Synthesis and Characterization of Cellulose acetate /titanium (IV) tungstomolybdate Nanocomposite Cation Exchanger for the Removal of Selected Heavy Metals from Aqueous Solution
2 Instituto de Catálisis y Petroleoquímica, CSIC, Spain, Email: taddesse@haramaya.edu.et
3 B Department of Materials Science and Engineering, National Taiwan University of Science and Technology, Taiwan, Email: diaz@icp.csic.es
Received: 01-Jul-2020 Accepted Date: Jul 13, 2020; Published: 25-Jul-2020
Citation: Minase B, Teju E, Taddesse AM, Diazc I. Synthesis and Characterization of Cellulose acetate /titanium (IV) tungstomolybdate Nanocomposite Cation Exchanger for the Removal of Selected Heavy Metals from Aqueous Solution. J Nanosci Nanomed 2020;4(2):1-10.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Novel Cellulose acetate titanium (IV) tungstomolybdate nanocomposite cation exchanger was synthesized by sol-gel method. Different techniques including FTIR, XRD, TGA SEM and BET were used to characterize the exchanger. The Cellulose acetate titanium (IV) tungstomolybdate (CATTM) behaved as a good cation exchanger with ion exchange capacity of 1.64 meq g-1 for Na+ ions. Thermal analysis of the material showed that the material retained 55 % of its ion exchange capacity up to 600°C. Adsorption behavior of metal ions in different solvents with varying concentration has also been explored and the sorption studies revealed that the material was selective for Cr(III) and Pb(II) ions. The analytical utility of the material was investigated by performing binary separations of selected metal ions in a column based on the distribution coefficients of the metals. Cr(III) and Pb(II) were selectively removed from synthetic mixtures of Cr(III)-Co(II), Cr(III)-Cd(II), Pb(II)-Co(II) and Pb(II)-Cd(II). Antimicrobial activity of the synthesized titanium (IV) tungstomolybdate compound was evaluated and showed a considerable antibacterial activity against Staphylococcus aureus, Streptococcus agalactiae, Escherichia coli and Shigella flexneri. The inorganic counterpart has also exhibited a promising antifungal activity against Aspergillus niger and Fusarium oxysporum.
Keywords
Antimicrobial; Cellulose acetate; Cation exchanger; Nanocomposite; Sol-gel method
Introduction
Heavy metals are unpleasantly affecting our ecosystem due to their toxicological and physiological effects in the environment [1]. If these metals are present beyond a certain concentration it can be a serious health hazard which can lead to many disorders in normal functioning of human beings and animal. Heavy metals are released into the environment from industrial applications, including mining, refining and production of textiles, paints, and dyes [2]. These pollutants greatly threaten the health of human populations and the natural ecosystems even at low concentration as they do not degrade biologically like most of organic pollutants, their presence in drinking water or industrial effluents is a public health problem due to their absorption and therefore possible accumulation in organisms [3]. The toxic heavy metal ions released from industries and automobile exhausts have many adverse effects on both aquatic and terrestrial life [4]. Therefore, heavy metal poisoning due to contamination of, surface water and soil has been a serious concern in many areas of the world [5].
Heavy metals are toxic because these ions are not biodegradable and tend to accumulate in living organisms [6]. Mercury, cobalt, Zinc, copper, nickel, mercury, cadmium, lead, and chromium are concern in the treatment of industrial waste waters [7-8]. The removal of these toxic metal ions from industrial effluents, water supplies and mine waters are a major global challenge in the 21st century. An increased level of lead in blood leads to increase in blood pressure, fertility problems, nerve disorders, muscle and joint pain, irritability and memory or concentration problems. Nickel exceeding its critical level might bring about serious lung and kidney problems aside from gastrointestinal distress, pulmonary fibrosis, and skin dermatitis [9]. It is very difficult for anyone to avoid exposure to any of the many harmful heavy metals that are so prevalent in our environment [10]. So removal of toxic metals from the industrial effluents has special importance from the Eco toxicological point of view it is very difficult for anyone to avoid exposure to any of the many harmful heavy metals that are so prevalent in our environment. Despite rapid development in the detectability of instrumental methods for analysis, a direct determination of trace metal ions in the samples of complex matrices still remains a difficult task because of insufficient sensitivity and selectivity of the methods used and strong interference from the sample matrix [11, 12].
Several methods, such as chemical precipitation, electrolysis, membrane separation, and ion exchange are available to remove toxic metals from aqueous waste streams. Among the heavy metal removal processes, ion exchange process is very effective to remove various heavy metals and can be easily recovered and reused by regeneration operation. Thus, it is probably one of the most attractive processes and, consequently, the one commonly used in industry, because of its simple and efficient application as well as cost effectiveness [13].
Despite their advantages, inorganic ion exchangers are non-reproducible, expensive and are incompetent to treat large volume of waste effluent. On the other hand, the organic ion exchangers have less thermal and radiation stability. Recently, attempts have been made to develop organic–inorganic composite ion exchangers by incorporation of organic monomers in the inorganic matrix to obtain materials of extra ordinary electrical, magnetic, ion exchange and optical properties, which arise from the synergism between the properties of the organic – inorganic components. The combination of organic and inorganic precursors yields hybrid materials that have mechanical properties not present in the pure materials [14].
The organic group can be reactive which implies that it is able to form an organic network as well as inorganic network [15,16]. Recently, several polymers based composite ion exchange materials have been synthesized in recent years synthesized a new inorganic exchanger, nano-titanium(IV) tungstomolybdate with promising separation potential towards metal ions such as Pb (IV), and Cr(III) and a redionucleotide UO22+ (uranyl ions) from a given mixture of toxic heavy metal ions These findings inspired us to work more on novel exchanger’s particularly hybrid exchangers. To the best of our knowledge No work has been done so far by supporting the inorganic exchanger reported previously with organic matrices such as polyaniline, cellulose acetate, and others [17, 18]. The present work therefore aimed at evaluating the ion exchange and antimicrobial activity of new exchange cellulose acetate tin (IV) tungstomolybdate ion exchanger. Accordingly, the latest development in this field is potential applications of these nanomaterials for ion exchange applications [10,19,20].
In the present study, cellulose acetate Ti (IV)tungstomolybdate has been produced via Sol gel method. The as-synthesized material has been characterized by X-ray diffraction (XRD), Scanning electron microscope SEM), infrared spectroscopy (FTIR), Brunauer – Emmett – Teller (BET) and Termogravmetric Analysis (TGA) Methods and the ion exchange property of the material has been studied via batch equilibration technique. The nanocomposites demonstrated considerably high affinity towards analytically important metal ions such as Cr(III) and Pb(II) in aqueous solution and it is good candidate for antibacterial Activity. [4, 17]
Experimental procedures
Preparation of Solution of Cellulose acetate
Solution of cellulose acetate was prepared in concentrated formic acid [16, 18]. This was done by dissolving cellulose acetate in concentrated formic acid (2g of cellulose acetate powder was dissolved in concentrated formic acid until gel like solution was formed).
Preparation of Reagents
Solution of 0.1M sodium tungstatedehydrate (Na2WO4.2H2O) and 0.1M sodium molybdate dehydrate (Na2MoO4.2H2O) solution was prepared in demineralized water (DMW) [13].And 0.1M solution of ti (IV) isopropoxide (C12H28O4Ti) was prepared in ethanol, HCl and DMW [19].
Preparation of Solution of Cellulose acetate
Solution of cellulose acetate was prepared in concentrated formic acid [16,20]. This was done by dissolving cellulose acetate in concentrated formic acid (2 g of cellulose acetate powder was dissolved in concentrated formic acid until gel like solution was formed).
Synthesis of Titanium (IV) Tungstomolybdate
The titanium precursor was prepared using sol-gel method as described by with certain modification to suit our purpose. Accordingly, 6ml titanium (IV) isopropoxide (TTIP) was dissolved in 50 mL absolute ethanol and stirred for 30 min [19]. A mixture of 2ml distilled water and 0.5 mL hydrochloric acid was added to the solution drop wise with constant stirring. A homogeneous solution was obtained after stirring vigorously for 2h. Then sol was formed and after aging for 24 h, the sol was transformed into gel. Upon the TiO2 precursor formed, 0.1M sodium molybdate and 0.1M sodium tungstate were added, and a homogeneous solution was obtained after stirring vigorously for 2h. After washing the sample twice with doubly distilled water to remove traces of chloride ions, and then dried at 50°C for 24h. The powder obtained was immersed into 1M nitric acid for 24h with gentle stirring to transform the ion exchanger into its hydrogenated form. To determine the chemical and physical properties of the prepared samples, the one which showed the largest IEC for Na+ ion was selected and characterized using physical and chemical characterization techniques [13].
Synthesis of Cellulose Acetate Titanium (IV) Tungstomolybdate Nanocomposite Cation Exchanger
In order to get a stable product with good ion exchange properties, a number of samples of cellulose acetate-Ti (IV) tungstomolybdate were synthesized by mixing a mixture of the aqueous solutions of 0.1M sodium tungstate and 0.1M sodium molybdate into the gel of Ti (IV) tungstomolybdate solution gradually with continuous shaking of the mixture in varying mixing ratios. The pH variation was adjusted by adding 1M nitric acid or 1M ammonia solutions to maintain the desired pH. The gel of cellulose acetate which was prepared in concentrated formic acid was added into the inorganic precipitate of Ti (IV) tungstomolybdate and mixed thoroughly with constant stirring for 1h. The gelatinous precipitate so formed, could stand for 24 h in the mother liquor for digestion [20,21]. The supernatant liquid was removed and the precipitate was washed with demineralized water several times to remove excess reagents. The product was dried at 50°C in an oven. The dried product was then kept in demineralized water for cracking and to obtain the particle of the size of (125 μm). then converted to H+ form by placing it in 1M HNO3 solution and was washed with demineralized water to remove excess acid and finally dried at 50°C. Hence, several samples were prepared, and based on ion exchange capacity and percentage yield, the proper sample was selected for detailed studies [22].
Evaluation and Chemical Characterization of the Synthesized Cellulose Acetate Titanium (IV) Tungstomolybdate Ion Exchanger
Ion-Exchange Capacity (IEC)
The ion exchange capacity, which is generally a measure of the hydrogen ion liberated by neutral salt to flow through the composite cation exchanger was determined by standard column process. 1.0 g (dry mass) of the composite ion exchange material in H+ form was placed in a glass column with a glass wool support at the bottom. It was washed with demineralized water to remove any excess of acid remained sticking on the particles. The hydrogen ions were then eluted with 0.1 M solution of different alkali and alkaline earth salts. The collected effluent was titrated against a standard solution of sodium hydroxide using phenolphthalein as an indicator. The hydrogen ions released was then calculated.
IEC = VNaOH CNaOH/W
where VNaOH, CNaOH and W are the volume of NaOH in milliliters, the concentration of NaOH in milli equivalents per milliliter and the weight of the dry exchanger sample in gram, respectively [4].
Thermal Effect on Ion Exchange Capacity
To determine the effect of heating temperature on ion exchange capacity of the material, 1.0 g sample of the cellulose acetate titanium (IV) tungstomlybdate in H+ form was heated at different temperatures in a muffle furnace for 1h and Na+ ion exchange capacity was determined after cooling them at room temperature by standard column process as described above.
pH-Titration
pH - titration studies of cellulose acetate Ti (IV) tungstomolybdate composite cation-exchanger was performed by method. In this procedure a total of 500 mg portions of the cation-exchanger in the H+ form was placed in each of several 250 mL conical flasks, followed by the addition of equimolar solutions of alkali metal chlorides and their hydroxides (NaCl- NaOH) in different volume ratios, the final volume being 50 mL to maintain the ionic strength constant. The pH of the solution was recorded every 24 h until equilibrium was attained which needed 5 days [23].
Chemical Stability
Each of 0.4 g portions of the composite cation exchange cellulose acetate Ti (IV) tungstomolybdate) in H+ form was treated separately with 50 mL of 0.1 M each of different reagent solutions such as: HNO3, H2SO4, NaOH, KOH and acetone, and finally with demineralized water (DMW) for 24 h with occasional shaking. After removal of excess reagent (liquid) the composite was dried in oven at 50°C.The ion exchange capacity of the remaining material was determined by the batch method [4, 24, 25].
Distribution Studies
To get the idea of partition behaviour of the exchanger towards the separation of metal ions of analytical interest, distribution coefficients (Kd) was determined in several solvents systems. A 0.4 g exchanger in H+ form was treated with 40 mL solution of metal ions in required solvent medium in a 100 mL Erlenmeyer flask. The mixture was agitated for 6 h at 250C in a shaker. The amount of metal ions before and after adsorption was determined by titration against a standard solution of 0.01 M di-sodium salt of EDTA. The Kd values were expressed as follows,
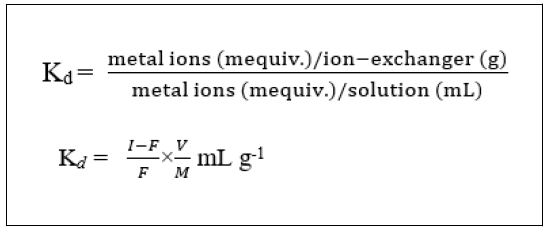
Where I is the initial amount of metal ion in the aqueous phase, F the final amount of metal ion in the aqueous phase, V the volume of the solution (mL) and M the amount of cation- exchanger (g) [26, 25].
Quantitative Separation of Metal Ions in Binary Synthetic Mixtures
A quantitative separation of some important metal ions of analytical utility was achieved in a column filled with cellulose acetate ti (IV) tungstomolybdate. 1.5 g of exchanger in H+ form was packed in a glass column of 0.9 cm internal diameter with a glass wool support at the end. The column was washed thoroughly with demineralized water and the mixture of two metal ions, having initial concentration 0.1M of each with different volume ratios, was loaded on it and allowed to pass through the column at a flow rate of 0.20 mL min−1 till the solution level was just above the surface of the material. The column was then rinsed with demineralized water so that the metal ions, which were not exchanged, could be removed. Individual metal ions adsorbed on the exchanger, was then eluted using the appropriate eluting reagents. The flow rate of the eluent was maintained at 0.5 mL min−1 throughout the elution process. The effluent was collected in 10 mL fractions and was titrated against the standard solution of 0.01 M di-sodium salt of EDTA [13].
Anti-microbial Studies
The organic polymer, inorganic, and composite exchangers were tested for their effect on selected microbes using the paper disc diffusion method. Antibacterial studies were conducted against four important bacteria (Escherichia coli and salmonella typhi from Gram-negative) and, staphylococcus aureus and streptococcus from Gram-positive) and two fungal species Aspergillus niger and Fusarium where they are using nutrient agar medium. Chloramphenicol, standard antibiotic drug, was used as reference in the ant bactericidal studies and Tilt was the standard antibiotic drug used as reference in antifungal studies. The effectiveness of the compounds was determined by measuring the diameter of inhibition zones [27].
Preparation of the Media
Bacteria E. coli and Salmonella type, Streptococcus and Staphylococcus aureus and fungi Aspergillius niger and Fusarium were transferred from the culture and then streaked on Mueler Hinton agar (MHA) plate and incubated for 24 h at 370C. The bacteria were transferred to the autoclaved MHA plate and cooled at about 450C in water bath and mixed vigorous by swirling of flasks and fungus using (PDA) medium. The media was transferred to sterilize petri-dishes. Finally, the media containing bacteria and fungus suspension were poured to sterilized plates and used for the antimicrobial tests. In this experiment piece of paper containing as synthesized nanocomposites, inorganic and organic polymer were placed on an agar plate where bacteria and fungi had been placed, and the plate was left to incubate for 24 h at 370C. Then, after, the inhibition zone of bacteria and fungi in each case was measured using ruler. The size of this zone depends on the effectiveness of nanocomposite at stopping the growth of the bacterium. Accordingly, the stronger antibiotic nanocomposite creates a larger zone [28].
Results and Discussion
Synthesis of Titanium (IV) Tungstomolybdate
Titanium (IV) tungstomolybdate was prepared by sol-gel method with titanium isopropoxide as precursor of TiO2. Aqueous solution with constant pH and peptizing the resultant suspension has been applied for preparation of the TiO2 Nano powder with narrow size distribution. The conditions used for the preparation have considerable effect on the degree of hydration and the composition of the exchanger. These two factors are responsible for the shape and size of cavities inside the ion exchanger and for other properties of the exchanger resulting in their unusual ion exchange behavior.
The Mixing Volume ratio of the Precursors
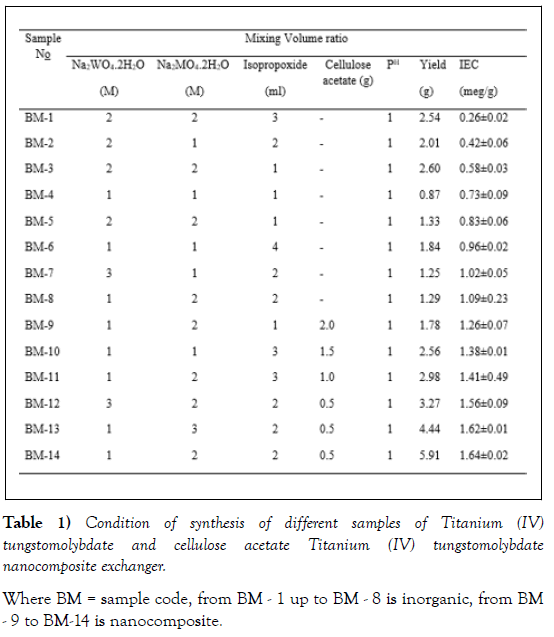
To determine the optimum mixing volume ratio of the precursors, different samples with varying mixing volume ratios were prepared keeping other parameters constant (Table 1). The mixing volume ratio of Nano composite exchangers (2:1:2) in sodium molybdate dehydrate, sodium tungstate dehydrates, and titanium isopropoxide, respectively, resulted in stable and better yield of precipitate with relatively higher IEC (1.64 meq/g) and was selected as an optimum mixing volume ratio for the entire synthesis work.
Synthesis of Cellulose Acetate Titanium (IV) Tungstomolybdate Nano Composite
Cellulose acetate Titanium (IV) tungstomolybdate nanocomposite was synthesized under varying condition by sol-gel mixing of inorganic precipitate titanium (IV) tungstomolybdate and cellulose acetate gel. Cellulose acetate is a commercially available white crystalline powder, and titanium (IV) tungstomolybdate is a light-white precipitate. The final product obtained cellulose acetate titanium (IV) tungstomolybdate, after changed to H+ form, dried and grained was found to be light yellow powder. The physical appearance of the material resembles more the organic resin cellulose acetate polymer with inorganic moiety titanium (IV) tungstomolybdate.
Characterization and Analytical Application of the Material Ion exchange capacity
Ion exchange capacity for sodium ion was a general parameter used as a measure for the ion exchange capacity of cation exchanger materials. The sodium ion exchange capacities of the newly synthesized composite cation exchange material are presented in (Table 1). Among the as-synthesized samples BM-14 possessed relatively good yield (5.91g) and better ion exchange capacity 1.64 meq/g and was selected for further studies.
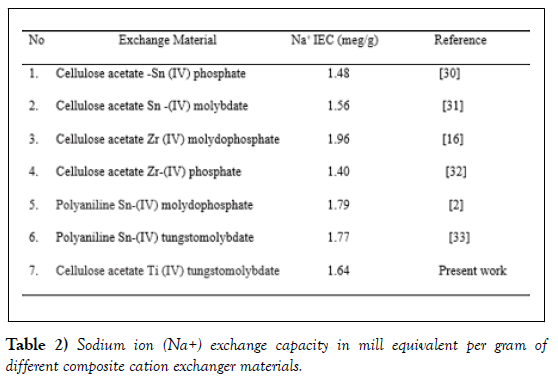
The composite cation-exchange material possessed a better Na+ ionexchange capacity (1.64 meq /g) as compared to inorganic counterpart, Titanium (IV) tungstomlybdate (1.09 meq/g). Cellulose acetate Titanium (IV) tungstomlybdate (BM-14) was found to exhibit comparable ion exchange capacity with composite exchangers where the organic moiety is cellulose acetate. However, better exchange capacity is also observed by composite exchangers where the organic counterpart is cellulose acetate [29]. In general, the as-synthesized exchanger is considered to exhibit moderate exchange capacity (Table 2).
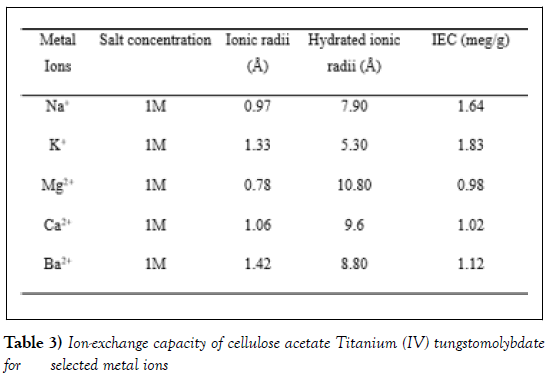
Ion-exchange capacity of the cellulose acetate Titanium (IV) tungstomolybdate (BM-14) for alkali and alkaline earth metal ions are depicted in (Table 3). As can be seen in Table 3, the affinity sequence for alkali metal ions was K+ >Na+ and for alkaline earth metal ions was Ba2+ >Ca2+ >Mg2+, This sequence is in accordance with the hydrated ionic radii. The ion-exchange capacity should increase with decreasing hydrated radii. The ions with large ionic radii have smaller hydrated radii and easily enter the pores of exchanger resulting in higher adsorption [30, 31].
pH Titration Curve
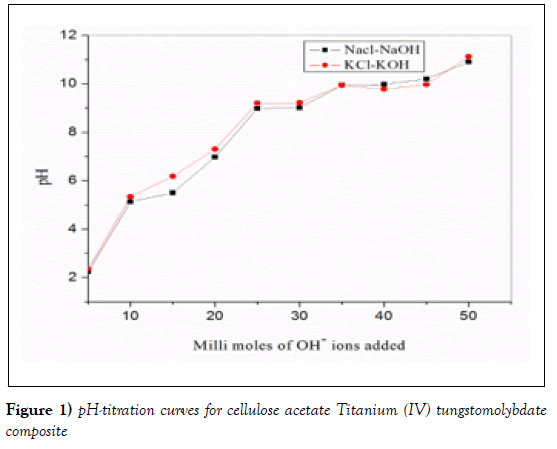
The pH-titration curves obtained under equilibrium conditions for NaOHNaCl and KOH-KCl systems is shown in (Figure.2). The titration curve showed two inflection points [32]. This indicates that cellulose acetate titanium (IV) tungstomolybdate has bi functional nature indicates that the synthesized nanocomposite appears to be strong cation exchanger as indicated by a low pH (~2.25) of the solution when no OH- ion was added. As the volume of NaOH added to the system is increased, more OH ions are consumed suggesting in the increase of the rate of ion exchange in basic medium due to the removal of H+ ions from the external solution, the rate of H+–Na+ exchange was faster than H+–K+ exchangers [33]. This might be due to the displacing capacity of the metal towards H+. As compared to K+, Na + is at the bottom of the activity series, so that the ability of Na+ to displace the H+ is lower than K+ i.e the pH of the solution increase since the hydrogen is kept with Na+ and what is remaining is the OH [34]. -
The pH titration in the presence of cellulose acetate Titanium (IV) tungstomolybdate was performed for NaCl-NaOH and KCl-KOH system. The pH titration curves show that pH increases when NaOH and KOH were 5-15-mmol per 0.2 g of composite cation exchange material and relatively very slow steep increase when hydroxide was added 20-35 mmol per 0.2 g of the composite material. The material is an acid cation exchanger because the pH titration curves usually showed a step edge at 35 mmol for the same mass of the material that is the –H functional group on the hybrid cation exchanger which were depleted and replaced with Na+ and K+ at these points (Figure 1)[35].
Chemical Stability
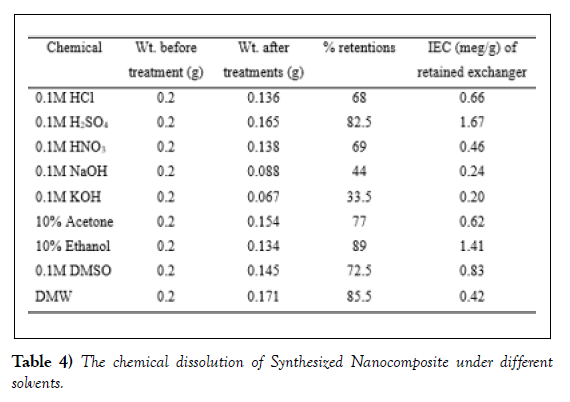
Chemical resistivity/stability of an ion exchanger in acids, bases and organic solvent media is important for its various applications in varied environments. The synthesized cellulose acetate titanium (IV) tungstomolybdate cation exchanger exhibit good chemical stability in some strong acids like, H2SO4 and in some organic solvents. However, it shows low stability in strong base, like NaOH. The values of IEC of cellulose acetate Titanium (IV) tungstomolybdate Nano composite in different solvents using titration of exchanger are given in (Table 4). As synthesized nanocomposite has good chemical stability, as it was resistant to 10% ethanol and 0.1M M H2SO4. This chemical stability may be due to the presence of binding polymer, which can prevent the dissolution of hetero polyacid salt or leaching of any constituent elements into the solution [36].
The composite cation exchanger also exhibited moderate stability towards 0.1 M HCl, 0.1 M HNO3, 0.1 M DMSO and 10% acetone. However, the material shows very high weight loss and extreme decrease in its IEC which shows high dissolution in basic medium. This may be related to the hydrolysis of exchanger beads or the high solubility of exchanger at higher pH.in 0.1M NaOH and 0.1M KOH poor stability of the nanocomposite is observed towards bases [37].
Thermal Studies
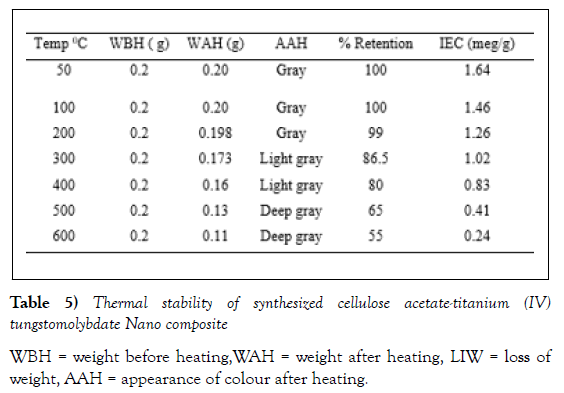
The ion exchange capacity of the synthesized nanocomposite material was changed by heating in different temperatures for 1h. The mass, physical appearance, and ion-exchange capacity of cellulose acetate Titanium (IV) tungstomolybdate was noted. The ion exchange capacity of the material decreased with increased heating temperature. The results revealed that the material is appreciably thermally stable since it retains about 55 % of its ion exchange capacity up to 6000C. Effect of heating temperature on the ion exchange capacity of the material is indicated in (Table 5). The thermal stability of the exchanger is comparable with previous reports such as cellulose acetate zirconium(IV) molybdophosphate that lost 58 % of its ion exchange capacity when heated to 6000C [16], polyaniline titanium (IV) tungstoarsenate which retained about 35 % its initial ion exchange capacity up to 6000C .On the other hand, some literatures revealed organicinorganic composite cation exchangers that possess high thermal stability. For example, acrylamide zirconium (IV) arsenate retains 84% of its ionexchange capacity up to 6000C [26]. Comparison of our finding with literatures stated above implicates the moderate thermal stability of the assynthesized exchanger.
Distribution Studies of Cellulose Acetate Titanium (IV) tungstomolybdate Nanocomposite
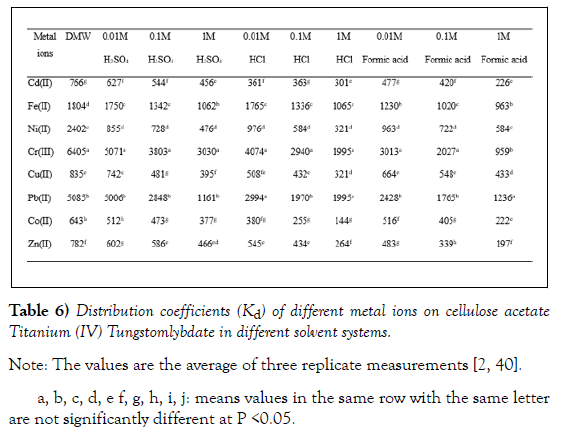
The distribution coefficient is the measure of the affinity of the cation exchanger towards different metal ions. Hence, the ion-exchange properties of the synthesized cation exchanger were studied by measuring the distribution coefficients (Kd) of common metal ions using column experiments in different solvent systems (Table 6). The Kd values of the metal ions were studied as a function of type and concentration of electrolyte solution and the results are presented in (Table 6). Measurement of distribution coefficient (Kd) of metal ions over a wide range of condition is a good way to avoid choosing eluting conditions for column separations by a strictly trial and error method. Although this distribution coefficient is measured on a batch basis, it can be used to predict elution behaviour of metal ions eluted from an ion exchange column. To separate two substances, conditions should be selected such that the distribution coefficient of one of them is low so that its elution from the column will be rapid, while the distribution coefficient of the other substance under the same conditions should be as large as possible so that this substance will be tightly held by the resin [32].
Table 6: Distribution coefficients (Kd) of different metal ions on cellulose acetate Titanium (IV) Tungstomlybdate in different solvent systems.
Note: The values are the average of three replicate measurements [2, 40].
a, b, c, d, e f, g, h, i, j: means values in the same row with the same letter are not significantly different at P <0.05.
In this work the distribution coefficient (Kd) values in different solvent systems were evaluated for eight metal ions towards cellulose acetate titanium (IV) tungstomolybdate by the batch equilibration method. The Kd values of the metal ions were studied as a function of type and concentration of electrolyte solution and the results are presented in (Table 6). Effect of different concentrations of electrolyte on metal ion uptake by the synthesized cation exchange material was studied. In general, the Kd values are lower in high concentration of electrolyte and vice versa. It was observed that Kd values decreases with increase in the concentration of all acids. This may be due to slower release of H+ ions from the exchange in strongly acidic medium so less adsorption of metal ions take place by the exchanger. Further, the Kd values in strong electrolyte media are lower as compared to weak electrolyte media as well as aqueous medium. This may be attributed to high competition amongst ions for exchange in strong electrolyte media. Kd values are higher for all metal ions in DMW than in electrolyte media. Smaller the size of the cation, greater is the tendency to be hydrated and greater the hydrated ionic radii. Larger ions being less hydrated, less energy is utilized for dehydration of the metal ions to occupy a site on the exchanger, which plays a prominent role in determining the selectivity of metal ions [24].
The promising feature of the synthesized composite material is its selectivity for Cr(III) (Kd= 6405 mLg-1) and Pb(II) (Kd= 5098 mLg-1) ions, which are the most toxic and polluting metal ions in the environment. The high selectivity of Pb(II) as compared with other metal ions is also observed in other tetravalent metal acid (TMA) salts based composites, vis. polyaniline tin(IV) molydophosphate (8509), Poly(methyl methacrylate) zirconium(IV) phosphate (5500), acrylamide zirconium(IV) arsenate retains (650) and Polyo- anisidine tin(IV) arsenophosphate (400), values in parenthesis are distribution coefficients (Kd) of the respective ion exchange materials in mLg−1. The high affinity of Cr(III) and Pb(II) towards cellulose acetate titanium(IV) tungstomolybdate in the present study suggests its possibility of separating these ions from other pollutants[2, 12, 26, 38, 39].
Separation of Metals Ions in Binary Mixtures
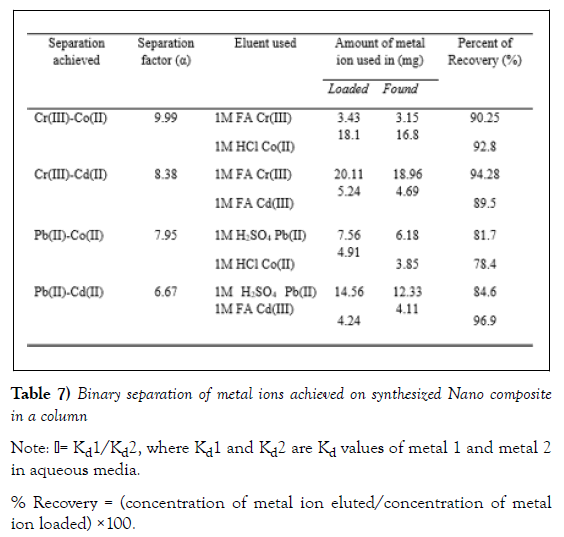
The separation capability of the material has been demonstrated by undertaking several binary separations of some important metal ions. Obviously, to achieve more clear separation of metal ions, large Δ Kd values should be selected on the same experimental conditions. The sequential elution of ions through column depends upon the metal–ligand stability. The weakly retained metal ions eluted first and strongly retained at last [16]. The study of the distribution behaviour of metal ions in aqueous as well as in various electrolyte media gives an indication of the possible binary metal ions separations as well as the eluents that could be used for separation. Kd values suggest the possibilities for many important binary separations. Based on separation factor α, binary separations have been carried out for four sets of metal ions: Cr(II)-Co(II), Cr(III)-Cd(II), Pb(II)- Co(II) and Pb(II)-Cd(II). The separation factors are guiding measures for separations. The details of these separation studies are presented in (Table 7).
To elute Cr(III) and Pb(II), 1M formic acid and 1M H2SO4 respectively was preferably used. This is since low Kd values of these metals in1M formic acid and 1M H2SO4 enables quantitative elution. While for Cd(II) ,1M of Formic acid was used in Pb(II)- Cd(II) system and1M H2SO4 in Pd(II)- Cd(II) system, 1M HCl was employed to elute Co(II) from Cr(III)- Co(II) system and 1M H2SO4 was used to elute it from Pb(II)- Co(II) mixture. All selections of solvents were done based on the Kd values of both metal ions in the solvent. Cellulose acetate titanium (IV) tungstomolybdate exhibits a78.4%- 96.9% separation efficiency for these metal ions.[11]
Physical Characterization of the Nanocomposite Ion Exchanger
FTIR analysis
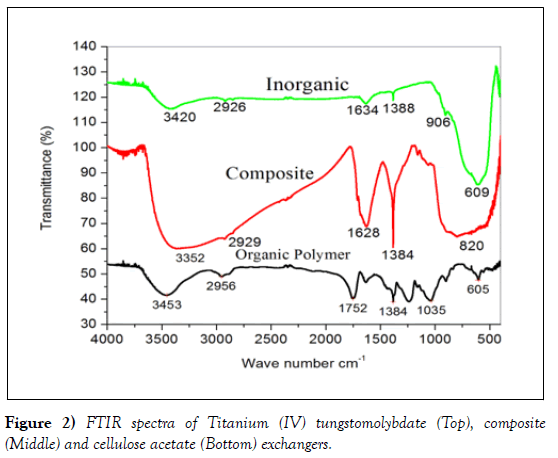
The FTIR spectra of the synthesized cation exchanger Ti(IV) tungstomolybdate are presented in (Figure 3). A broad band in the region 3600-2800 cm-1 is due to the O-H stretching vibration of the water bonded to TiO2. A sharp peak around 1634cm-1corresponds to the deformation/ bending vibration of water molecules (H-O-H). A band at around 1388cm-1 is also described to the presence of water bonded with TiO2. Finally, from the IR analysis it can be deduced that bands at around 906 cm-1and 609 cm-1 and may be due to the presence of Ti-O, W-O and Mo-O stretching vibration mode, respectively.
The FT-IR spectra of the synthesized nanocomposite exhibits the following absorption peaks. A strong and broad peak at 3352 cm-1 is attributed to – OH bond stretching vibration of water molecule, the peak at 2929 cm−1 is could be due to C−H stretch modes of the substituent methyl group. A peak at 1628 cm-1 may be due to deformation vibration of free water molecule, the absorption band around at 1735 cm-1 may be assigned to the C=O stretching of ester group in cellulose acetate, the peak depicted at 1384 cm-1 may be due to -CH bands [41]. A broad peak at around 820 cm-1 is due to the presence of molydate group and an assembly of sharp peaks in the region 500–550 cm-1 is due to the superposition of metal–oxygen stretching vibrations which shows the binding of inorganic precipitate with organic polymer and formation of ‘organic–inorganic’ hybrid. An assembly of bands in the region 400 – 800 cm-1 was due to the metal – oxygen stretching vibration.
Cellulose acetate spectrum shows a band at 1384 cm-1 can be assigned to stretching vibration of carbonyl group of cellulose acetate [42]. The peak ~964 cm-1 may be usually assigned to an out of plane bending vibration of C-H, bond of cellulose acetate. Also, at 1752 cm-1 which are indicated as ester group, and a peak at 1035 cm-1 indicating the presence of acetyl =CO. The presence of acetyl C-O indicates that the OH group in cellulose molecules has been substitute by =CO group producing the cellulose acetate and the peak range from 906cm-1 -604 cm-1 for the metal-oxygen bond and sharp peak at 1638 cm-1 corresponds to the deformation vibration of free water molecule in the organic polymer (Figure 2) [43].
XRD analysis
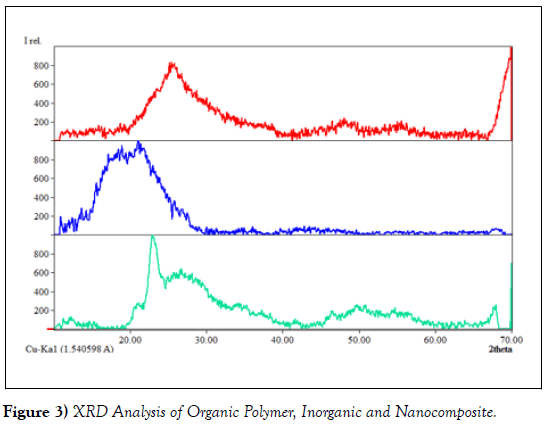
The XRD studies were conducted on the organic polymers (cellulose acetate), and accordingly, diffraction peaks observed at scattering angle 2θ of the data are illustrated in (Figure 3). The XRD diffractogram showed peaks at 2θ equals to 17.05o, 18.22 o,18.87 o representing a typical cellulose acetate having semi crystalline nature [44]. The amorphous nature was observed in the powder XRD of Titanium (IV) tungstomolybdate and diffraction peaks belong to anatase phase of TiO2. However, the presence of some sharp intensity peaks at 2θ values 23.24o and 24.33o indicates the formation of some crystal at very small scale up on formation of nanocomposite structure [2]. The X-ray diffraction pattern of Nano composite showed a few weak intensity peaks on a broad background suggesting the semi crystalline or amorphous nature of the composite. The strong peaks at 2θ values 25.71o and 26.65° indicates the formation of some crystal at very small scale up on composite formation [11, 45].
The average crystallite size of each of the cellulose acetate (organic polymer), titanium (IV) tungstomolybdate, and cellulose acetate titanium (IV) tungstomolybdate nano composite was calculated using the Debye- Scherrer’s formula:
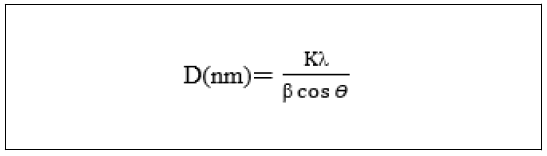
Where, D= crystallite size in nm, K = the shape factor constant taken as 0.9; is the full width at half maximum (FWHM) in radians and is the wave length of the X-ray (0.15406 nm) for Cu target Kα1, whereas cos is radiation and is the Bragg’s angle.
Based on the above approach, we estimated the average crystalline size of assynthesized cation exchangers. And all the as-synthesized materials were found to be in the Nano range that means (organic polymer (24.9nm) titanium(IV) tungstomolybdate (38.8nm and composite was found to be, to 55.41nm)[46].
Thermogravimetric Analysis (TGA)
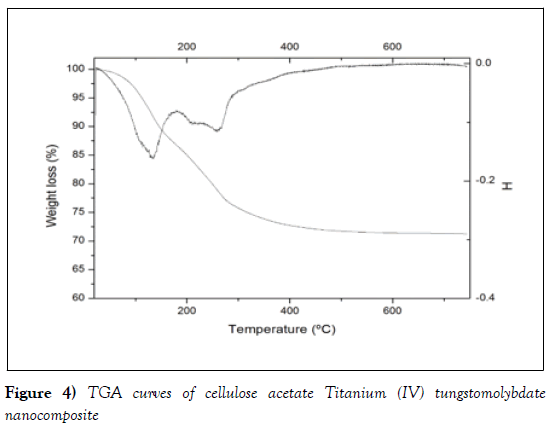
The TGA curve of cellulose acetate titanium (IV) tungstomolybdate nanocomposites is presented in Figure 10. As can be seen in the figure, the sample exhibited three stages of weight losses. The first step occurs between 25°C- 100°C, shows a continuous weight loss of mass may be due to loss external water molecule present. The second step appeared in the temperature range of 100-150°C may be accounted for the evaporation of internal water molecules as the result of condensation of –OH groups from metal oxide [41]. The third step appeared in the temperature range of 150°C-400°C may be due to complete decomposition of the organic polymer part of the material. After these range on wards, a smooth horizontal section shows the complete formation of oxide form of the material (Figure 4) [42-45].
Surface area
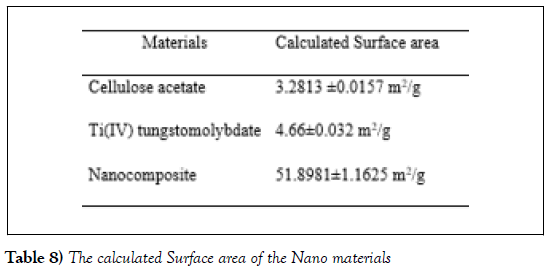
Specific surface areas of the nanocomposites were determined using Brunauer–Emmett– Teller (BET) methods. The average specific surface area of cellulose acetate, Titanium (IV) tungstomolybdate and the nanocomposite cation exchanger cellulose acetate Titanium (IV) tungstomolybdate is indicated in (Table 8). Nanomaterials with high surface activity, high specific surface area and high surface energy, show promising potential in the preparation of high-performance ion exchange and thus are widely used as ion exchanger. The nanocomposite shows highest surface area compared with organic polymer and Titanium (IV) tungstomolybdate. The difference in their surface area was due to the synergetic effect among the components such as titanium, molybdate and tungstate in the composite system [6]. The increase in the surface area was significant; hence, the larger surface area of nanocomposite will benefit for the spatial separation of redox sites in the crystals which can enhance electron-transfer and ion exchange properties of the nanocomposite [17].
SEM-EDX analysis
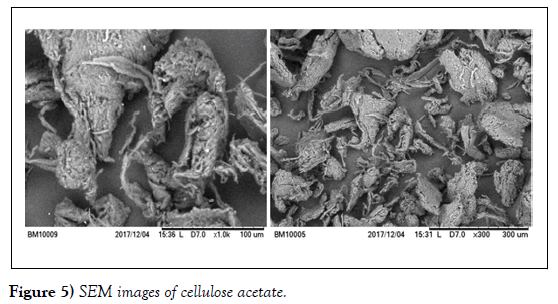
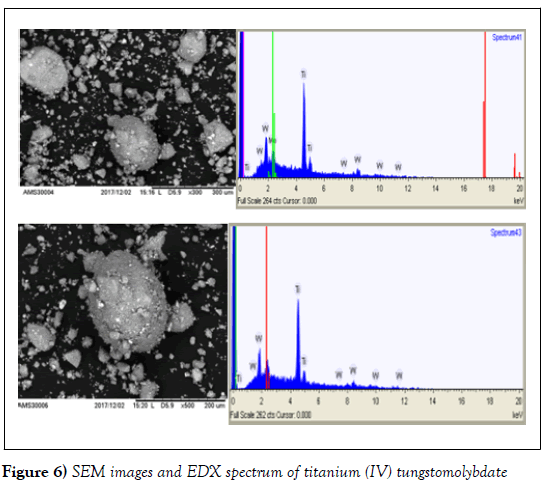
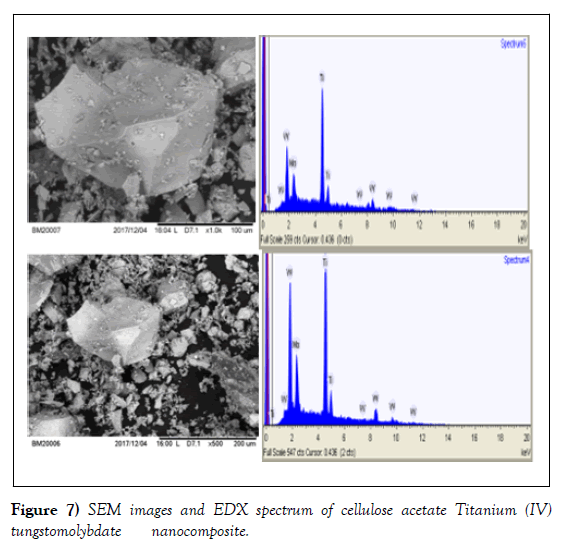
The SEM images of the organic polymer, the as-synthesized inorganic and nanocomposite exchangers with selected magnification are shown in (Figures 5-7). The SEM image of cellulose acetate presents (Figure 5) the inorganic exchanger (Figure 6) the image of inorganic and finally (Figure 7).
The energy dispersive x-ray analysis was done for elemental composition present in the as synthesized inorganic and composite Nano powders. The presence of Ti, Mo and W is confirmed from the EDX spectra (Figures7). The amount of Ti ranged from 45.0 to 62.1%, Mo from 13.8 to 18.6% and W from 24.1 to 36.3%; the average being 55%, 16% and 29% respectively. The wider range of composition exhibited by Ti and W indicates the heterogeneity of the exchanger. The ratio Ti: Mo:W. as inferred from the EDX is found to be (3:1:2). In the inorganic exchanger, the presence of Ti, Mo and W is confirmed from its EDX spectral analysis (Figure 7); the amount of Ti ranged from 65.5 to74.2%, Mo from 7.2 to 11.9 % and W from 19.8 to 25.8 %, the average being 69.56 %, 9.6% and 22.7% respectively. Based on this information, the ratio of the three elements as inferred from the EDX is found to be (7:1:2) in Ti: Mo:W [13, 22, 25].
Antimicrobial Activities of as Synthesized of Nano Composite
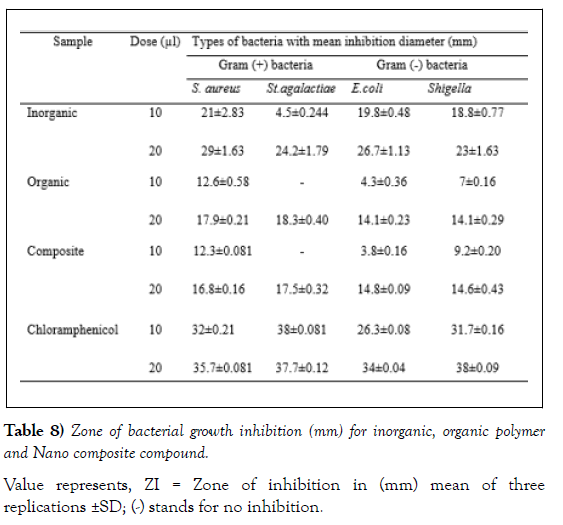
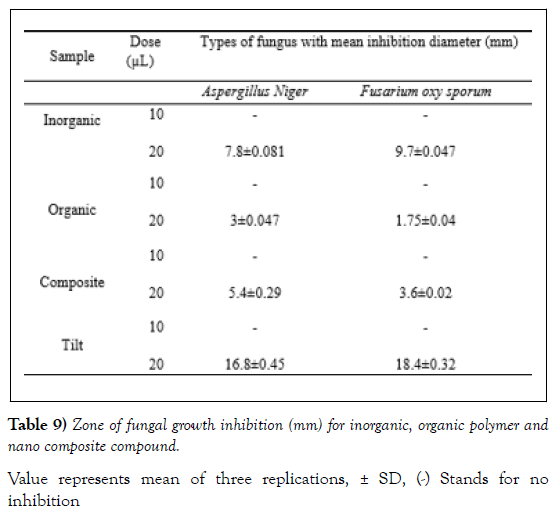
In the present study, organic polymer and the composite were investigated for their respective antimicrobial potential. For this purpose, four bacterial species out of which two are Gram positive (S. aureus and St. agalactiae) and the remaining two are Gram negative (E.coli and Shigella) were selected for the antibacterial study. Similarly, for the antifungal test two fungal species (Aspergillus niger and Fusarium oxysporum) were used. The results of the bioassay tests for the bacterial and fungal species are presented in (Tables 8 and 9), respectively.
Antibacterial Activity
All the three tested groups of chemicals including the inorganic compound, organic polymer and the composite were found to be active against St. aureus, St. agalactiae, E. coil and Shigella at concentrations of 10 and 20 μL. The results of the zones of inhibition for the three chemical compounds against the tested bacterial species are presented in (Figure 8) (Table 7) [46,47].
Value represents, ZI = Zone of inhibition in (mm) mean of three replications ±SD; (-) stands for no inhibition.
Anti-Fungal Activities
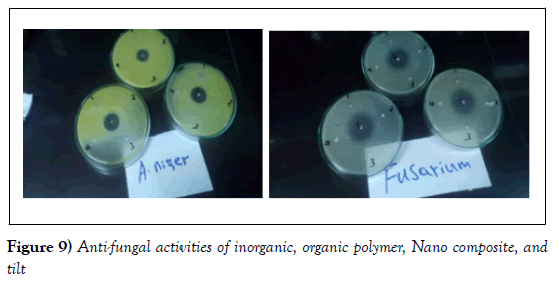
The antifungal activities of the inorganic, organic polymer and the composite were also investigated against two fungal species (Aspergillus niger and Fusarium oxysporum). During this bioassay, a positive result was observed, and the results are presented in (Table 9) and the figures corresponding for this bioassay (Figure 9).
Value represents mean of three replications, ± SD, (-) Stands for no inhibition
Conclusion
A new composite cation exchanger, cellulose acetate titanium (IV) tungstomolybdate, has been synthesized which has high thermal stability unlike the organic resins which have one of the severest limitations of their poor thermal stability. The material was successfully used for the separation of metal ions quantitatively from synthetic aqueous solution. The sodium ion exchange capacity of the material was found to be 1.64 meq/g. From the pH titration curves obtained under equilibrium conditions for NaOHNaCl and KOH KCl systems; it was observed that cellulose acetate titanium (IV) tungstomolybdate has bi functional nature and strong acidic cation exchanger.
The thermal study results revealed that the material is appreciably thermally stable in terms of ion exchange capacity since it retains about 55% of its ion exchange capacity up to 600°C. The selectivity that cellulose acetate titanium (IV) tungstomolybdate of towards different heavy metal ion in demineralized water and other electrolytic solvents with varying concentrations was evaluated using distribution coefficients of the metal ions and it was found that the material is highly selective for Cr(III) and Pb(II) which are important heavy metal. Its analytical utility for the separation of these metal ions from synthetic binary mixtures was assessed based on the separation factor (α) and quantitative separation of metal ions with reasonably high efficiency (68.03%–98.98%) was achieved using the as synthesized composite exchanger.
Antimicrobial activity tests showed some antimicrobial potency of the synthesized Nano composite even though it was very lower as compared to standard anti-biotic organic polymer compound was active against the four microbial pathogens tested. The synthesized inorganic compound showed the highest degree of antibacterial activity against Staphylococcus aureus, Streptococcus agalactiae, Escherichia coli and Shigella flexneri as compared to the organic and nano composite. Additionally the synthesized inorganic compound exhibited higher activity towards both fungal (A. niger and F.oxysporium) than organic polymer and nano composite. From this study, it can be concluded that this inorganic compound is very active than organic polymer and synthesized nano composite. But antimicrobials activities were comparatively lower than that of the standard drug used in the study.
Acknowledgements
ID is grateful to CSIC for her research leave at AAU and HU. The financial support from the CSIC for Development (Project MAT2012-31127), the Research and Extension Office of Haramaya University (HURG-2016-03-03) and Chemistry Department of HU are acknowledged. This work has been also (partially) funded by the Spanish State Research Agency (Agencia Española de Inves- tigación, AEI) and the European Regional Development Fund (Fondo Europeo de Desarrollo Regional, FEDER) through the Project.
REFERENCES
- Lenntech K. Water treatment and air purification. Netherlands. Rotter Dam Seweg. 2004.
- Semagne B. Synthesis, characterization and analytical application of polyaniline tin (IV) molybdophosphate composite with nanocrystalline domains. J Reactive and Functional Polymers. 2016;98:17-23.
- Awual Md. A novel facial composite adsorbent for enhanced copper (II) detection and removal from waste water. J Chemical Engineering . 2015;266:368-375.
- Abd El-Latif MM, Elkady MF. Synthesis, characterization and evaluation of nano-zirconium vanadate ion exchanger by using three different preparation techniques’ Materials Research Bulletin. 2011;46(1):105-118.
- Trotman, Lloyd C. Ubiquitination regulates PTEN nuclear import and tumor suppression Cell. 2007;128(1):141-156.
- Wang, Jiangxue. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. JToxicology. 2008;254(1-2):82-90.
- Yuliani K, Sahat S. The Development of Learning Devices Based Guided Discovery Model to Improve Understanding Concept and Critical Thinking Mathematically Ability of Students at Islamic Junior High School of Medan. J education and practice. 2015;6(24):116-128.
- Parisa S, Mehran J, Saeed P. Novel proton exchange membranes based on proton conductive sulfonated PAMPS/PSSA-TiO2 hybrid nanoparticles and sulfonated poly (ether ether ketone) for PEMFC. J International of Hydrogen Energy. 2019;44(5):3099-3114.
- Fu F, Qi W. Removal of heavy metal ions from wastewaters: a review. J environmental management. 2011;92(3):407-18.
- Bezabih K, Abi M T, Izabel D et al. Nano-crystalline titanium (IV) tungstomolybdate cation exchanger: Synthesis, characterization and ion exchange properties. J environmental chemical engineering. 2017;5(1):1004-14.
- Sudibta S, Prasun K, Chatterjee. Hybrid ion exchanger supported nanocomposites: Sorption and sensing for environmental applications. J Chemical engineering. 2011;166(3):923-31.
- Mahmod T, Arjumand A, Keneth D et al. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral research. 2005;67(2):107-119.
- SudiptaS, Prasunk K, Chatterjee et al. Synthesis, characterization and analytical applications of a new composite cation exchanger cellulose acetate-Zr (IV) molybdophosphate. JColloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;316(1-3):217-25.
- Liming Y, Liya E, Yu. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water research. 2008;42(13):3480-88.
- Babar A, Nasir A, Saiba,S et al. Essential oils used in aromatherapy: A systemic review. J Tropical Biomedicine Asian Pacific. 2015;5(8):601-11.
- Karkare, Manasi Manoj. Choice of precursor not affecting the size of anatase TiO 2 nanoparticles but affecting morphology under broader view. International Nano Letters. 2014;4(3):111.
- Ayoub A, Mu N, Mohammed A et al. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism. J Cleaner Production. 2017;156:426-436.
- Kuljit K, Rajeev J, Riteeka t. Chitosan–gelatintin (IV) tungstatophosphate nanocomposite ion exchanger: synthesis, characterization and applications in environmental remediation. J Polymers and the Environmen. 2019;27(1):19-36.
- Sayed A, Mu. N, Inamuddin. Synthesis and characterization of a new inorganic cation-exchanger—Zr (IV) tungstomolybdate: Analytical applications for metal content determination in real sample and synthetic mixture. J hazardous materiasl. 2007;142(1-2):404-411.
- Topp NE, Pepper KW. Properties of ion-exchange resins in relation to their structure. Part I. Titration curves. J Chemical Society (Resumed). 1949:3299-303.
- Abou-Mesalam M. Sorption kinetics of copper, zinc, cadmium and nickel ions on synthesized silico-antimonate ion exchanger. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2003;225(1-3):85-94.
- ArchanaN, Suresh Ch, Navin Ch. Mechanical and thermal properties of nano-cellulose obtained from sisal fiber reinforced polyvinyl alcohol (PVA) bio-composites. J Sci. Res. Rev. 2012;1(3):40-50.
- Syed A, Nabi, Habid HS. Synthesis, characterization and analytical application of hybrid; acrylamide zirconium (IV) arsenate a cation exchanger, effect of dielectric constant on distribution coefficient of metal ions. J hazardous materials. 2009;163(2-3):657-664.
- Josep S, Lina K, Marta L, et al. Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2012;402(7):2335-45.
- MarcellaM, Marina V, luihi F: a novel inhibitor of lactatedehydrogenase. ChemMedChem. 2012;7(2):311-17.
- Sayed A, Mu N, Inamuddin Nabi. Synthesis and characterization of a new organic–inorganic Pb2+ selective composite cation exchanger acrylonitrile stannic (IV) tungstate and its analytical applications. J Chemical Engineering. 2009;152(1):80-87.
- Bhim Sh, Gaurav SH, Deepaak P et al. Synthesis, characterization and antibacterial activity of cellulose acetate–tin (IV) phosphate nanocomposite. Carbohydrate polymers. 204;103:221-27.
- Vinod K, Shilpi A, inderjeet T et al. Synthesis, characterization and analytical application of cellulose acetate-tin (IV) molybdate nanocomposite ion exchanger: binary separation of heavy metal ions and antimicrobial activity. Ionics. 2015; 21(7):2069-78.
- Vinod K, Shilpi A, inderjeet T et al. Cellulose acetate–zirconium (IV) phosphate nano-composite with enhanced photo-catalytic activity. Carbohydrate polymers. 2013;95(1):434-40.
- Zeid A, Mohammed A, Mu.N et al. Electrical conductivity and thermal stability studies on polyaniline Sn (IV) tungstomolybdate nanocomposite cation-exchange material: application as Pb (II) ion-selective membrane electrode. Int. J. Electrochem.Sci. 2015;10(3):2663-84.
- Asif A, Tabassuma A. Preparation, physico-chemical characterization and electrical conductivity measurement studies of an organic–inorganic nanocomposite cation-exchanger: poly-o-toluidine Zr (IV) phosphate. Electrochimica Acta. 2008;53(17):5540-48.
- Yiliang C, Bingcai P, Haiyan L et al. Selective removal of Cu (II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environmental science & technology. 2010;44(9):3508-13.
- Chanyan B, Nilesh D, Subhash C et al. Nano-cerium vanadate: A novel inorganic ion exchange for removal of americium and uranium from simulated aqueous nuclear waste. J hazardous materials. 2014;280:63-70.
- Ersin E, Eran J, Cuneyt A. Preparation of lead zirconate by homogeneous precipitation and calcination. J American Ceramic Society. 1997;80(10):2714-16.
- Kumari, Swati. Synthesis and characterization of ferrous nanoparticles and polymer-grafted ferrous nanoparticles with an examination of thermal and magnetic properties. Mississippi State University, 2016.
- Marceline N, ManuL, Ari V et al. Preparation and characterization of sodium iron titanate ion exchanger and its application in heavy metal removal from waste waters. J of hazardous materials. 2008;152(2):640-47.
- Weqar A, Shakeel A. Khan. Synthesis, characterization and ion-exchange properties of a new and novel ‘organic–inorganic’hybrid cation-exchanger: poly (methyl methacrylate) Zr (IV) phosphate.Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2007;295(1-3):193-9.
- Asif AK, Umair B, Mohd K. Electrically conductive polyaniline-titanium (IV) molybdophosphate cation exchange nanocomposite: synthesis, characterization and alcohol vapour sensing properties’ of Industrial and Engineering Chemistry. 2013;19(4):1226-33.
- Nabi SA, Bushra R, Othman ZA et al. Synthesis, characterization, and analytical applications of a new composite cation exchange material acetonitrile stannic (IV) selenite: adsorption behavior of toxic metal ions in nonionic surfactant medium. Separation Science and Technology. 2011;46(5):847-57.
- Pawel J, Malgorzata Ch, Kataryna KJ et al. Network films composed of conducting polymer-linked and polyoxometalate-stabilized platinum nanoparticles. Chemistry of materials. 2004;16(21):4128-34.
- Su-His L,ruey-Shin Heavy metal removal from water by sorption using surfactant-modified montmorillonite.J of hazardous materials. 2002;92(3):315-26.
- Mohammad Z, Ahamadi SJ, Nosnati SA et al. Preparation and characterization of zirconium (IV) molybdo tungsto vanado silicate as a novel inorganic ion exchanger in sorption of radionuclides. J of hazardous materials. 2009;169(1-3):808-812.
- Yavari R, Ahmadi SJ, Huang Y.O et al. Synthesis, characterization and analytical application of a new inorganic cation exchanger—Titanium (IV) molybdophosphate. Talanta. 2009;77(3):1179-84.
- Subhash c, Seema, Arti. Synthesis, characterization and ion exchange properties of a new ion exchange material: bismuth (iii) Iodophosphate. Recent research in Science and technology 2011.
- El-Gammal B, Shady SA. Chromatographic separation of sodium, cobalt and europium on the particles of zirconium molybdate and zirconium silicate ion exchangers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2006;287(1-3):132-8.
- Beyene H, Tekilt G. Application of sustainable nanocomposites for water purification process. Sustainable Polymer Composites and Nanocomposites. Springer, Cham. 2019;387-412.
- Abebe B, Tadesse AM, Kebede T et al. Fe-Al-Mn ternary oxide nanosorbent: Synthesis, characterization and phosphate sorption property. J of Environmental Chemical Engineering. 2017;5(2):1330-40.