Studies on the sclerotia of Aspergillus sclerotioniger and the alkaloid extracted
Received: 03-Dec-2017 Accepted Date: Mar 05, 2018; Published: 12-Mar-2018
Citation: Abu El-Souod SM, Mahmoud YA, Awadalla OA, et al. Studies on the sclerotia of Aspergillus sclerotioniger and the alkaloid extracted. J Exp Clin Microbiol. 2018;1(1): 01-07.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
In the present study the sclerotia of different Aspergilli (Aspergillus sclerotioniger, A. sclerotiorum, A. candidus, A. flavus, A. piperis, A. ochraceus, A. robustus, A. sepultus, A. petrakii, A. melleus, A. parasiticus and A. sclerotii carbonarius) were examined for the presence of alkaloids in their formed sclerotia separately where, A. sclerotioniger exhibited the best result. Accordingly, the culture conditions (temperature, pH, carbon source, nitrogen source and carbon/ nitrogen (C/N) ratio were optimized for the formation of sclerotia by A. sclerotioniger. The best weight of sclerotia was achieved at temperature 30°C, PH 6, maltose as carbon source, KNO3 as nitrogen source and (C/N) ratio of 43.33.
The total alkaloids content of A. sclerotioniger sclerotia was detected using Van Urk’s reagent and recording the optical density on spectrophotometer at 580 nm, then separated on thin layer chromatography (TLC). The separated alkaloid was defined using the percentage of carbon, hydrogen and nitrogen (CHN) percentage, as well as ultraviolet (UV) spectrum, nuclear magnetic resonance (H1NMR) analysis, gas spectroscopy mass spectral analysis (GC-MS mass) and infrared (IR) analysis. The alkaloid was found to be a member of indole alkaloids and was defined as chloro derivative of aflavinine.
Keywords
Aspergillus sclerotioniger; Sclerotia; Alkaloids; Culture conditions
Introduction
Sclerotia are resting bodies produced by several fungi as a mechanism for their long-term survival and propagation of the species [1,2]. The sclerotia are morphologically variable but they are nutrient-rich of multi-hyphal structures which enable them to remain dormant or quiescent when their environment is adverse; and then when conditions improve, germinate to reproduce the fungus [3]. Several species of black Aspergilli are able to produce sclerotia including A. carbonarius, A. ellipticus, A. aculeatus, A. costaricaensis, A. piperis, A. sclerotioniger, A. aculeatinus and A. sclerotii carbonarius. Among them, A. sclerotioniger give positive result with Ehrlish reagent at the sclerotia region which means that the sclerotia contain alkaloids [4]. Ergot alkaloids are a complex family of indole derivatives with diverse biological activities [5] and produced mainly by fungi of the genera Claviceps, Penicillium and Aspergillus [5,6]. These ergot alkaloids and related indole metabolites were considered to protect the sclerotia from predation especially those of the genus Claviceps [7]. These important indole metabolites are also associated with the sclerotia of Aspergilli such as, A. flavus, but not the vegetative mycelium or conidia [8]. In the meantime, three new diketopiperazine metabolites (N-methylepiamauromine, epiamauromine and cycloechinulin) were distinguished from the sclerotia of A. ochraceus [9]. Also, variecolactol was isolated from the organic extracts of the sclerotia of A. auricomus [10]. On the other hand, three new metabolites with the aflavinine ring, were isolated from the sclerotia of A. tubingensis (NRRL 4700), and their structures were assigned primarily through nuclear magnetic resonance (NMR) studies [11]. The crude extracts of the sclerotia of Aspergillus niger showed effective insecticidal activity against the corn borer, Ostrinia furnacalis [12].
A. sclerotioniger is a member of Aspergillus niger group which was discovered in 2004 [13]. This fungus produces distinct globose to subglobose and red brown sclerotia. For collecting more knowledge about this species few researches were conducted concerning its physiological characters. Accordingly, this work was designed to study the sclerotia formation of different Aspergilli (Aspergillus sclerotioniger, A. sclerotiorum, A. candidus, A. flavus, A. piperis, A. ochraceus, A. robustus, A. sepultus, A. petrakii, A. melleus, A. parasiticus and A. sclerotii carbonarius) concerning the alkaloid extracted. On the other hand, the study included the mycelial growth and formation of sclerotia of A. sclerotioniger with special attention to the alkaloid compounds in these sclerotia as well as factors affecting their production.
Materials and Methods
The tested fungi
For the present study some sclerotia forming Aspergilli were chosen. Aspergillus sclerotioniger, A. sclerotiorum, A. candidus, A. flavus, A. piperis, A. ochraceus, A. robustus, A. sepultus, A. petrakii, A. melleus, A. parasiticus and A. sclerotii carbonarius were purchased from the mycological center, Assiut University, Egypt and maintained on Czapek’s agar medium (CZA).
Chemicals and standard alkaloids
Ρ-dimethyl-amino-benzaldehyde was used in preparation of color reagents. It was purchased from El Gomhoria Company, Cairo, Egypt. Pure samples of α-ergocryptine and ergocornine were purchased from Sigma-Aldrich Company and used as standard alkaloids.
Media and reagents
CZA medium was used in the present study. The citrate-phosphate and boric acid-borax buffers were prepared according to references [14] and [15] for adjusting the pH values of CZA medium. On the other hand, Ehrlich’s and Van Urk’s reagents were prepared according to [16] and [17] for detection of alkaloids.
Survey for the occurrence of alkaloids in the used aspergilli
Alkaloids were detected in sclerotia of tested Aspergilli by reacting with Ehrlich reagent [4,18] using a filter paper method. Four mm square agar plug is cut out from the sclerotia region of each Aspergillus culture separately grown on CZA. A round piece (1 cm diam.) of filter paper, Whatman No. 1, wetted with Ehrlich reagent was placed on the mycelial side of the plug. If a violet ring appears after 2-6 min, the culture contains cyclopiazonic acid or related alkaloids. If the reaction comes after 7-10 min, it is regarded as weak. After 10 min the violet ring will fade away. Some fungi produce alkaloids that will react with Ehrlich reagent to give pink to red or yellow rings.
Effect of different factors on the growth and weight of the sclerotia produced from A. sclerotioniger
A. sclerotioniger gave positive result with Ehrlich reagent more than other tested used species; so, it was chosen to optimize its growth conditions for yielding large amount of sclerotia for further work on sclerotial alkaloids.
Effect of different degrees of temperature: This experiment was designed to determine the optimum temperature for the mycelial growth and the best yield of sclerotia produced from A. sclerotioniger using a modified method adopted by the researchers in reference [19]. For this purpose, CZA plates were inoculated with 10 mm disc from five days old culture of A. sclerotioniger, then incubated at different temperature (15, 20, 25, 30, 35 and 40°C) separately. Colony diameter and the weight of sclerotia formed per plate were estimated after six days.
Effect of different pH values: This experiment was carried out using modified method adopted by the same reference. CZA medium with different pH values (4, 5, 6, 7, 8 and 9) was prepared using citrate-phosphate and boric acid-borax buffers. The plates of each pH degree were inoculated separately with 10 mm disc from five days old culture of A. sclerotioniger. The inoculated plates were incubated at 30°C. Colony diameter and weight of sclerotia formed per petri plate were estimated after six days. Periods (days) taken by mycelium to fill petri plate was also recorded.
Effect of different carbon and nitrogen sources: This experiment was carried out using the modified method of [20,21] using CZA medium. Sucrose was replaced in the CZA medium with equivalent amounts of carbon from five different carbon sources (fructose, galactose, glucose, lactose and maltose). Also, NaNO3 was replaced by equivalent amounts of nitrogen from seven different nitrogen sources (asparagine, glycine, tryptophan, urea, (NH4)2SO4, NaNO2 and KNO3) using the using the following equation:
X conc/L = wt. of X source (g) × Number of X atoms × X molecular weight / molecular wt. of X source
Where: X referred to carbon or nitrogen.
The pH of the medium was adjusted to 6 by citrate-phosphate buffer. The inoculated plates with A. sclerotioniger were incubated at 30°C. Colony diameter and the weight of sclerotia formed per each plate were estimated after six days. Periods (days) taken by mycelia to fill petri plate was also recorded.
Effect of carbon nitrogen (C⁄N) ratio: From the previous experiments, it was found that maltose and KNO3 were the best carbon and nitrogen sources respectively for both mycelial growth and sclerotia formation. The C⁄N ratio was 25.5 where the carbon and nitrogen conc. in maltose and KNO3 were 12.6 and 0.49 respectively. To study the effect of different C⁄N ratio on the growth and sclerotia formation of A. sclerotioniger, a modified method of [22] was used. Different concentrations from maltose and KNO3 were utilized to prepare different C⁄N ratios (Table 1). The citrate-phosphate buffer was used in preparation of the media for obtaining PH 6. The media were sterilized in autoclave at 121°C and 1.5 atm. for 20 min. Petri plates containing uniform quantities (20 ml) of sterilized media were inoculated, incubated and treated as previously mentioned. All the studied culture conditions were considered and optimized for the production of the maximum amount of sclerotia.
| Maltose concentration (g / L) | KNO3 concentration (g / L) | C / N ratio% Calculated as C and N occurred in each corresponding source |
|---|---|---|
| 19 | 5.05 | 13.3 |
| 23.77 | 4.33 | 16.6 |
| 21.39 | 2.89 | 22.5 |
| 30 | 3.56 | 25.5 |
| 26.14 | 2.89 | 27.5 |
| 21.39 | 2.16 | 30 |
| 23.77 | 1.8 | 40 |
| 30.9 | 2.16 | 43.33 |
Table 1: Carbon/Nitrogen ratio (%)
Studies on the alkaloids extracted from sclerotia of A. sclerotioniger
Extraction of alkaloids: The total alkaloids of A. sclerotioniger were estimated [23]. Mature sclerotia were gently picked from the mycelia after 6 days incubation. Sclerotia were subsequently placed in a sieve and washed repeatedly with distilled water to remove any mycelia that remained attached with sclerotia then kept on the sieve to dry at room temperature. The alkaloids in the tested sclerotia were then extracted [16]. A random weight about 500 mg of sclerotia was powdered using a porcelain mortar and extracted by 6 ml of extraction mixture (98% methanol: water, 8:2, v/v alkalinized with ammonium hydroxide (NH4OH). In the dark the mixture was stirred by a magnetic stirrer for 2h and then re-extracted without stirring for 12 h before filtration. The filtrate was loaded on silica gel plates for separation of alkaloids from the other components.
Quantification Analysis For Total Alkaloids
Preparation of standard curve using α-ergocryptine: Standard curve was prepared using a modified method of [24]. From α-ergocryptine 5 mg were dissolved in 5 ml ethanol for preparing a stock solution of concentration 1000 μg⁄ml. Different dilutions (10, 20, 40, 60, 80 and 100 μg⁄ml] from the stock solution were then prepared. From each dilution 2 ml were added separately to 4 ml Van Urk’s reagent in test tubes. Two ml ethanol was added to 4 ml Van Urk’s reagent as a blank. Treatments were incubated in a water bath at 30°C for 15 min for developing the expected color indicating the presence of alkaloid. The optical density of each treatment was recorded using spectrophotometer at λ=580 nm. A standard curve was plotted using the optical density (OD) values against the known concentrations of α-ergocryptine.
Determination of total alkaloids extracted from tested sclerotia of A. sclerotioniger: Alkaloids were extracted from 7.5 mg of the tested sclerotia. According to the procedures modified from [24], 2 ml from this filtrate was added to 4 ml Van Urk’s reagent in a test tube and 2 ml extraction mixture was added to 4 ml Van Urk’s reagent in another test tube as a blank. The tubes were then incubated in a water bath at 30°C for 15 min for developing definite colors. The OD was recorded using spectrophotometer at λ=580 nm. The total alkaloids content of the tested sample was calculated using the previously prepared standard curve.
Identification of extracted alkaloid: A modified method from [25] was used for the preparation of silica gel plates which were used for the identification of the studied alkaloid. The method of [16] was used for the identification of the extracted alkaloid by thin layer chromatography (TLC). In the present work it was used for the tested alkaloid and standard alkaloids (ergocornine and α-ergocryptine); from each sample 300 μl, were spotted using automatic pipette on a line about 1.5 cm above the bottom edge of the silica plates. Each plate was then exposed to ammonia vapor for about 15 min. The plates were developed in the chromatographic jar containing the solvent system chloroform or methylene chloride - acetone (3:1) v ⁄ v for about 45 min. After complete running of the plates in the solvent system, they were sprayed by Ehrlich reagent or, examined under ultra violet irradiation at 365 nm. The retention factor (Rf), which is defined as the distance travelled by the compound divided by the distance traveled by the solvent system, was then calculated for the resulted spots.
The tested alkaloid spot was scraped from other silica plates each of spots not sprayed with Ehrlich reagent, and then dissolved in 5 ml extraction mixture. The resulted solution was centrifuged for about 10 min to discard the silica gel; this step was repeated few times to separate all the alkaloid amount from the silica. Then the supernatant was filtered through Whattman No.1 filter paper, the clear filtrate was set to evaporate the solvent to dryness to achieve the alkaloid as powder.
The tested alkaloid powder was dissolved in 5 ml from the extraction mixture solution and then analyzed using ultraviolet (UV) spectrophotometer to detect its UV spectrum in chemistry department faculty of science, Tanta University. Also, infrared (IR) spectra were carried out at room temperature using a BRUKER FT-IR spectrometer tensor 27 on the range 200 to 4000 cm-1 at Central Lab., Tanta University. The elemental analysis for the unknown alkaloid recognizes the percentage of carbon, hydrogen and nitrogen in the tested alkaloid sample. Thus, it was obtained by burning the solid sample in oxygenated atmosphere. This leads to turning of C, H and N to carbon dioxide (CO2), water vapor (H2O) and nitrogenous gases. The resulted gases were separated and the ratio of each of C, H and N was recorded. The percentage of chlorine in the alkaloid sample was also recorded. The nuclear magnetic resonance (H1NMR) analysis was carried out with 300.0687855 MHZ; the sample was dissolved in DMSO. The molecular weight of the unknown alkaloid was achieved by mass spectral analysis using gas chromatography mass spectrometer (GC-MS). The elemental analysis, H1NMR and GC-MS were carried out at micro analytical center, Cairo University.
Statistical analysis
Statistical analysis of the present study was conducted, using the mean, standard error, standard deviation, F-value and analysis of variance (ANOVA) using the computer programme SigmaPlot 12.3.
Results and Discussion
Survey for the occurrence of alkaloids in the used Aspergilli
The Ehrlich reaction method was designed to detect some indole secondary metabolites produced by fungi. The results of Ehrlich reaction are illustrated in Figure 1. It is evident that A. sclerotioniger produced the most obvious ring color among all the used Aspergillus spp. (yellow and blue) which indicated the presence of alkaloids associated with sclerotia. On the other hand, A. melleus gave negative results where no color was detected. Other tested Aspergilli formed yellow rings which indicated the presence of other types of alkaloids as illustrated in Figure 1.
Figure 1) Occurrence of alkaloids using Ehrlich color reaction of tested Aspergilli
Since the A. sclerotioniger revealed the best results in Ehrlich test so a large amount of its sclerotia should be obtained for extraction of alkaloids. Thus, the growth factors were of great consideration and should be studied to be optimized.
Effect of different factors on the growth and weight of the sclerotia produced from A. sclerotioniger
Effect of different degrees of the temperature: Table 2 illustrates the effect of different temperature degrees on the radial growth and weight of sclerotia of A. sclerotioniger. The different temperature degrees gave a statistically significant difference in radial growth and weight of sclerotia at P ≤ 0.001 and P=0.023 respectively. The best growth at which the best weight of sclerotia was obtained when the plates were incubated at 30°C where the mean radial growth was 8.667 cm and the weight of sclerotia was 0.110 g ⁄ plate after 6 days of incubation. The lowest weight value of sclerotia was recorded at 20°C (weight= 0.00397 g ⁄plate). The temperature degrees (15°C and 40°C) exhibited no sclerotia at all.
| Temperature (°C) | Radial growth after 6 days (cm) (Mean ± SD) |
Weight of sclerotia after 6 days (g /plate) (Mean ± SD) |
|---|---|---|
| 15 | 1.65 ± 0.01 | Sclerotia not observed |
| 20 | 2.525 ± 0.305 | 0.00397 ± 0.00344 |
| 25 | 4.3 ± 0.265 | 0.0403 ± 0.0238 |
| 30 | 8.667 ± 0.577 | 0.110 ± 0.0799 |
| 35 | 9 ± 0 | 0.0405 ± 0.0351 |
| 40 | 4.611 ± 0.102 | Sclerotia not observed |
| F-value | 336.380* | 3.993** |
SD Standard deviation; *Significant at P = 0.001; **Significant at P = 0.023
Table 2 : Effect of different temperature degrees on radial growth and weight of sclerotia of A. sclerotioniger
Effect of different pH values on the radial growth and weight of sclerotia of A. sclerotioniger: Table 3 represents the effect of different ranges of pH values on the radial growth and weight of sclerotia of A. sclerotioniger. The results indicated that the range of pH values from 4 to 7 induced the mycellial growth and formation of sclerotia. There was a significant difference (P ≤ 0.001) between the means of radial growth and weight of sclerotia according to the effect of different pH values. PH 6 gave the highest weight of sclerotia (0.0545 g ⁄ plate) after 6 days. The lowest radial growth was obtained at PH 8 after 6 days and no sclerotia were formed, while at pH 9 the growth of the tested fungus was not detected.
| pH value | Radial mycelial growth after 6 days (cm) (Mean ± SD) |
Weight of sclerotia after 6 days (g /plate) (Mean ± SD) |
|---|---|---|
| 4 | 9 ± 0 | 0.0529 ± 0.0219 |
| 5 | 8.425 ± 0.479 | 0.0317 ± 0.0137 |
| 6 | 8.05 ± 0.52 | 0.0545 ± 0.0167 |
| 7 | 7.625 ± 0.33 | 0.0348 ± 0.0144 |
| 8 | 2.95 ± 0.58 | 0 ± 0 |
| 9 | 0 ± 0 | 0 ± 0 |
| F-value | 340.449* | 11.794* |
SD Standard deviation; *Significant at P = 0.001
Table 3 : Effect of different pH values on the radial growth and weight of sclerotia of A. sclerotioniger.
Effect of different carbon sources on radial growth and weight of sclerotia of A. sclerotioniger: Carbon was calculated as the number of C atoms present in the used carbon sources. Table 4 showed a significant difference between the carbon sources on the radial growth and weight of sclerotia at P ≤ 0.001. The highest radial growth (9 cm) was recorded after 6 days when the medium containing fructose as sole carbon source was used but the yield of sclerotia was relatively low (0.0107 g ⁄plate). Maltose followed fructose as a suitable carbon source for mycelia growth recorded as 8.79 cm; however, it gave the highest weight of sclerotia (0.0283 g⁄plate) after 6 days. Glucose and sucrose markedly induced the radial growth and weight of sclerotia but to less extent than maltose (8.706 cm, 0.0203 g ⁄plate and 8.16 cm, 0.0218 g ⁄plate respectively). The media containing galactose and lactose were not favorable for growth and production of sclerotia of A. sclerotioniger where both results reported the lowest values.
| Carbon source | Radial growth (cm) (Mean ± SD) |
Weight of sclerotia (g /plate) (Mean ± SD) |
|---|---|---|
| Maltose | 8.793 ± 0.285 | 0.0283 ± 0.0116 |
| Sucrose | 8.16 ± 0.862 | 0.0218 ± 0.0057 |
| lactose | 6.207 ± 0.406 | 0.0045 ± 0.0011 |
| Glucose | 8.706 ± 0.405 | 0.0203 ± 0.016 |
| Fructose | 9 ± 0 | 0.0107 ± 0.00198 |
| Glactose | 4.79 ± 0.732 | 0.0099 ± 0.0027 |
| F-value | 52.425* | 5.674* |
SD Standard deviation; *Significant at P = 0.001
Table 4 : Effect of different carbon sources on radial growth and weight of sclerotia of A. sclerotioniger after 6 days of incubation.
Effect of different nitrogen sources: Nitrogen was calculated as the number of N atoms in used nitrogen sources. From Table 5, it is evident that the effect of tested nitrogen sources on the radial growth and weight of sclerotia of A. sclerotioniger were of statistically significant differences at P ≤ 0.001. The inorganic sources (NaNO3 and KNO3) enhanced the mycelial growth of A. sclerotioniger than (NH4)2SO4 and the organic sources (asparagine, glycine, tryptophan and urea). The radial growth was 9 cm and 8.494 cm when NaNO3 and KNO3 were used as nitrogen sources respectively. On the other hand, the highest sclerotial weight was achieved when using KNO3 as a sole nitrogen source where the mean weight was 0.1012 g ⁄plate after 6 days. Although NaNO3 gave the best radial growth (9 cm), it gave the lowest weight of sclerotia (0.0169 g⁄plate) after 6 days. On the level of organic sources, asparagine recorded the best weight of sclerotia (0.0959 g⁄plate), while urea recorded the lowest weight (0.0263 g ⁄plate).
| Nitrogen source | Radial growth (cm) (Mean ± SD) |
Weight of sclerotia (g /plate) (Mean ± SD) |
|---|---|---|
| Asparagine | 6.636 ± 1.064 | 0.0992 ± 0.0089 |
| Tryptophan | 7.633 ± 0.153 | 0.0746 ± 0.0158 |
| Glycine | 7.031 ± 0.0491 | 0.067 ± 0.013 |
| Urea | 5.136 ± 0.627 | 0.0263 ± 0.0126 |
| (NH4)2SO4 | 6.983 ± 0.265 | 0.0610 ± 0.0084 |
| KNO3 | 8.494 ± 0.206 | 0.101 ± 0.0204 |
| NaNO3 | 9 ± 0 | 0.0170 ± 0.0006 |
| F-value | 20.436* | 15.898* |
SD Standard deviation; *Significant at P = 0.001
Table 5 : Effect of different nitrogen sources on radial growth and weight of sclerotia of A. sclerotioniger after 6 days of incubation.
Effect of carbon nitrogen (C ⁄ N) ratio on radial growth and weight of sclerotia of A. sclerotioniger: Each of C and N were calculated as the number of C and N atoms in each of the used sources. The effect of different C/N ratios (13.3, 16.6, 22.5, 25.5, 27.5, 30, 40 and 43.33 %) on radial growth and weight of sclerotia is illustrated in Table 6. On the media containing different C/N ratios separately the results indicated that no difference was detected in the radial growth affected by different C/N ratios where the mycelial growth was completed (9 cm) after 6 days. This means that there is statistically insignificant in radial growth values in response to different C/N ratios. However, the highest weight of sclerotia was recorded when using C/N ratio of 43.33 followed by C/N ratio 40 and 27.5% where the weights of sclerotia were 0.245, 0.228 and 0.218 g/plate respectively. There was a significant difference between the C/N ratios on the weight of sclerotia at P ≤ 0.001.
| C / N (%) | Radial growth after 6 days (cm) (Mean ± SD) |
Weight of sclerotia after 6 days (g /plate) (Mean ± SD) |
|---|---|---|
| 13.3 | 9 ± 0 | 0.142±0.008 |
| 16.6 | 9 ± 0 | 0.13±0.0078 |
| 22.5 | 9 ± 0 | 0.168±0.0141 |
| 25.5 | 9 ± 0 | 0.167±0.0031 |
| 27.5 | 9 ± 0 | 0.218±0.0019 |
| 30 | 9 ± 0 | 0.166±0.0068 |
| 40 | 9 ± 0 | 0.228±0.0056 |
| 43.33 | 9 ± 0 | 0.245±0.00028 |
| F-value | ______ | 61.048* |
SD Standard deviation; *Significant at P = 0.001
Table 6: Effect of carbon nitrogen (C/N) ratio on radial growth and weight of sclerotia of A. sclerotioniger
All the obtained results in optimizing the culture growth conditions for the sclerotia formation of A. sclerotioniger were in accordance with those of other workers [20] on Aspergillus caelatus where the optimal synnema/ sclerotium formation occurred between 28°C–30°C, pH 6 and 10. Also the same author reported that the mono-saccharides (arabinose, dextrose and xylose) and the disaccharides (cellobiose, melibiose, sucrose and trehalose) stimulated the most synnema/ sclerotium formation in A. caelatus isolate (NRRL 25528). He also stated that serine, threonine, potassium nitrate and sodium nitrate induced most of sclerotium formation in A. caelatus NRRL 25528. But in contrast to our results he found that the maximum production for both synnemata and sclerotia by the A. caelatus isolates NRRL 25528 and NRRL 26119 occurred at C/N ratio of 8.6. On the other hand, other researchers, [26] recorded abundant sclerotium production at 28-32°C temperatures which support maximum growth in A. ochraceus. The sclerotia of A. ochraceus were formed at pH values ranged from 4 to 9 while there was no visible growth at pH 3 which occurred without sclerotia production at pH range of 11 to 13. While the sucrose and glutamic acid containing media as carbon and nitrogen sources exhibited the maximum number of sclerotia. Comparing to our work, earlier study found that the sclerotia were a distinguishing feature of A. sclerotioniger at 25°C [4].
Studies on the alkaloids extracted from sclerotia of A. sclerotioniger
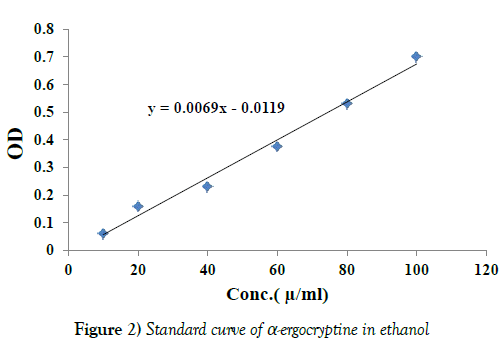
Quantification analysis for total alkaloids content extracted from the tested sclerotia of A. sclerotioniger using standard curve of α-ergocryptine: The standard curve of α-ergocryptine was designated by plotting known concentrations (10, 20, 40, 60, 80 and 100 μ/ml) of α-ergocryptine in ethanol against their OD at wavelength 580 nm. From Table 7 and Figure 2 it is evident that the OD was markedly increased as the concentration of α-ergocryptine increased reaching 0.7 at 100 (μ/ml) concentrations.
| Conc. of a-ergocryptine (µ/ml) | OD |
|---|---|
| 10 | 0.06 |
| 20 | 0.16 |
| 40 | 0.23 |
| 60 | 0.375 |
| 80 | 0.53 |
| 100 | 0.7 |
Table 7: OD of different concentrations of a-ergocryptine in ethanol at 580 nm
The equation indicating the correlation between the values of concentration and OD was obtained from Microsoft Excel 2010 program and represented in Figure 2. The equation was:
Y=0.0069 X-0.0119
Where Y: OD and X: concentration.
Accordingly, the unknown concentration of total alkaloids of the extraction from 7.5 mg of sclerotia of A. sclerotioniger was 96.85 so, the total alkaloids resulted from one mg sclerotia was calculated to be 12.913 μ/mg.
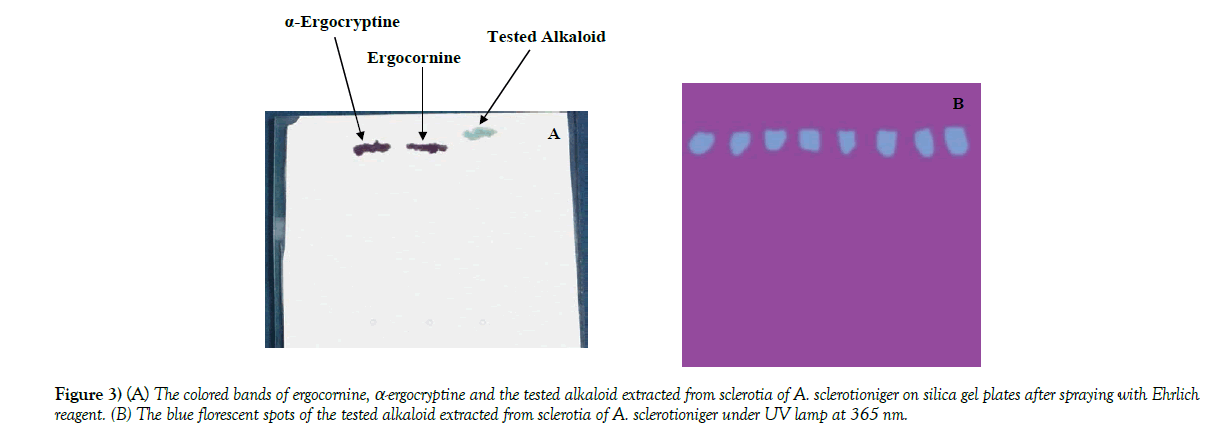
Identification of extracted alkaloid: When spraying with Ehrlich reagent the extracted alkaloid from sclerotia of A. sclerotioniger was detected using the silica gel plates as blue band while the standard alkaloids (ergocornine and α-ergocryptine) revealed violet color bands (Figure 3A). In the meantime, the extracted alkaloid was also detected as blue florescent spots on silica gel plates when tested with UV irradiation at 365 nm (Figure 3B). The blue color of tested alkaloid indicated that this alkaloid belongs to indole alkaloids as mentioned by [27]. So that the chemical structure of the tested alkaloid was expected to contain the following indole ring:
Figure 3) (A) The colored bands of ergocornine, α-ergocryptine and the tested alkaloid extracted from sclerotia of A. sclerotioniger on silica gel plates after spraying with Ehrlich
reagent. (B) The blue florescent spots of the tested alkaloid extracted from sclerotia of A. sclerotioniger under UV lamp at 365 nm.
The Rf is defined as the distance reached by the compound divided by the distance reached by the solvent system. For the tested alkaloid Rf was found to be 0.976 while Rf of ergocornine and α-ergocryptine were 0.696 and 0.72 respectively.
The above results indicated that the extracted alkaloid belongs to a group of alkaloids named indole alkaloids. Many reports revealed that sclerotia of some species of Aspergillus section nigri produce indole alkaloids. The black fungus, Aspergillus indologenus was isolated from a soil in India which produced the insecticidal compounds okaramins A, B, H, and two types of indol-alkaloids which have not been elucidated [28]. The same author also reported that A. violaceofuscus was found to be related to A. japonicus, and produced some of the same interesting indol-alkaloids as A. indologenus. While, A. sclerotii carbonarius which is a biseriate species similar to A. carbonarius and A. ibericus, produces abundant sclerotia and some unique indol-alkaloids [29].
The UV spectrum analysis of the tested alkaloid sample was found between 276 and 283 nm. Also, the percentages of carbon, hydrogen, nitrogen and chlorine were found to be 76.47, 10.24, 2.67 and 15.61 % respectively.
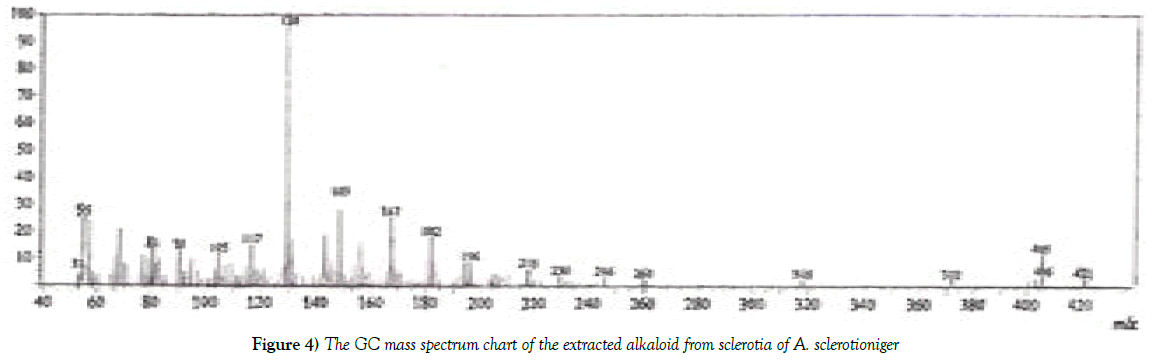
The molecular weight of the unknown extracted alkaloid was recorded using GC mass spectrum analysis. The molecular weight of the tested alkaloid was found to be 421 (Figure 4).
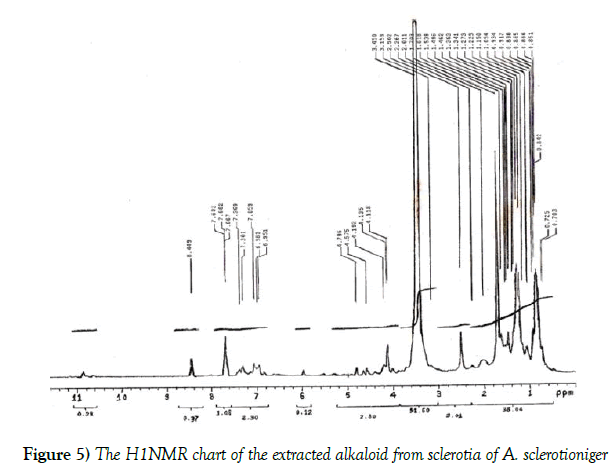
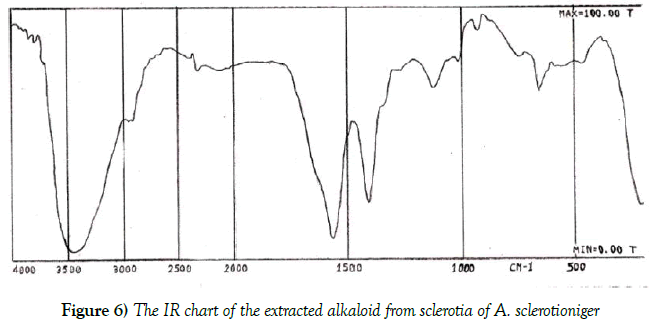
Figures 5 and 6 illustrated the H1NMR and IR analysis charts for the tested alkaloid respectively. These charts indicated the presence of OCH3, CH2, CH3, OH, C=O, NH or NH2 groups and aromatic system in its structure.
From all the previously mentioned analysis it was obvious that the extracted alkaloid from the sclerotia of A. sclerotioniger may be predicted as a chloro derivative from Aflavinine compound. The chemical structure of Aflavinine could be designed as follow:
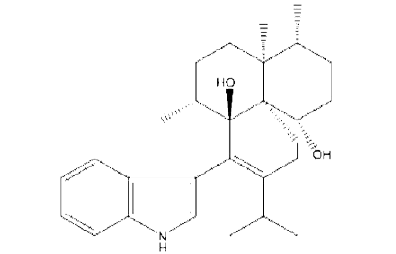
This finding was supported by earlier study [13] who studied different species of A. niger group and stated that all the tested strains of the black aspergilli produced a large number of known and as yet unknown extrolites. Many of these extrolites belonged to aflavinines. They reported that A. costaricaensis and A. piperis produce 14-epi-14-hydroxy 10,23-dihydro-24,25 dehydroaflavinine and 10,23-dihydro-24,25-dehydroflavinine. Also, [30] found that A. tubingensis produced:
• 14-epi-14-hydroxy-10,23dihydro-24,25-dehydroaflavinine,
• 10,23-dihy-dro24,25 dehydroaflavinine and
• 10,23-dihydro-24,25dehydro-21-oxo-aflavinine.
Conclusion
From the present study it could be concluded that, the best radial growth and weight of sclerotia of A. sclerotioniger were obtained when the pH of used CZA medium was adjusted at pH 6 while the best incubation temperature was 30°C. Maltose and KNO3 recorded the best radial growth and weight of sclerotia of A. sclerotioniger when used as carbon and nitrogen sources in CZA medium at C/N ratio 43.33%. The resulted sclerotia of A. sclerotioniger were tested for the production and identification of alkaloids where the quantity of total alkaloids content resulted was equals 12.913 μ/ml. The blue color of tested alkaloid on TLC when sprayed with Ehrlich reagent indicated that this alkaloid belongs to indole alkaloids. Also, according to the CHN percentage, UV spectrum, GC mass spectrum, H1NMR and IR analyses, the extracted alkaloid from the sclerotia of A. sclerotioniger was predicted to be a chloro derivative of aflavinine.
REFERENCES
- Gloer JB. Antiinsectan aflavinine derivatives from the sclerotia of Aspergillus flavus. J Org Chem. 1988;53(23):5457-60.
- Wicklow DT. Sclerotial metabolites of Aspergillus flavus toxic to a detritivorous maize insect (Carpophilus hemipterus, Nitidulidae). Trans Br Mycol Soc. 1988;91(3):433-38.
- Willetts H, Bullock S. Developmental biology of sclerotia. Mycol Res. 1992;96(10):801-16.
- Samson RA. Diagnostic tools to identify black aspergilli. Studi Mycol. 2007;59:129-45.
- Schardl CL, Panaccione DG, Tudzynski P. Ergot alkaloids–biology and molecular biology. The alkaloids: Chem Biol. 2006;63:45-86.
- Flieger M, Wurst M, Shelby R. Ergot alkaloids—sources, structures and analytical methods. Folia microbiologica. 1997;42(1):3-30.
- Floss HG. Biosynthesis of ergot alkaloids and related compounds. Tetrahedron. 1976;32(8):873-912.
- Wicklow DT, Cole RJ. Tremorgenic indole metabolites and aflatoxins in sclerotia of Aspergillus flavus: an evolutionary perspective. Can J Bot. 1982;60(5):525-8.
- de Guzman FS. New diketopiperazine metabolites from the sclerotia of Aspergillus ochraceus. J Nat Prod. 1992;55(7):931-9.
- de Guzman F. Variecolactol: a new sesterterpene lactone from the sclerotia of Aspergillus auricomus (Gueguen) Saito. Sci Diliman. 2007;11(1).
- TePaske MR. Tubingensin A: an antiviral carbazole alkaloid from the sclerotia of Aspergillus tubingensis. The J Org Chem. 1989;54(20):4743-6.
- Laurito A, Doyungan S. Insecticidal activity of crude sclerotial and mycelial extracts from Aspergillus Niger. Philippine Scientist (Philippines).2001.
- Samson RA. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud Mycol. 2004;50: 45-61.
- McIlvaine T. A buffer solution for colorimetric comparison. J Biol Chem. 1921;49(1):183-6.
- Holmes W. Boric acid borex buffer. Anat Record. 1943;86:163.
- Pažoutová S. Chemoraces and habitat specialization ofClaviceps purpurea populations. Appl Environ Microbiol. 2000;66(12):5419-25.
- Blaney B. Alkaloids of the sorghum ergot pathogen (Claviceps africana): assay methods for grain and feed and variation between sclerotia/sphacelia. Aust J Agric Res. 2003;54(2):167-75.
- Lund F. Differentiating Penicillium species by detection of indole metabolites using a filter paper method. Letters in Appl Microbiol. 1995;20(4):228-31.
- Coung N, Dohroo N. Morphological, cultural and physiological studies on Sclerotinia sclerotiorum causing stalk root of cauliflower. Omonrice. 2006;14:71-7.
- McAlpin CE. Synnema and sclerotium production in Aspergillus caelatus and the influence of substrate composition on their development in selected strains. Mycologia. 2004;96(5):937-47.
- Younis M, Mehmood K. Effect of carbon, nitrogen sources and ascorbic acid on the colony growthand acervulus production of Pestalotia psydii. Int J Agr Biol (Pakistan). 2004.
- Mustafa U, Kaur G. Effects of carbon and nitrogen sources and ratio on the germination, growth and sporulation characteristics of Metarhizium anisopliae and Beauveria bassiana isolates. Afr J Agric Res 2009;4(10):922-30.
- Ryan S. An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzyme Microb Technol. 2003;33(6):766-74.
- Chen YM, Lu SI. A new and simplified method for the determination of ergot alkaloids. World J Microbiol Biotechnol. 1986;2:493-8.
- Ghosh AK, Bhattacharya S. Planar chromatographic studies on Abies webbiana leaves. Int J Chem Tech Res. 2009;1:807-14.
- Paster N, Chet I. Effects of environmental factors on growth and sclerotium formation in Aspergillus ochraceus. Can J Bot. 1980;58(16):1844-50.
- Tyler V, Groeger D, Investigation of the alkaloids of amanita species1–II. Amanita. Citrina and Amanita porphyria. Planta medica. 1964;12(04):397-402.
- János Varga FK, Hamari BTZ, Téren JJ. Genotypic and phenotypic variability among black aspergilli. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. 2000;pp:397.
- Noonim P. Two novel species of Aspergillus section Nigri from Thai coffee beans. Int J Syst Evol Microbiol. 2008;58(7):1727-34.
- TePaske MR. Three new aflavinines from the sclerotia of Aspergillus tubingensis. Tetrahedron. 1989;45(16):4961-8.