; 223(6):B27-B29.
Google Scholar Cross Ref - Michaels AY, Sood S, Frates MC. Vein of Galen Aneurysmal Malformation. Ultrasound Q. 2016; 32(4):366-9.
Google Scholar Cross Ref - D'Amico A, Tinari S, D'Antonio F, et al. Outcome of fetal Vein Galen aneurysmal malformations: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021; 22: 1-6.
Google Scholar Cross Ref - Rodesch G, Hui F, Alvarez H, et al. Prognosis of antenatally diagnosed vein of Galen aneurysmal malformations. Childs Nerv Syst. 1994; 10(2):79-83.
Google Scholar Cross Ref - Sepulveda W, Platt CC, Fisk NM. Prenatal diagnosis of cerebral arteriovenous malformation using color Doppler ultrasonography: case report and review of the literature. Ultrasound Obstet Gynecol. 1995;6(4):282-6.
Google Scholar Cross Ref - Sepulveda W, Vanderheyden T, Pather J, et al. Vein of galen malformation: prenatal evaluation with three-dimensional power Doppler angiography. J Ultrasound Med. 2003; 22(12):1395-8.
Google Scholar Cross Ref - Brinjikji W, Krings T, Murad MH, et al. Endovascular Treatment of Vein of Galen Malformations: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2017; 38(12):2308-14.
Google Scholar Cross Ref - Recinos PF, Rahmathulla G, Pearl M, et al. Vein of Galen malformations: epidemiology, clinical presentations, management. Neurosurg Clin N Am. 2012; 23(1):165-77.
Google Scholar Cross Ref - Deloison B, Chalouhi GE, Sonigo P, et al. Hidden mortality of prenatally diagnosed vein of Galen aneurysmal malformation: retrospective study and review of the literature. Ultrasound Obstet Gynecol. 2012;40(6):652-58.
Google Scholar Cross Ref - Taffin H, Maurey H, Ozanne A, et al. Long-term outcome of vein of Galen malformation. Dev Med Child Neurol. 2020;62(6):729-34.
Google Scholar Cross Ref - Mohanty CB, Srinivas D, Sampath S. Spontaneous thrombosis of a vein of galen malformation. Asian J Neurosurg. 2016; 11(1):69.
Google Scholar Cross Ref - Pulido LF, Murcia Salazar D, Gómez Amarillo D, et al. Spontaneous thrombosis of a vein of Galen malformation associated with acute sinusitis: a case report. Childs Nerv Syst. 2021;37(10):3271-36.
Google Scholar Cross Ref - Kariyappa KD, Krishnaswami M, Gnanaprakasam F, et al. Spontaneous thrombosis of vein of Galen malformation. J Pediatr Neurosci. 2016; 11(3):264-6.
Google Scholar Cross Ref - Tubbs RS, Krishnamurthy S, Verma K, et al. Cavum velum interpositum, cavum septum pellucidum, and cavum vergae: a review. Childs Nerv Syst. 2011; 27(11):1927-30.
Google Scholar Cross Ref - Paladini D, Deloison B, Rossi A, et al. Vein of Galen aneurysmal malformation (VGAM) in the fetus: retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet Gynecol. 2017; 50(2):192-9.
Google Scholar Cross Ref - Brunelle F. Brain vascular malformations in the fetus: diagnosis and prognosis. Childs Nerv Syst 2003; 19:524–8.
Google Scholar Cross Ref - Alberico RA, Barnes P, Robertson RL, et al. Helical CT angiography: dynamic cerebrovascular imaging in children. AJNR Am J Neuroradiol 1999; 20:328–34.
Google Scholar Cross Ref - Zerah M, Garcia-Monaco R, Rodesch G, et al. Hydrodynamics in vein of Galen malformations. Childs Nerv Syst 1992; 8:111–7.
Google Scholar Cross Ref - Malarbi S, Gunn-Charlton JK, Burnett AC, et al. Outcome of vein of Galen malformation presenting in the neonatal period. Arch Dis Child. 2019; 104(11):1064-9.
Google Scholar Cross Ref - Frawley GP, Dargaville PA, Mitchell PJ, et al. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Arch Dis Child Fetal Neonatal Ed 2002;87:F144–F149
Google Scholar Cross Ref - Chevret L, Durand P, Alvarez H, et al. Severe cardiac failure in newborns with VGAM. Prognosis significance of hemodynamic parameters in neonates presenting with severe heart failure owing to vein of Galen arteriovenous malformation. Intensive Care Med 2002; 28:1126–30.
Google Scholar Cross Ref - Sivasankar R, Limaye US, Wuppalapati S, et al. Endovascular Management of Vein of Galen Aneurysmal Malformations: A Retrospective Analysis over a 15-Year Period. J Vasc Interv Neurol. 2019; 10(3):23-9.
Google Scholar Cross Ref - Khullar D, Andeejani AM, Bulsara KR. Evolution of treatment options for vein of Galen malformations. J Neurosurg Pediatr. 2010; 6(5):444-51.
Google Scholar Cross Ref - Hrishi AP, Lionel KR. Periprocedural Management of Vein of Galen Aneurysmal Malformation Patients: An 11-Year Experience. Anesth Essays Res. 2017; 11(3):630-5.
Google Scholar Cross Ref - Chevret L, Durand P, Alvarez H, et al. Severe cardiac failure in newborns with VGAM. Prognosis significance of hemodynamic parameters in neonates presenting with severe heart failure owing to vein of Galen arteriovenous malformation. Intensive Care Med. 2002; 28(8):1126-30.
Google Scholar Cross Ref - Kumar MA. Red blood cell transfusion in the neurological ICU. Neurotherapeutics. 2012; 9(1):56-64.
Google Scholar Cross Ref - Halbach VV, Dowd CF, Higashida RT, et al. Endovascular treatment of mural-type vein of Galen malformations. J Neurosurg. 1998; 89(1):74-80.
Google Scholar Cross Ref