A Unique Case of COVID-19 Related Acute Coronary Thrombosis Complicated By Severe Hypokalemia
Received: 07-Oct-2020 Accepted Date: Nov 17, 2020; Published: 24-Nov-2020
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
We report the case of a 52-year-old white male who was recently diagnosed with symptomatic COVID-19 and presented to the hospital with incessant VT/VF cardiac arrest, ST elevation myocardial infarction (STEMI) and profound hypokalemia. The patient was successfully treated with primary percutaneous coronary intervention (PCI) and concurrent aggressive potassium repletion. To the authors’ knowledge, this is the first case of COVID-19 presenting not only with an acute coronary thrombosis but also severe hypokalemia, both of which contributed to his cardiac arrest. The association of COVID-19 with acute coronary thromboses, including the challenges surrounding the diagnosis and management in this patient population is discussed. Additionally, the effect of COVID-19 on the renin-angiotensin-aldosterone-system (RAAS) is reviewed with a focus on hypokalemic presentations
Keywords
COVID-19; Hypokalemia; RAAS; Thrombosis
Introduction
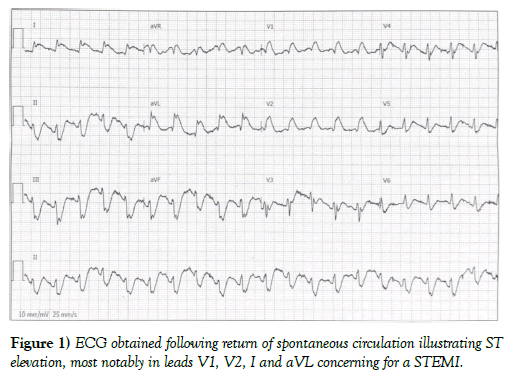
The patient is a 52-year-old white male with a history of hypertension who presented to the emergency department with chest pain and, shortly after arrival, developed cardiac arrest. Eight days prior to this presentation the patient developed fever, cough, body aches and subsequently tested positive for COVID-19 by polymerase chain reaction. Approximately four days after being diagnosed with symptomatic COVID-19 he began having intermittent substernal chest discomfort which continued until six days after COVID-19 diagnosis when he developed unremitting chest discomfort that prompted his wife to bring him to the emergency department. Once he arrived to the emergency department he was immediately escorted to an isolation room at which time he lost consciousness. A pulse was not palpable and immediate ACLS protocols were engaged. The patient was noted to have multiple unstable dysrhythmias to include ventricular fibrillation and pulseless polymorphic ventricular tachycardia. The patient underwent 14 defibrillations for the incessant VT/VF cardiac arrest. Additionally, the patient was intubated in the isolation room and intravenous epinephrine, magnesium, and amiodarone were given. Upon achieving sinus rhythm with return of spontaneous circulation, the patient was noted to have ST segment elevation in leads I, aVL, V1, and V2 concerning for an acute anterolateral myocardial infarction (Figure 1). A quick point of care ultrasound of the heart confirmed an anteroapical wall motion abnormality consistent with a likely STEMI. Of note, initial laboratory analysis showed serum potassium of 2.6 mmol/L. The patient was given aspirin per rectum, a intravenous heparin bolus and emergently taken to the cardiac catheterization lab with simultaneous initiation of potassium repletion.
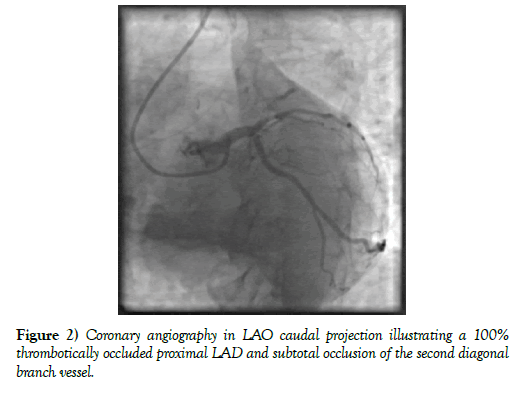
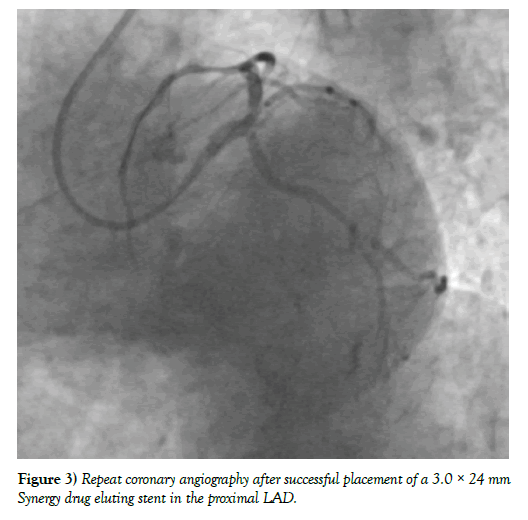
Coronary angiography, performed after full PPE with contact and droplet precautions was donned, revealed single vessel coronary artery disease involving an acute 100% thrombotically occluded proximal left anterior descending (LAD) artery. There was a large amount of thrombus in the proximal LAD that extended across the ostium of a moderate sized second diagonal branch resulting in TIMI 0 flow in the LAD and TIMI 1 flow in the diagonal branch (Figure 2). Intravenous cangrelor was administered and, due to the presence of a large amount of thrombus, aspiration thrombectomy was performed which retrieved a large amount of thrombus. TIMI 3 flow in the LAD and diagonal branch was subsequently restored with a door to device time of 101 minutes. A 3.0 × 24 mm Synergy drug eluding stent was then successfully deployed using intracoronary ultrasound guidance (Figure 3). Unfortunately, despite the aggressive use of microvascular vasodilators, flow into the apical LAD and diagonal branch remained suboptimal with minimal myocardial blush that was suspected to be secondary to a significant amount of distal embolization that occurred despite thrombectomy. The patient was, consequently, started on an eptifibatide infusion in an attempt to further improve microvascular flow, pharmacologically. Residual, mild to moderate coronary artery disease of the mid LAD and first diagonal branch up to 40-50% was otherwise noted and deferred to medical management.
Figure 2: Coronary angiography in LAO caudal projection illustrating a 100% thrombotically occluded proximal LAD and subtotal occlusion of the second diagonal branch vessel.
Figure 3: Repeat coronary angiography after successful placement of a 3.0 × 24 mm Synergy drug eluting stent in the proximal LAD.
The patient was transferred to the ICU using appropriate COVID precautions and successfully extubated within an hour of case completion. A repeat EKG was performed and revealed evidence of an anteroseptal and anterolateral infarct with improved, albeit, persistent ST elevation. A chest x-ray obtained at that time showed worsening multifocal airspace opacities as compared to one performed six days prior to admission. He was started on dual antiplatelet therapy with aspirin and ticagrelor and his cangrelor infusion was discontinued. The eptifibatide infusion was discontinued after 18 hours and the amiodarone was discontinued after 24 hours as his acute ischemia had resolved and his serum potassium had normalized following aggressive intravenous repletion.
A formal transthoracic echocardiogram was performed on hospital day 2 and showed a left ventricular ejection fraction of 35% with anterior, anteroseptal, and apical wall akinesis. Given the increased risk of left ventricular thrombus formation amidst significant wall motion abnormalities the patient was started on systemic anticoagulation with rivaroxaban which was also felt to help mitigate any further thrombotic complications of his acute COVID-19 infection. Dual antiplatelet therapy was continued given his low bleeding risk and recent evidence supporting a higher risk for stent thrombosis in the setting of an acute COVID-19 infection 1. Finally, guideline directed medical therapy for an acute myocardial infarction was initiated.
Interestingly, a repeat EKG performed on hospital day 2 showed worsening ST elevations across the anterior precordial leads in the absence of chest pain. A CT coronary angiogram was performed which revealed a patent LAD stent and a patent diagonal branch without evidence of ventricular aneurysm. Additionally, there was no left ventricular thrombus noted. Diffuse peripheral areas of consolidation and ground glass opacities consistent with COVID-19 pneumonia were noted in the lungs. The patient was transferred to the step down unit and, over the next several days, continued to improve on medical therapy. He was discharged with a Life Vest on hospital day 10 with close follow up in the heart failure and infectious disease clinics.
Discussion
This case highlights the acute cardiovascular morbidity associated with COVID-19 infection with specific attention to the increased risk of acute coronary thrombosis. Additionally, although the acute, thrombotically occluded proximal LAD contributed to the initial cardiac arrest, the profound presenting hypokalemia provided a firm foundation for further electrical instability in this setting and underscores the detrimental effect the virus can have on the renin-angiotensin-aldosterone axis. To our knowledge, this is the first case to demonstrate the morbid interplay of simultaneous, COVID-19-related acute coronary thrombosis with COVID-19 related profound hypokalemia.
COVID-19 is a novel enveloped RNA beta-coronavirus that has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus enters human cells primarily through angiotensin converting enzyme 2 (ACE2) binding. This enzyme is primarily expressed in alveolar cells, cardiac myocytes, and vascular endothelium [1,2]. Although respiratory symptoms are the primary clinical manifestation of COVID-19, a variety of cardiovascular complications can occur to include acute coronary thrombosis, acute myocardial injury without obstructive coronary artery disease, arrhythmias, heart failure (with or without cardiogenic shock), pericardial effusion, and thromboembolic complications 3.
The frequency, relative incidence, and phenotype of COVID-19 related acute coronary thrombosis versus other associated cardiac manifestations is not well defined [3]. Recent studies have highlighted that not all symptomatic COVID-19 cases with focal ST elevation on EKG are secondary to an acute coronary thrombosis. One retrospective analysis of 28 coronavirus positive patients admitted for STEMI showed 11 of these patients did not have obstructive CAD (39.3%) [4]. Another paper reviewed the cases of 18 patients with COVID-19 and STEMI, of which 50% underwent angiography, and of those only 67% had obstructive CAD [5].
The initial management of confirmed or suspected COVID-19 positive patients who present with ST elevation on EKG, therefore, can pose a management dilemma in the emergency department. On the one hand, performing emergent coronary angiography on all patients with presenting ST elevation on EKG provides unnecessary viral exposure to the cardiac catheterization team in cases without an acute coronary thrombosis. On the other hand, a missed or delayed diagnosis of an acute coronary thrombosis in the setting of non-specific symptoms can increase short and long term cardiovascular morbidity and mortality. Indeed, one of the ways these risks have been mitigated is with the use of systemic thrombolysis in suspected or confirmed COVID-19 patients presenting with a possible STEMI [6]. However, given the data supporting primary PCI over thrombolysis as a means to reduce death, non-fatal re-infarction and stroke and the increased risk of bleeding associated with thrombolytic therapy, a thrombolytic strategy for all COVID-19 patients with ST elevation presenting at PCI capable hospitals is not being routinely recommended in the United States [7,8]. Alternatively, the use of point of care ultrasound to assist in the immediate management of these patients can be helpful with the presence of focal wall motion abnormalities used to decide if the patient should be taken emergently to the cardiac catheterization lab [8,9].
Outside of the existing guidelines for management of acute coronary thrombosis, evidence based treatment of COVID-19 associated acute coronary thrombosis is limited. However, potential drug interactions between current therapies for acute coronary thrombosis and investigational therapies for COVID-19 are worthy to note and, thus may require a more thoughtful approach to what specific acute coronary thrombosis therapies are chosen. Although no known interactions between parenteral antithrombotic therapies and investigational therapies for COVID-19 are known to exist, potential interactions between antiplatelet therapies used in acute coronary thrombosis and those therapies used in COVID-19 do exist as a result of the co-interaction with the cytochrome system. Lopinavir/Ritonavir inhibits CYP3A4 metabolism which can lead to a reduction in the effective dosage of clopidogrel, or actually increase the effects of ticagrelor. Remdesivir, a nucleoside-analog inhibitor of RNA-dependent RNA polymerase, is an inducer of CYP3A4 but does not currently require dose adjustments for oral antiplatelet medications. Tocilizumab, an IL-6 inhibitor, results in increased expression of CYP3A4 and 2C19 with no dose adjustments on oral antiplatelet agents currently recommended. Sarilumab, which inhibits IL-6 mediated signaling, also increases expression of CYP3A4 but with no dose adjustments on oral antiplatelet agents currently recommended. Other investigational therapies including azithromycin, bevacizumab, hydroxychloroquine, eculizumab, interferon, losartan, methylprednisolone, pirfenidone, and ribavirin are not currently identified as having drug-drug interactions with anti-platelet agents [2,3].
Although the propensity of COVID-19 to cause thromboses has been well described, the association with hypokalemia is only recently being recognized [10-12]. COVID-19 enters human cells primarily through angiotensin converting enzyme 2 (ACE2) binding, an enzyme that plays an important role in angiotensin II regulation. Under normal conditions, ACE converts Angiotensin I to Angiotensin II which then can exert multiple Angiotensin II Type 1 Receptor (AT1R) mediated effects, to include increased water reabsorption and production of aldosterone which increases sodium resorption and enhances potassium secretion. ACE2 counteracts ACE, converting Angiotensin II to Angiotensin I-7, thus prohibiting angiotensin II from initiating its downstream effects. The downregulation of ACE2 as a consequence of COVID-19 binding and COVID-19 viral mediators can therefore shift the balance between these two enzymes and contribute to a relative state of hyperaldosteronism with subsequent hypokalemia as exhibited by our patient [13,14]. Data is limited, but there is currently one prospective cohort study evaluating the prevalence of hypokalemia and response to treatment with potassium supplementation in patients with COVID-19. 175 patients were enrolled. 18% of patients had potassium less than 3 mmol/L and 37% had potassium less than 3.51 mmol/L, overall indicating a high prevalence of hypokalemia in this population. Patients responded well to potassium supplementation. Additionally, more severe hypokalemia appeared to be associated with more severe disease activity [15,16].
Conclusion
COVID-19 is a pro-thrombotic illness that may increase the likelihood of acute coronary thrombosis. Additionally, ACE2 downregulation by COVID-19 can have deleterious effects on the balance of the reninangiotensin- aldosterone axis promoting hypokalemia. This case represents the first known case of both of these COVID-19 related complications acting synergistically to cause incessant VT/VF arrest. Indeed, an increased incidence of out of hospital cardiac arrest has been seen in the COVID era. Although this is likely multifactorial from increased risk of COVID related respiratory failure, coronary and pulmonary thromboses, myocarditis, dysrhythmia and a possible hesitancy to seek emergent healthcare amidst a global pandemic, the associated profound electrolyte abnormalities seen as a direct result of the deleterious effect of COVID-19 on the RAAS cannot be ruled out as a potential contributor.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Prieto-Lobato A, Ramos-Martínez R, Vallejo-Calcerrada N, et al. A case series of stent thrombosis during the COVID-19 pandemic. JACC: Cas Rep. 2020.
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: A JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950-73.
- Hendren NS, Drazner MH, Bozkurt B, et al. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020;141:1903-14.
- Stefanini GG, Montorfano M, Trabattoni D, et al. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation. 2020;141:2113-16.
- Bangalore S, Sharma A, Slotwiner A, et al. ST-Segment elevation in patients with COVID-19 - A Case Series. The New England journal of medicine. 2020;382:2478-80.
- Jing ZC, Zhu HD, Yan XW, et al. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J. 2020;41:1791-94.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet (London, England). 2003;361:13-20.
- Mahmud E, Dauerman HL, Welt FG, et al. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76: 1375-84.
- The American Heart Association's Get With The Guidelines-Coronary Artery Disease Advisory Work G, Mission Lifeline P, The American Heart Association's Council On Clinical C, The American Heart Association's Council On Clinical Cardiology's Committee On Acute Cardiac C, General Cardiology C and The American Heart Association's Council On Clinical Cardiology's Committee Interventional Cardiovascular Care C. Temporary Emergency Guidance to STEMI Systems of Care During the COVID-19 Pandemic: AHA's Mission: Lifeline. Circulation. 2020.
- Klok FA, Kruip M, Van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020;191:145-47.
- Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Throm Res. 2020;191:9-14.
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Int Care Med. 2020;46:1089-98.
- Groß S, Jahn C, Cushman S, et al. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J Mol Cell Cardiol. 2020;144:47-53.
- Brojakowska A, Narula J, Shimony R, et al. Clinical implications of SARS-CoV-2 Interaction with renin angiotensin system: A JACC review topic of the week. J Am Coll Cardiol. 2020;75:3085-95.
- Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA network open. 2020;3:2011122.
- Baldi E, Sechi GM, Mare C, et al. COVID-19 kills at home: The close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;1-20.