Prospective single-center study of the Venclose radiofrequency ablation system for saphenous vein incompetence
2 Specialist in Dermatology, Phlebology, Vein Center at Brühl, Leipzig, Germany
3 Distinguished Fellow, Medical- Affairs, Becton, Dickinson and Company, Colorado Springs, Colorado, United States
Received: 29-Mar-2023, Manuscript No. puljpl-23-6290; Editor assigned: 31-Mar-2023, Pre QC No. puljpl-23-6290 (PQ); Accepted Date: Apr 04, 2023; Reviewed: 02-Apr-2023 QC No. puljpl-23-6290(Q); Revised: 03-Apr-2023, Manuscript No. puljpl-23-6290 (R); Published: 05-Apr-2023, DOI: 10.37532/ puljpl.2023.16(1).01-07
Citation: Mattausch T, Wenzel H-C, Settlage R. Prospective single-center study of the Venclose radiofrequency ablation system for saphenous vein incompetence. J Phlebol Lymphology 2023;16(1):1-4
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: To evaluate the clinical performance of the Venclose radiofrequency system for thermal ablation of incompetent great saphenous veins.
MATERIALS AND METHODS: Twenty-five patients were enrolled and treated at the Vein Center at Brühl, Leipzig, Germany in a prospective exploratory study. Primary outcome measures were the percentage of limbs with vein occlusion without reflux in the treated vein and the number and type of adverse events through 12 months.
RESULTS: Four of 25 patients received bilateral great saphenous vein treatments (29 treated limbs). All patients completed the study through 12 months. Twenty-eight great saphenous veins (97%) were ablated and successfully occluded with no reflux while one vein developed segmental recanalization starting at the 3-month follow up. Six adverse events were reported through 12 months; two were determined by the investigator as probably related to the device or procedure. All adverse events were reported as resolved by 6 months.
CONCLUSION: In this exploratory study, use of the Venclose radiofrequency ablation system achieved vein occlusion without reflux in all but one vein with six adverse events treated and resolved.
Key Words
Thermal ablation; Radiofrequency ablation; Saphenous vein incompetence; Chronic vein insufficiency
Introduction
Endovascular Radiofrequency (RF) ablation has become a common treatment for chronic Great Saphenous Vein (GSV) insufficiency and resultant varicose veins [1]. RF ablation was developed to reduce the morbidity and complications associated with surgical vein stripping such as anesthesia associated risks, post-procedural pain, scarring at the incision sites, saphenous nerve injury, and deep vein thrombosis [1-4]. RF ablation reduces the time back to activities of daily living and has been recommended in the recently published 2023 Clinical Practice Guidelines “for patients with symptomatic varicose veins and axial reflux in the great saphenous vein, who are candidates for intervention…” [1]. We examined a newer iteration endovascular RF ablation system designed with two heating lengths (i.e., 2.5 and 10 cm) to optimize treatment with one smaller-profile (6 F) catheter. The current exploratory study was designed to observe vein occlusion and the number and type of Adverse Events (AEs) after treatment of GSV insufficiency with the device.
Materials and Methods
Study design
Twenty-five patients were treated in the prospective, single-center, exploratory study of a RF system used for segmental thermal ablation of incompetent GSVs. Patients were treated at the Vein Center at Brühl, Leipzig, Germany between May and July 2020 under a protocol approved by the institution’s ethics committee. Patients provided written informed consent prior to participation in the study; procedures were conducted in accordance with the Declaration of Helsinki, good clinical practices, and applicable healthcare laws. Data were collected by the investigators using standardized clinical report forms. The study, sponsored by Venclose, Inc., was intended to provide clinical outcomes and evidence of safe use for conformity with the Essential Requirements of the European Union Medical Device Directives. The study was registered on clinicaltrials.gov [NCT04236245] prior to first patient enrollment.
Study eligibility and study device
Patient inclusion and exclusion criteria are detailed in Table 1. Eligible patients had significant GSV reflux by duplex ultrasound (DUS) that could be treated endovascularly. Significant reflux was defined as reverse flow with a reflux duration greater than 0.5 seconds after the Valsalva maneuver or distal augmentation while the patient was standing or in reverse Trendelenburg position. Patients were excluded from study participation if there was evidence of thrombus in the diseased vein segment and, in the judgement of the investigator, the patient was at risk if heat energy was delivered to the incompetent vein
TABLE 1. Inclusion and exclusion criteria
| Inclusion Criteria |
|
|---|---|
|
|
|
|
|
|
|
|
|
|
| Exclusion Criteria |
|
|
|
|
|
|
|
|
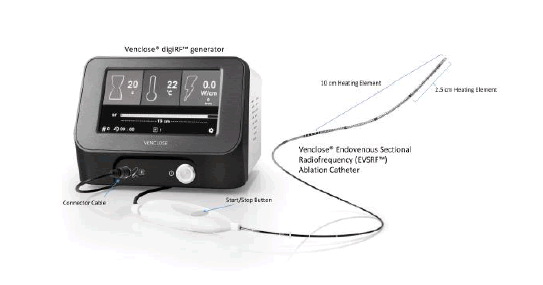
Patients were treated with the Venclose Endovenous Sectional Radiofrequency Ablation System (Tempe, Arizona, USA), composed of a disposable 6 F RF ablation catheter with both 2.5 cm and 10 cm heating elements. The catheter was attached to a generator that completed automated 20-second-long treatment cycles generating resistive RF ablation via bipolar energy to heat (120°C) the wall of an incompetent vein and ultimately occlude the vessel (Figure 1).
Figure 1: The Venclose Endovenous Sectional Radiofrequency Ablation System (Venclose/Becton, Dickinson and Company, USA) is a two-piece system consisting of a of Radiofrequency generator and a 6 F ablation catheter with two heating element lengths (2.5 cm and 10 cm) to thermally ablate a superficial refluxing vein
Outcome measures
Primary outcome measures were the percentage of limbs with vein occlusion with an absence of reflux in the treated vein and the number and type of AEs. Recanalization and reflux rates were measured by DUS and clinical assessment at 3 days and 3, 6, and 12-months post-procedure. The rate of limbs with vein occlusion was defined as the absence of blood flow from 3 cm inferior to the Saphenofemoral Junction (SFJ) along the entire length of the treated vein documented with DUS. Flow in the stump of the GSV up to 3 cm below the SFJ was considered normal and did not constitute a failure [5,6]. The reflux-free rate was also measured with DUS and was defined as no reflux in the treated vein segment (i.e., reverse flow >0.5 s with the patient standing). Secondary clinical outcomes were evaluated using the Clinical Etiologic Anatomic Pathophysiologic (CEAP) clinical classification, the Venous Clinical Severity Score (VCSS), and quality of life was measured with the Chronic Venous Insufficiency Quality of Life Questionnaire (CIVIQ-20) [7]. Measurements were recorded at baseline and 3, 6, and 12 months.
Study procedures and follow up
Patients received a comprehensive examination by the investigator. Demographics data, medical history (e.g., previous venous interventions, risk factors, comorbidities, and concomitant conditions), surgical clearance, and a detailed venous DUS examination were performed prior to treatment. If the patient met the study eligibility criteria, the investigator explained the benefits and risks of study participation, and the patient was enrolled after providing voluntary written informed consent. The patient then underwent a minimally invasive procedure with the Venclose system to deliver controlled RF energy to ablate the GSV with adjunctive sclerotherapy and/or miniphlebectomy performed as needed. The ablation procedure was conducted in accordance with the device instructions for use and all procedures were performed by investigators trained and experienced with the RF system. Procedure details and peri-procedural events were recorded immediately following the procedure. After the ablation procedure, patients were treated per the institution standard of care which included post-operative compression, deep-venous thrombosis prophylaxis (e.g., the patient was instructed to ambulate frequently for several days after treatment), a recommendation that the patient refrain from strenuous activities for several days, and non-steroidal anti-inflammatory drugs as needed. Patients were evaluated at 3 days where bandages were removed, wounds were evaluated, DUS was performed to assess the deep venous system, and the index GSV was assessed for closure. At 3 months, 6 months, and 12-months post-procedure the patient received a DUS examination of the treated limb in a standing position to assess occlusion; completed CEAP, VCSS, and CIVIQ-20 evaluations; and AEs were assessed.
Statistical analysis
The study was designed as exploratory with a sample size based on the potential adequacy of data to meet the study objectives. The analysis population consisted of all patients and limbs treated with the study device. There were no statistically-tested hypotheses; data were observational and summarized using descriptive statistics including frequency counts and percentages for categorical variables and means and standard deviations for continuous variables. AEs were described and reported through 12 months
Result
Baseline patient characteristics and procedure details
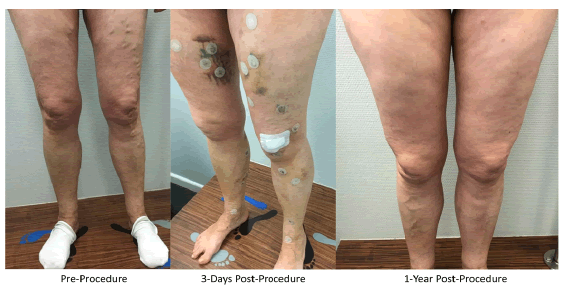
Twenty-five patients were consented and enrolled; four patients had bilateral limb treatments resulting in 29 treated limbs. Patients were on average 54 years ± 10.8 years old, 80% were female, and 100% were White. Risk factors included hypertension (40%), past or present smoking (36%), and obesity (16%). Procedure details are summarized in Table 2. Patients had pronounced varicose veins. The mean maximum GSV diameter at baseline, measured in the standing position, was 9.2 mm ± 2.4 mm while on the table at the time of the procedure (i.e., supine position), the vein diameter at the SFJ was 7.3 mm ± 2.9 mm. The Venclose catheter 10 cm heating element was used in all cases. The mean procedure duration was 23 minutes ± 4 minutes with a total treatment time of just over two minutes (127 seconds ± 18 seconds). The mean total length of vein treated was 53 cm ± 9 cm. Adjunctive procedures performed at the time of the index procedure included sclerotherapy (100%) and phlebectomy (93%). An example of a typical case in shown in Figure 2a-2c.
TABLE 2. Procedure details
| Procedure Characteristics | Treated Limbs (N=29)* |
|---|---|
| Mean Maximum GSV Diameter at Baseline**, mm ± SD | 9.2 ± 2.4 |
| Mean Vein Diameter at the SFJ***, mm ± | 7.3 ± 2.9 |
| SD Mean Maximum Vein Diameter in Treatment Zone ***, mm ± SD | 4.7 ± 2.0 |
| Number of Refluxing Perforating Veins in Treatment Zone | 0 |
| Vessel Shape of the SFJ (N=28) | |
| Bulbous | 6 (21%) |
| Cylinder | 16 (57%) |
| Funnel | 6 (21%) |
| Mean Procedure Time, min ± SD | ± 4 |
| Mean Treatment Time, sec ± SD | 127 ± 18 |
| Heating Lengths Applied | |
| 2.5 cm | 0 (0%) |
| 10 cm | 25 (100%) |
| Mean Treatment Length, cm ± SD | 53 ± 9 |
| Adjunctive Procedures Performed | |
| Phlebectomy | 27 (93%) |
| Sclerotherapy | 29 (100%) |
| RF Ablation Catheter Used to Treat AASV | 1 (3%) |
GSV=Great Saphenous Vein; SFJ=Saphenofemoral junction; RF= Radiofrequency; AASV = Anterior Accessory Saphenous Vein *29 limbs treated in 25 patients; **Measured in the standing position at baseline; ***Measured in the supine position at the time of the procedure
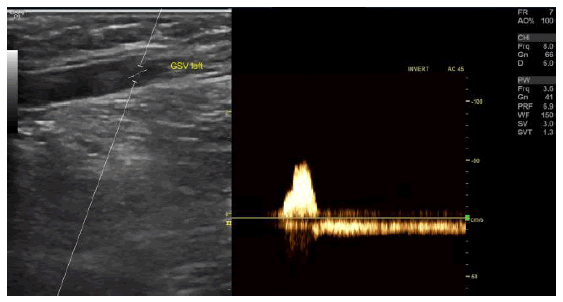
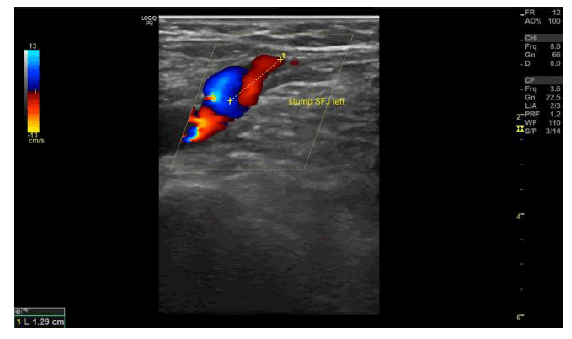
Figure 2a): Case photographs of a 61-year-old woman with a family history of varicose veins who presented with varicosis, pain, and swelling in her legs especially after prolonged standing and sitting. Duplex ultrasonography pre-procedure revealed bilateral great saphenous vein trunk insufficiency (Hach III) and reflux but no evidence of deep vein outflow disease or hemodynamically significant arteriosclerosis.
Figure 2b): Duplex ultrasound image of left Great Saphenous Vein (GSV) reflux preprocedure. The GSV in both legs was ablated with the Venclose radiofrequency ablation system (120ÃÃÂ??°C-20 second intervals) along with concomitant mini-phlebectomy and sclerotherapy of lateral branch varicose veins. After treatment, the patient experienced little pain, but had a local paresthesia in the right lower leg due to the phlebectomy (resolved by the 3-month follow-up visit). One-week post-procedure the patient could perform normal activities, returned to work, and her leg pain and swelling were resolved
Post-procedure follow-up and primary outcomes
Primary outcomes are summarized in Table 3. All 25 patients (100%) completed the study. The combined primary outcome measure was the percentage of limbs with vein occlusion and the absence of reflux in the treated vein. There was 100% occlusion and no reflux along the treated GSV at 3 days (29/29 limbs) and 97% of limbs (28/29) were occluded and had no reflux at the 3, 6, and 12-month visits. One limb had segmental recanalization first reported at the 3-month visit. Six AEs were reported through 12 months. Two patients had non-occlusive deep vein thrombosis, one determined by the investigator as probably related to the device or procedure. One patient developed paresthesia at nine days post procedure that was also determined by the investigator as probably related to the device or procedure. Three additional AEs were reported (i.e., musclevein thrombosis, increased pain, lymphedema all reported as not related to device or procedure. All adverse events were reported as resolved prior to the 6-month visit.
TABLE 3. Primary outcomes
| Primary Outcomes | 3 Day | 3 Month | 6 Month | 12 Month |
|---|---|---|---|---|
| Occlusion of the Treated Vein, % (n) | ||||
| Complete Occlusion | 100 (29) | 97 (28) | 97 (28) | 97 (28) |
| Segmental Recanalization | 0 (0) | 3 (1) | 3 (1) | 3 (1) |
| Reflux Along the Treated Vein, % (n) | ||||
| No | 100 (29) | 97 (28) | 97 (28) | 97 (28) |
| Yes | 0 (0) | 3 (1) | 3 (1) | 3 (1) |
| Status of the Deep Venous System, % (n) | ||||
| Normal | 93 (27) | 100 (29) | 100 (29) | 100 (29) |
| Abnormal | 7 (2)1 | 0 (0) | 0 (0) | 0 (0) |
*One muscle-vein thrombosis and subtotal thrombosis v. popliteal (Non-occlusive DVT) and one endo-thermal heat induced thrombosis (Non-occlusive DVT)
Secondary outcomes
Secondary outcomes, summarized in Table 4, showed improvement in the CEAP, VCSS, and CIVIQ-20 scores at the 3, 6, and 12 month time points. Three-day results did not show improvement from baseline; most patients wore compression stockings for two to three weeks after the procedure, and 3 days was too soon to measure procedural improvement. By the 3 month visit, 75% of limbs had a CEAP classification of C0 (3%), C1 (38%), or C2 (34%) as compared to Baseline where 100% of the limbs were categorized as C3 or higher. At 12 months, 85% of the limbs were classified as C0 (3%), C1 (72%), or C2 (10%). Results with the VCSS score showed a similar pattern with a mean baseline score of 4.4 improving to 1.6 at 3 months, 0.7 at 6 months, and 0.6 at 12 months. Finally, the CIVIQ-20 Global Index score improved from baseline (13.5 ± 10.5) to 12 months (2.6 ± 8.3), indicating an improvement in quality of life with respect to chronic venous insufficiency. A subjective satisfaction survey was added post hoc to the analysis plan, and patients reported pain (scale of 1 being low to 10 being high) as 1.2 ± 1.1 at baseline and 0.5 ± 1.5 at 12 months with an overall procedure satisfaction of 92% being very satisfied and 2% somewhat satisfied.
TABLE 4. Secondary outcomes
| Secondary Outcomes | Baseline | 3 Month | 6 Month | 12 Month |
|---|---|---|---|---|
| N=29* | N=29 | N=29 | N=29 | |
| CEAP Score, % (n) | ||||
| C0: no visible or palpable signs of venous disease | 0 (0) | 3 (1) | 3 (1) | 3 (1) |
| C1: telangiectasies or reticular veins | 0 (0) | 38 (11) | 65 (19) | 72 (21) |
| C2: varicose veins | 0 (0) | 34 (10) | 17 (5) | 10 (3) |
| C3: edema | 83 (24) | 14 (4) | 3 (1) | 3 (1) |
| C4a: pigmentation or eczema | 14 (4) | 7 (2) | 7 (2) | 7 (2) |
| C4b: lipodermatosclerosis or atrophie blanche | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| C5: healed venous ulcer | 3 (1) | 3 (1) | 3 (1) | 3 (1) |
| C6: active venous ulcer | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VCSS, mean + SD | 4.4 + 1.8 | 1.6 + 1.3 | 0.7 + 1.0 | 0.6 + 0.9 |
| CIVIQ-20, mean + SD | 13.5 + 10.5 | 5.1 + 6.6 | 3.1 + 5.6 | 2.6 + 8.3 |
| Subjective Patient Survey | ||||
| Pain Sore2, mean + SD | - | 0.5 + 0.7 | 0.3 + 0.5 | 0.5 + 1.5 |
| Satisfaction with Procedure, % (n) | ||||
| Very Satisfied | - | 64 (16) | 84 (21) | 92 (23) |
| Somewhat Satisfied | - | 32 (8) | 16 (4) | 8 (2) |
| Not Satisfied | - | 4 (1) | 0 (0) | 0 (0) |
*29 limbs treated in 25 patients; **Scale of 0 to 10 -0 being no pain and 10 being the worst possible pain
Discussion
Use of the Venclose RF ablation system in this exploratory study achieved vein occlusion without reflux in all but one incompetent GSV with six AEs treated and resolved by 6 months. There were also improvements in qualitative evaluations (e.g., CEAP, VCSS, and CIVIQ-20) through 12 months, and over 90% of patients reported little pain and stated that they would choose the procedure again for future treatment.
We observed a high occlusion rate (97%) through 12 months with only one partially recanalized GSV. Although not directly comparable to other trials, this observed rate was similar to previously reported occlusion rates with the ClosureFast RF ablation catheter of 92%–98% at one year [8-12]. We used the device in this trial per current standard of care and placed the tip of the catheter at least 2 cm distal to the SFJ. In the one partially recanalized case that we observed, the recanalization of the GSV occurred approximately one cm distal to where the catheter tip was placed which may have allowed for recanalization of the GSV stump as well as the Posterior Accessory Saphenous Vein (PASV). Some phlebologists in Germany are deviating from this standard approach and positioning the catheter almost flush to the GSV ostium while also treating the Anterior Accessory Saphenous Vein (AASV) possibly the PASV, and side branches greater than 2 mm in diameter that end near the SFJ with heat ablation or foam sclerotherapy [13]. This flush ablation (i.e., endovascular crossectomy), is designed to prevent revascularization of a long residual GSV stump as well as the AASV/ PASV and may have prevented the partial recanalization in this trial.
Further observations during the study were of note. In our hands, the Venclose catheter had a slightly curved shape and its overall flexibility allowed positioning of the catheter without the need for an additional guide wire. The heating element could be switched off making it possible to treat accessory vein segments and longer side branch varices with the same catheter. The VCSS and CIVIQ-20 scores improved from baseline values by over 80% (VCCS: 4.4 to 0.6 and CIVIQ-20: 13.5 to 2.6 at 12 months, respectively), patients reported low pain (0.5 at 12 months on a scale of 1-10, with 1 being low pain), and were satisfied with the procedure (94% very or somewhat satisfied).
Limitations of our study should be noted. Although prospective, this was a small single-center exploratory study. Results were observational; there were no statistically powered or tested hypotheses and no concurrent control. Patients were predominantly female (80%), and all were White; results, therefore, are not generalizable to a broader more diverse patient population. Results were also based on investigator visual inspections and judgment; DUS images were not reviewed by a core laboratory and adverse events were not adjudicated by a clinical events committee. The RF ablation system tested in the current study may have performed differently than devices used in other trials and other patient groups. Differences between thermal ablation devices and methods were not examined and would require properly powered, randomized concurrentlycontrolled studies within similar patient populations. Finally, rates of occlusion and other outcomes may vary when the device is used by operators outside of our center in Leipzig.
Conclusion
In this exploratory work, the Venclose system provided a reliable RF ablation system for the treatment of incompetent GSVs. The catheter flexibility and dual heating elements allowed us to treat the GSV and side branch varices with one catheter. Occlusion rates were high, AEs were minimal and reported resolved by 6 months, qualitative venous measures improved from baseline, and there was a high level of patient satisfaction
Acknowledgement
The authors thank Mai-Ly Wilcox for her oversight and management of the trial.
Funding
The Venclose Study for Incompetent Saphenous Veins was supported by Venclose, a wholly-owned subsidiary of Becton, Dickinson and Company.
Conflict of Interest
TM and HCW report support from Becton, Dickinson and Company as Investigators in this exploratory study. RS is an employee of Becton, Dickinson and Company.
References
- Gloviczki P, Lawrence PF, Wasan SM, et al. The 2022 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part I. Duplex Scanning and Treatment of Superficial Truncal Reflux: Endorsed by the Society for Vascular Medicine and the International Union of Phlebology, J. Vasc. Surg.: Venous Lymphat. Disord., 2023;11(2):231-61.
- Critchley G, Handa A, Maw A, et al. Complications of varicose vein surgery. Ann. R. Coll. Surg. Engl.. 1997;79(2):105.
- Whing J, Nandhra S, Nesbitt C, et al. Interventions for great saphenous vein incompetence. Cochrane Database Syst. Rev., 2021(8).
- Morrison C, Dalsing MC. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: prevalence, severity, and relevance for modern practice, J. vasc. surg.. 2003;38(5):886-90.
- Proebstle T, van den Bos R. Endovenous ablation of refluxing saphenous and perforating veins. Vasa. 2017;46(3):159-66.
- Creton D, Pichot O, Sessa C, et al. Radiofrequency-powered segmental thermal obliteration carried out with the ClosureFast procedure: results at 1 year. Ann. vasc. surg. 2010;24(3):360-6.
- Eklöf B, Rutherford RB, Bergan JJ, et al. Revision der CEAP-Klassifizierung für chronische Venenleiden. Phlebologie. 2005;34(04):220-5.
- Braithwaite SA, Braithwaite BD. Clinical utility of the Covidien Closure Fast™ endovenous radiofrequency ablation catheter. Med. Devices: Evid. Res..2014:179-85.
- Almeida J.I., Kaufman J., Göckeritz O., et al., 2009. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). J. Vasc. Interv. Radiol., 20(6):752-9.
- Braithwaite SA, Braithwaite BD. Clinical utility of the Covidien Closure Fast™ endovenous radiofrequency ablation catheter. Med. Devices: Evid. Res. 2014:179-85. [Google Scholar]
- Rasmussen LH, Lawaetz M, Bjoern L, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins, J. Br. Surg.. 2011;98(8):1079-87.
- Bisang U, Meier TO, Enzler M, et al. Results of endovenous ClosureFast treatment for varicose veins in an outpatient setting. Phlebology. 2012;27(3):118-23.
- Fink, C., Hartmann, K., Mattausch, T., et al., Impact of a synchronous prophylactic treatment of the anterior accessory saphenous vein on the recurrent varicose vein rate in patients undergoing thermal ablation of an insufficient great saphenous vein (SYNCHRONOUS-Study): study protocol for a prospective, multicentre, controlled observational study. BMJ open, 2022. 12(6): e061530.