Role of serum levels of Aspergillus-galactomannan antigen index in evaluating cause of acute exacerbation of COPD
Received: 11-Aug-2023, Manuscript No. PULCLR-23-6657; Editor assigned: 14-Aug-2023, Pre QC No. PULCLR-23-6657 (PQ); Reviewed: 28-Aug-2023 QC No. PULCLR-23-6657; Revised: 24-Jan-2025, Manuscript No. PULCLR-23-6657 (R); Published: 31-Jan-2025
Citation: Napa P. Role of serum levels of Aspergillus-galactomannan antigen index in evaluating cause of acute exacerbation of COPD. J Chest Lung Res. 2025;5(1):1-4.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Microbial agents account for the etiology of 75% of Acute Exacerbations of COPD (AE-COPD). However, the role of non-usual microorganisms such as Aspergillus spp. has not been well established. Aspergillus-Galactomannan Antigen (AGA) can be easily measured in serum samples, and thus has been widely used as a complementary diagnostic marker for the diagnosis of Aspergillus disease.
Materials and methods: A hospital based observational study was conducted at department of chest and TB of a tertiary care centre. Study included 50 diagnosed cases of acute exacerbation of chronic obstructive pulmonary disease. Serum galactomannan levels were investigated in all cases from November 2020-February 2022. Cut-off for the same to predict AE-COPD was calculated based on ROC analysis.
Results: The prevalence of Invasive Pulmonary Aspergillosis (IPA) as an etiology for AECOPD was 70%. On ROC analysis, for a cut-off of >=1, the sensitivity and specificity of AGA was 97.1% and 73.3% with overall accuracy of 90% for diagnosing IPA.
Conclusion: Study concluded that serum AGA index is a useful diagnostic modality for identification of invasive Aspergillosis as a cause of AECOPD. At a cut-off value of >1, it has an excellent sensitivity and good specificity. Majority of these patients had longer hospital stay as compared to non-IPA cases.
Keywords
Acute exacerbations of COPD; Aspergillus galactomannan antigen index; Chronic obstructive pulmonary disease; Invasive pulmonary Aspergillosis
Introduction
The prevalence of Chronic Obstructive Pulmonary Disease (COPD) is constantly increasing, while its incidence is growing in old age [1-3]. COPD is also a leading cause of morbidity worldwide, particularly in developing countries. Whereas COPD is an obstructive and progressive airway disease, it is also associated with a significant reduction in physical activity, and psychological problems, all of which contribute to the patient’s disability and poor Health-Related Quality of Life (HRQoL).
World Health Organization (WHO) predicts that it will become the third leading cause of mortality by 2030 COPD often coexists with other comorbidities which may have a significant impact on its prognosis. Cardiovascular diseases, osteoporosis, lung cancer, diabetes, metabolic syndrome, and depression are among a few of them [4,5]. Cardiovascular disease is a major comorbidity in COPD patients and probably the most frequent and most important coexisting illness as these conditions have many common risk factors such as age, male sex, cigarette smoking although its actual prevalence is unknown.
COPD exacerbations are defined as, “An event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough, and/or sputum that is beyond normal day-to-day variations, is acute on onset, and may warrant a change in regular medication in a patient with underlying COPD” [6].
Overall, these microbial agents account for the etiology of 75% of Acute Exacerbations of COPD (AE-COPD) [7]. However, the role of non-usual microorganisms such as Aspergillus spp. has not been well established. Previous published data investigating Aspergillus spp. in COPD are retrospective or were conducted in a small series of patients [8, 9]. However, Aspergillus spp. may be responsible for important clinical events from saprophytic colonization of the airways to rapidly invasive and lifethreatening disseminated diseases, depending on the host immune status and the presence of underlying lung disease. Patients with severe COPD who often receive broad-spectrum antibiotics and corticosteroids are now acknowledged to be one of the main risk groups for pulmonary aspergillosis [10].
As little is known about the risk of pulmonary aspergillosis in severe COPD patients, some retrospective studies have analysed the incidence of Aspergillus fumigatus isolation from lower respiratory tract samples in non-immunocompromised patients and shown that COPD patients are an important group which are affected by either colonization or proven aspergillosis [11]. It is often difficult to diagnose Aspergillus disease in the clinical setting. However, Aspergillus-galactomannan antigen can be easily measured in serum samples, and thus has been widely used as a complementary diagnostic marker for the diagnosis of Aspergillus disease [12].
In this study, we aimed to find the role of serum levels of Aspergillus– galactomannan antigen index in evaluating cause of Acute Exacerbation of COPD (AE-COPD).
Materials and Methods
A hospital based observational study was conducted at department of chest and TB of a tertiary care centre. Study included 50 cases with acute exacerbation of chronic obstructive pulmonary disease. Patients on antifungals or on fluids like TPN, plasmalyte, platelets transfusion were excluded.
COPD and AE-COPD were diagnosed according to the global initiative for chronic obstructive lung disease. Written informed consent was taken from each patient. On admission, the following examinations were carried out on all patients:
• Full medical history from the patient (if possible) or his relatives: History of smoking (current, ex, or nonsmoking), history of chest symptoms (cough, expectoration, dyspnea, and wheeze), history of previous intubation and/or ventilator support, prior evidence of cor pulmonale with or without congestive heart failure, comorbidities, and drug therapy.
• Full clinical examination (general and local).
• Plain chest radiography (posteroanterior, anteroposterior, and/or lateral), HRCT chest.
• Pulmonary function test: Measurements of Forced Expiratory Volume in the first second (FEV1) for assessment of severity of disease will be obtained. Patients will be classified according to their postbronchodilator FEV1 into mild (FEV1 ≥ 80% predicted), moderate (50% ≤ FEV1, <80% predicted), severe (30% ≤ FEV1, <50% predicted) and very severe (FEV1<30% predicted) [13].
• Cut-off for serum galactomannan levels was calculated based on ROC analysis.
• Sputum/tracheal secretions culture
• Outcome variables included: Need for assisted ventilation, length of hospitalization and survival/death.
Sample size calculation
Sample size was calculated using formulae:
=Zα2 (5%) p (1-p)/E2
n=Sample size
Z=Level of significance (at 95% confidence level, its value is 1.96)
P=Prevalence of Aspergillosis in AE-COPD (16%)
Q=1-P (1-0.16=0.84)
E=Allowable error (taken as 10%)
n=(1.96)2 * (0.16)* (0.84)/(0.1)2
n=50 (approx.)
So, final sample size was taken as 50 diagnosed cases of acute exacerbation of chronic obstructive pulmonary disease.
Statistical analysis
The quantitative data was represented as their mean ± SD. Categorical and nominal data was expressed in percentage. The t-test was used for analysing quantitative data, or else non parametric data was analyzed by Mann Whitney test and categorical data was analyzed by using chi-square test. ROC Curve analysis was done for diagnostic accuracy. The significance threshold of p-value was set at <0.05. All analysis was carried out by using SPSS software version 21.
Results
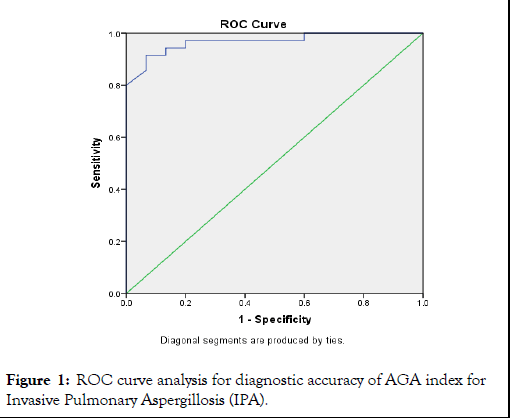
Mean age of the study cases was 61.58 years with over half of the cases (58%) were in elderly age group (>60 years). Male predominance was seen in the study group with 86% males to 14% females, giving a male to female ratio of 6.14:1. Out of the total 50 cases, 8% had moderate impact of COPD, 76% had severe impact while 16% had very severe impact of COPD on life respectively. We observed that prevalence of invasive pulmonary aspergillosis as an etiology for AECOPD was 70%. Mean AGA levels were significantly more among cases positive for IPA as compared to negative cases for IPA (2.59 vs. 0.64; p<0.01). On ROC analysis, area under curve for AGA index for diagnosing IPA was 0.968 (95% CI-0.924-1.0; p<0.01). The optimal cut-off for AGA as per ROC analysis was >=1 (Table 1 and Figure 1). At a cut off of AGA>=1, sensitivity and specificity of AGA was 97.1% and 73.3% with overall accuracy of 90%.
|
Area under the curve |
|||||
|---|---|---|---|---|---|
|
Test variable |
Area |
SE |
p-value |
95% CI |
|
|
Lower bound |
Upper bound | ||||
|
S. AGA |
0.968 |
0.022 |
<0.01 |
0.924 |
1 |
Table 1: ROC curve analysis for diagnostic accuracy of AGA index for Invasive Pulmonary Aspergillosis (IPA).
Figure 1: ROC curve analysis for diagnostic accuracy of AGA index for Invasive Pulmonary Aspergillosis (IPA).
Mean age of cases with and without IPA was 61.77 years and 60.8 years respectively (p-0.59). No association was observed between IPA with age and any specific gender (p>0.05). Mean CAT score was significantly higher in cases with IPA as compared to cases without IPA (27.4 vs. 25.8; p<0.01). Mean hospital stay was significantly more in cases with IPA as compared to cases without IPA (7.06 vs. 5.13; p<0.01). Ventilator requirement was seen in 65.7% cases of IPA as compared to only 20% among non-IPA cases (p<0.01). Incidence of mortality was 5.7% among IPA cases while it was 0% in non-IPA cases (p-0.34) (Table 2).
| Variable | IPA | p-value | ||||
|---|---|---|---|---|---|---|
| No (n-15) | Yes (n-35) | |||||
| Age in years (mean/SD) | 60.8 ± 5.63 | 61.77 ± 6.01 | 0.59 | |||
| Gender | Female | 1 | 14.30% | 6 | 85.70% | 0.65 |
| Male | 14 | 32.60% | 29 | 67.40% | ||
| CAT score (mean/SD) | 25.8 ± 3.43 | 27.4 ± 4.1 | <0.01 | |||
| Day of hospital stay (mean/SD) | 5.13 ± 2.17 | 7.06 ± 2.5 | <0.01 | |||
| Ventilator requirement | No | 12 | 80.00% | 12 | 34.30% | <0.01 |
| Yes | 3 | 20.00% | 23 | 65.70% | ||
| Mortality | No | 15 | 100.00% | 33 | 94.30% | 0.034 |
| Yes | 0 | 0.00% | 2 | 5.70% | ||
Table 2: Association of Invasive Pulmonary Aspergillosis (IPA) with demography and clinical outcome.
Discussion
In present study, we observed that prevalence of invasive pulmonary aspergillosis as an etiology for AECOPD was 70%.
An accurate estimation of IPA incidence in COPD patients is difficult, partly due to the lack of infection surveillance measures. In a study including 595 patients with IPA, 9% suffered from non-detailed pulmonary diseases [14,15]. Huerta A, et al., in their study observed the prevalence of Aspergillus spp. isolation was 16.6% on admission. Yoshimura K et al. in their study observed prevalence of IPA as 40.3% [16,17].
In present study, mean CAT score was significantly higher in cases with IPA as compared to cases without IPA (27.4 vs. 25.8; p<0.01). This showed that IPA had a significantly more impact on the patient’s life. Mean hospital stay was significantly more in cases with IPA as compared to cases without IPA (7.06 vs. 5.13; p<0.01). Similarly, ventilator requirement was also more in cases of IPA (65.7% vs. 20%; p<0.01). This showed that severity of the disease was more with aspergillosis infection. Incidence of mortality was 5.7% among IPA cases while it was 0% in non-IPA cases (p-0.34).
Studies have shown that higher risk of IPA occurrence may be correlated with advanced stages of COPD. As reported in a recent series of 57 probable IPA, 36 patients (63.2%) were stage III and 21 (33.8%) were stage IV [18]. Yoshimura K et al., in their study observed that IPA cases had significantly higher incidence of severe AE-COPD (P=0.0039, Gray’s test) and respiratory-related mortality (P=0.0176, log-rank test). Gu Y et al. also observed that pulmonary aspergillosis group (PA) had significantly higher inhospital mortality and 180-day mortality than the non-PA group (45% (27/60) vs. 0% (0/15), p=0.001, and 52.5% (31/59) vs. 6.7% (1/15), p700 mg (HR 2.452, 95% CI 1.134–5.300, p=0.023) and respiratory failure at admission (HR 5.983, 95% CI 2.487–14.397, p<0.01) were independently associated with increased mortality [19]. Tong X et al. in their study observed that duration of hospitalization was longer in the Aspergillus colonization group than in the control group (15 ± 5 days vs. 12 ± 4 days, p=0.011) [20].
Galactomannan is a polysaccharide that is a major constituent of Aspergillus cell walls. The Platelia® galactomannan assay is the approved test using the serum and Bronchoalveolar Lavage Fluid (BALF).
In present study, mean AGA levels were significantly more among cases positive for IPA as compared to negative cases for IPA (2.59 vs. 0.64; p<0.01). On ROC analysis, Area under curve for AGA index for diagnosing IPA was 0.968 (95% CI -0.924-1.0; p<0.01). The optimal cut-off for AGA as per ROC analysis was >=1. At a cut off of AGA>=1, sensitivity and specificity of AGA was 97.1% and 73.3% with overall accuracy of 90%.
He H et al., aimed to investigate what the optimal cut-off value would be for AGA. According to the Receiver Operating Characteristics (ROC) curve, an Optical Density (OD) ratio of 0.8 was chosen as the cut-off value for AGA. Compared to serum GM and LRT Aspergillus isolation, BALF GM yield a better sensitivity, specificity, positive and negative predictive values of 88.9%, 100%, 100% and 94.4%, respectively. Areas under the ROC curve was 0.879 (95% CI-0.691 to 0.972) for serum AGA results from the first day of ICU admission. It yielded a sensitivity and specificity of 77.8% and 100% respectively with overall efficacy of 92.3%.
AGA levels were significantly higher in IPA patients (2.88 ± 2.09 versus 0.87 ± 0.47, P=0.023). Sulahian A et al., in their study observed the sensitivity and specificity of AGA as 97% and 49% respectively. Yoshimura K et al., explore whether serum levels of Aspergillusgalactomannan antigen could be used to evaluate the risk of severe Acute Exacerbation of COPD (AE-COPD) [20]. Study concluded that serum Aspergillus-galactomannan antigen was detected in patients with COPD, and elevated serum Aspergillus-galactomannan antigen was associated with severe AE-COPD.
Thus, to summarize, serum Aspergillus-Galactomannan Antigen (AGA) Index is a useful diagnostic modality for identification of invasive aspergillosis as a cause of in these cases. At a cut-off value of >1, it has an excellent sensitivity and good specificity [21,22].
Conclusion
Our study concluded that invasive pulmonary aspergillosis is an important serious infectious etiology for acute exacerbation of COPD. Serum Aspergillus Galactomannan Antigen (AGA) Index is a useful diagnostic modality for identification of invasive aspergillosis as a cause of in these cases. At a cut-off value of >1, it has an excellent sensitivity and good specificity. Majority of these patients had longer hospital stay as compared to non-IPA cases. Due to the associated poor outcome, a high index of suspicion is necessary for the timely treatment of these patients and serum AGA index is a valuable tool for the same.
References
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498-04.
[Crossref] [Google Scholar] [PubMed]
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27(2):397–412.
[Crossref] [Google Scholar] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
- World Health Organization. World Health Statistics. 2008.
- Nici L, ZuWallack R. Chronic obstructive pulmonary disease co-morbidities and systemic consequences. USA: Humana Press; 2012.
- Evensen AE. Management of COPD exacerbations. Am Fam Physician. 2010;81:607–13.
[Google Scholar] [PubMed]
- Wedzicha JA, Seemungal TA. COPD exacerbations: Defining their cause and prevention. Lancet. 2007;370(9589):786–96.
[Crossref] [Google Scholar] [PubMed]
- Guinea J, Torres-Narbona M, Gijon P, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin Microbiol Infect. 2010;16(7):870–77.
[Crossref] [Google Scholar] [PubMed]
- Soler N, Huerta A, Torres A. The importance of Aspergillus species infection in chronic obstructive pulmonary disease. Clin Pulm Med. 2011;18:161–68.
- Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011;37(4):865–72.
[Crossref] [Google Scholar] [PubMed]
- Barberan J, Alcazar B, Malmierca E, et al. Repeated Aspergillus isolation in respiratory samples from non-immunocompromised patients not selected based on clinical diagnoses: Colonisation or infection?. BMC Infect Dis. 2012;12:295.
[Crossref] [Google Scholar] [PubMed]
- Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004;4(6):349–57.
[Crossref] [Google Scholar] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 195(5):557-82.
[Crossref] [Google Scholar] [PubMed]
- Petty TL. History of COPD. Int J COPD. 2006;1(1):3–14.
[Crossref] [Google Scholar] [PubMed]
- Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore). 2000;79:250–60.
[Crossref] [Google Scholar] [PubMed]
- Huerta A, Soler N, Esperatti M, et al. Importance of Aspergillus spp. isolation in Acute exacerbations of severe COPD: Prevalence, factors and follow-up: The FUNGI-COPD study. Respir Res. 2014 Dec;15(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Yoshimura K, Suzuki Y, Inoue Y, et al. Utility of serum Aspergillus-galactomannan antigen to evaluate the risk of severe acute exacerbation in chronic obstructive pulmonary disease. PLoS ONE. 2018;13(6).
[Crossref] [Google Scholar] [PubMed]
- Guinea M, Torres-Narbona P, Gijon T, et al. Invasive pulmonary aspergillosis in patients with COPD: A description of 57 cases collected in a single tertiary hospital (1999–2008). Abstract M-2161. Washington, D.C. 48th ICAAC/IDSA 46th Annual Meeting; October 25–28, 2008.
- Gu Y, Ye X, Wang Y, et al. Clinical features and prognostic analysis of patients with Aspergillus isolation during acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2021:21(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Tong X, Cheng A, Xu H, et al. Aspergillus fumigatus during COPD exacerbation: A pair-matched retrospective study. BMC Pulm Med. 2018;18:55.
[Crossref] [Google Scholar] [PubMed]
- He H, Ding L, Sun B, et al. Role of galactomannan determinations in bronchoalveolar lavage fluid samples from critically ill patients with chronic obstructive pulmonary disease for the diagnosis of invasive pulmonary aspergillosis: A prospective study. Crit Care. 2012:16(4):1-8.
[Crossref] [Google Scholar] [PubMed]
- Sulahian A, Porcher R, Bergeron A, et al. Use and limits of (1-3)-β-D glucan assay (Fungitell), compared to galactomannan determination (Platelia Aspergillus), for diagnosis of invasive aspergillosis. J Clin Microbiol. 2014;52:2328-2333.