Arachidonic acid and its metabolites as the surveillance differentiation inducers to protect healthy people from becoming cancer patients
Received: 21-Dec-2020 Accepted Date: Jan 04, 2021; Published: 11-Jan-2021
Citation: Liau MC, Kim JH, Fruehauf P. Arachidonic acid and its metabolites as the surveillance differentiation inducers to protect healthy people from becoming cancer patients. Clin Pharmacol Toxicol Res. 2021;4(1):7-10.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Introduction
We have previously brought up the likelihood that most human cancers were originated from PSCs because PSCs and CSCs were very much alike on cell features and biological missions [1,2]. It requires only a single hit by silencing TET-1 enzyme to convert PSCs to CSCs, which can be easily accomplished by PSCs since these cells are equipped with abnormally active Methylation Enzymes (MEs). MEs are a ternary enzyme complex consisting of Methionine Adenosyl Transferase (MAT)-Methyl Transferase (MT)-SAdenosyl Homocysteine Hydrolase (SAHH) [3]. The association of MEs with telomerase turns MEs to become abnormally stable and active [4], so that hypomethylation of DNA necessary to activate differentiation related genes cannot take place [5,6]. Normal primitive stem cells expressing telomerase such as ESCs and PSCs are still able to carry out differentiation programs, relying on TET-1 to achieve DNA hypomethylation [7,8]. TET-1 is frequently silenced in cancer cells [9-11]. Thus, a single hit to silence TET-1 completely eliminates differentiation capability of CSCs and the progenies derived from CSCs. The blockade of differentiation is an important issue of cancer, which enables cancer cells to keep on dividing once an oncogene is activated or a suppressor gene is inactivated. Perpetual cell proliferation is the hallmark of cancer.
The association of telomerase with MEs alters the regulation and kinetic properties of MEs [4,12]. The cancer MAT-SAHH isozyme pair display Km values 7-fold higher than the normal isozyme pair. The increased Km value of the cancer MAT isozyme suggests that MEs of cancer cells have elevated levels of bound S-adenosylmethionine (AdoMet), which may exercise a positive influence on the stability of MEs. Binding of AdoMet to β-cystathionine synthase has been shown to protect this enzyme against protease digestion [13]. Consistent with this model, it was reported that the pool sizes of AdoMet and S-adenosylhomocysteine (AdoHcy) were greatly diminished in cancer cells undergoing drug-induced Terminal Differentiation (TD) [14]. Thus, abnormal MEs were not only play an important role to block differentiation; they also play a critical role in the promotion of malignant growth.
PSCs with abnormally active MEs if allowed to proliferate uncontrollably, the chance of aberrantly introducing methyl groups at the promoter site of TET-1 is very high. Fortunately, human body produces metabolites active as DIs and DHIs that can effectively prevent the buildup of cells with abnormal MEs. The concept of chemo-surveillance was based on the findings that healthy people could maintain a steady level of DIs and DHIs in the plasma, whereas cancer patients tended to show deficiency of such metabolites due to excessive urinary excretion [15]. DIs are chemicals capable of eliminating the association of telomerase from abnormal MEs, and DHIs are chemicals inhibitory to the ternary MEs. DIs and DHIs are hydrophobic metabolites that can be purified from urine by reverse phase chromatography, using C18 or XAD-16 as adsorbents. CDA-2 was a preparation using XAD-16 as the adsorbent [16], which has been approved by the Chinese FDA for the therapy of MDS in 2017 [2]. MDS is a disease attributable entirely to CSCs [17]. CDA-2 had a slightly better therapeutic efficacy in comparison to Vidaza and Decitabine, the two drugs approved by USA for the therapy of MDS, and totally devoid of serious adverse effects [18,19], whereas Vidaza and Decitabine were proven carcinogens [20,21] and damaging to DNA [22,23].
Organic acids constitute all DIs of CDA-2 [24], 40% as liposomal complexes with pregnenolone and 60% in association with cell membrane fragments. Active organic acids do not display UV absorption. We have previously found PGE2 active as a DI [25]. The study of PGE2 as a DI was prompted by the finding that it was involved in wound healing [26]. Wound healing is a biological mission of PSCs, and, therefore, is closely related to chemosurveillance to keep a check on PSCs.
The study of PGE2 as a DI is controversial. PGs are involved in multiple biological functions through different receptors. PGE2 as a crucial mediator of PSCs [27] and an active DI is good for wound healing and antitumor effect. The involvements of PGs in inflammation [28] and carcinogenesis [29,30] discourage the consideration of PGE2 as an antitumor agent. Nevertheless, beneficial antitumor effect of PGE2 was also reported [31]. In this study, we tried to stay away from controversy and to focus on more stable and less controversial PGs such as PGJ2 and 16, 16-dimethylPGE2. AA is not known as an antitumor agent. Since it is the substrate of PGs, we took up the study to see if it was related to the organic acids of CDA-2.
Materials and Methods
Chemicals and reagents
Chemicals and cell culture supplies used in this study were purchased from Sigma, St. Louise, MO unless otherwise indicated. PGJ2, 16, 16-dimethylPGE2, and bicycloPGE2 were purchased from Cayman Chemical Co., Ann Arbor, MI. 35 × 10 mm cell culture dishes were from CytoOne, USA Scientific.com.
Culture of HL-60 cells
HL-60 cells were purchased from ATCC, Manassan, VI, which were initially maintained in ISCOVE’s modified medium, supplemented with 10% fetal bovine serum, 2 mM glutamine, 50 units/ml penicillin-50 μg/ ml streptomycin for a few generations, and then transferred to RPMI 1640 medium to replace ISCOVE’s modified medium. Cells were subcultured every 4 days at an initial concentration of 5-10 × 104 cells/ml.
Nitro Blue Tetrazolium (NBT) assay
NBT assay was conducted according to previously described procedure [25]. Each 35 × 10 mm cell culture dish contained 2 ml of RPMI 1640 culture medium. HL-60 cells at an initial concentration of 5-10 × 104 cells were incubated with or without drugs for 4 days. Approximately 2 × 105 cells were sedimented at 600 xg for 5 min. The cell pellet was suspended in 3 drops from a Pasteur pipet of NBT reagent consisting of 1 mg NBT and 5 μg phorbol-12-myristate 13 acetate per ml Hank Balanced Salt Solution (HBSS), and incubated at 37˚C for 30 min. The reaction was terminated by the addition of a drop from a Pasteur pipet of 4% paraformaldehyde in HBSS. NBT+cells were counted using a hemacytometer. NBT assays were repeated at least 3 times, and the results were reported as mean ± SD.
Results
DI activities of stable PGs
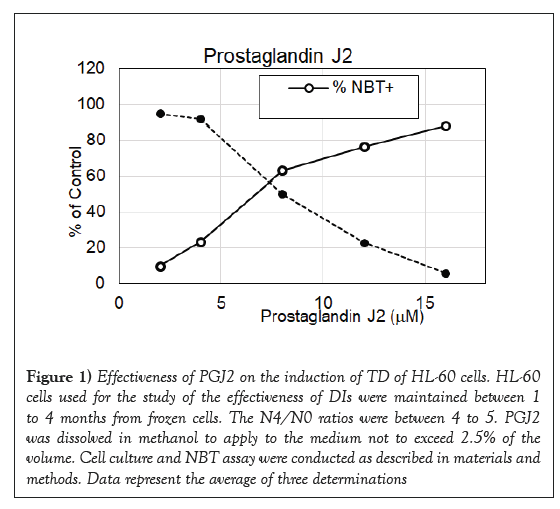
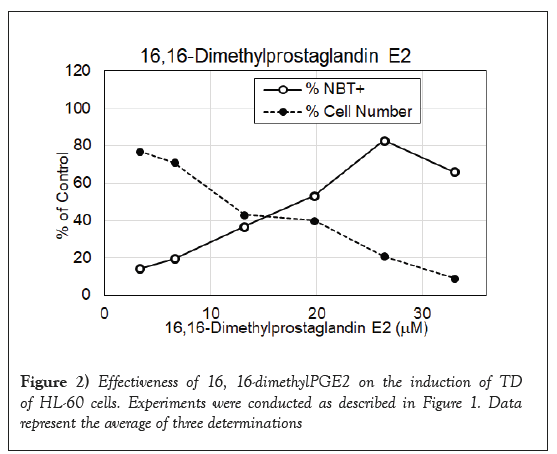
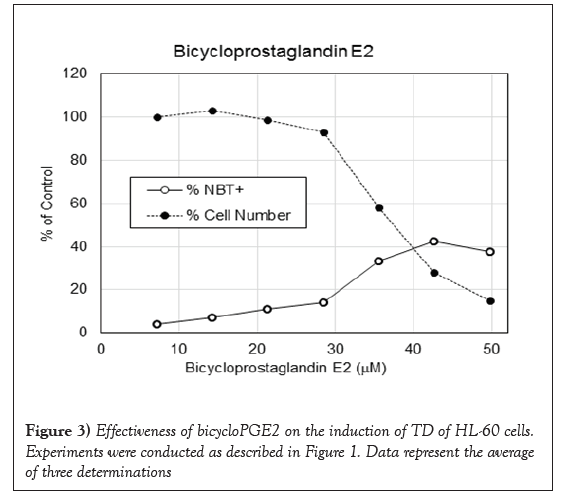
PGJ2 has a cyclopentenone ring with reactive α, β-unsaturated carbonyl group that forms covalent Michael adduct with key cysteines in proteins. It becomes a stable adduct with albumin in the plasma [32]. It has been shown to display multifaceted functions including anti-inflammatory and cytoprotective effects, and anticancer activity [33-35]. As shown in Figure 1, it had impressive DI activity much better than PGE2. PGJ2 was active in the dosage ranges between 4 and 17 μM with a maximum to induce 86% NBT+cells at 17 μM, which was approximately one quarter of the dosage of PGE2 to reach the maximum DI activity. 16, 16-dimethylPGE2 is a competitive inhibitor of 15-hydroxyPG dehydrogenase to prolong the very short half-life of PGE2. This more stable PGE2 metabolite has been shown to display consistent antitumor effect [36]. Result presented in Figure 2 indicates that 16, 16-dimethylPGE2 was active in the dosage ranges between 10 and 32 μM with a maximum to induce 83% NBT+cells at 27 μM, which was exactly one half of the dosage of PGE2 to reach the maximum DI activity. 16, 16-dimethylPGE2 definitely is a better DI than PGE2. It is more active and more stable. BicycloPGE2 is the stable end product of PGE2. This stable end product, however, did not have good DI activity as shown in Figure 3. BicycloPGE2 was only modestly active in the dosage ranges between 27 and 50 μM with a maximum to induce 42% NBT+cells at 42 μM.
Figure 1: Effectiveness of PGJ2 on the induction of TD of HL-60 cells. HL-60 cells used for the study of the effectiveness of DIs were maintained between 1 to 4 months from frozen cells. The N4/N0 ratios were between 4 to 5. PGJ2 was dissolved in methanol to apply to the medium not to exceed 2.5% of the volume. Cell culture and NBT assay were conducted as described in materials and methods. Data represent the average of three determinations
DI activities of AA
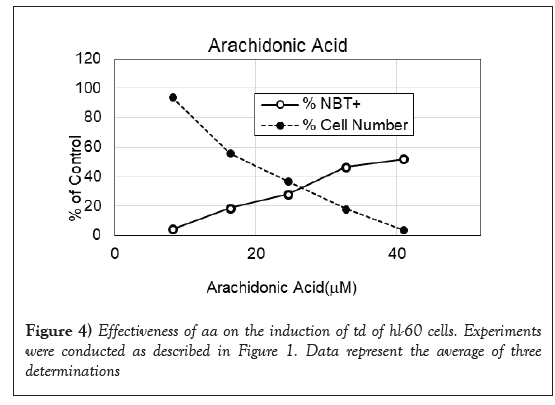
AA is not known to possess antitumor effect. Since it is the substrate of PGs, we took up the study to examine its activity as a DI. As shown in Figure 4, its activity as a DI is very close to that of bicycloPGE2. It was only modestly active in the dosage ranges between 20 and 42 μM with a maximum to induce 52% NBT+cells at 42 μM. Although AA is only modestly active as a DI, it had a strong synergistic effect with pregnenolone to promote TD of HL-60 cells as shown in Table 1. The synergistic effect of pregnenolone to potentiate DI activity of AA was much better than the synergistic effect to potentiate DI activity of ATRA. These results suggest that pregnenolone is more compatible to AA than the DI induced by ATRA. Thus, pregnenolone can boost relative inactive AA to become acceptable DI.
| Additions | %NBT+cells | Fold potentiation | ||
|---|---|---|---|---|
| Pregnenolone | 7.9 µM | 6.3 ± 0.4 | ||
| 15.8 µM | 11.4 ± 0.5 | |||
| ATRA | 0.15 µM | 13.3 ± 0.7 | ||
| ATRA, 0.15 µM+Pregnenolone, 7.9 µM | 35.8 ± 1.5 | 1.83 | ||
| ATRA, 0.15 µM+Pregnenolone, 15.8 µM | 57.5 ± 1.9 | 2.32 | ||
| AA, 12 µM | 10.9 ± 0.8 | |||
| AA, 12 µM+Pregnenolone, 7.9 µM | 59.5 ± 1.7 | 3.45 | ||
| AA, 12 µM+Pregnenolone, 15.8 µM | 83.4 ± 2.8 | 3.74 | ||
Note: Cell culture and NBT assay were conducted as described in Figure 1. ATRA, AA and pregnenolone were dissolved in methanol to apply to the medium not to exceed 2.5% of the volume. Data represent the average of three determinations. 4.5 ± 0.2% of the blank of %NBT+cells has been subtracted from each experimental datum. Fold potentiation is calculated from the formula % NBT+cells of (DI+DHI)/% NBT+cells of DI+% NBT+cells of DHI.
Table 1 : Synergistic effect of pregnenolone on the potentiation of TD of HL-60 cells induced by ATRA or AA
Discussion
Wound healing and the evolution of cancer are closely related to involve PSCs as critical elements [2,26]. Wound healing is a process to involve the breakdown of membrane bound phospholipid to release AA for the synthesis of PGs. The breakdown of membrane bound phospholipid facilitates membrane permeability for the release of inhibitory metabolites such as DIs and DHIs inside PSCs to allow the buildup of PSCs. Increase of membrane permeability is bad in terms of cancer development, because the proliferation of PSCs runs a risk to evolve into CSCs, but the buildup of PSCs is necessary to repair the damage. Acute destruction taking place in healthy people does not pose a threat on cancer development, because the damage is usually healed quickly to return to the normal state. Chemo-surveillance is intact in healthy people, which can effectively prevent unnecessary proliferation of PSCs beyond wound healing. Besides, PGs synthesized in response to destruction also play a role to control unnecessary buildup of PSCs. Chronic destruction is the problem. Damage response becomes cumulative and progressive to involve immunological damage control that produces cytokine such as tumor necrosis factor, otherwise also known as cachectin, to inhibit reabsorption of renal tubule to result in excessive excretion of low molecular weight metabolites, a known symptom commonly shared by inflammatory patients and cancer patients. Excessive urinary excretion of surveillance chemicals eventually affects the patient’s ability to control the proliferation of PSCs and their evolution into CSCs. Thus, inflammation is a necessary evil for efficacious repair of the destruction damage; it can also lead to cancer if chemo-surveillance is also damaged in the process. The protection of the integrity of chemo-surveillance is very important for the prevention of cancer.
The evolution of CSCs from PSCs is so simple, requiring just a single hit to silence TET-1 enzyme, which is within the reach of PSCs with abnormally active MEs. Effective chemo-surveillance to keep a check on the proliferation of PSCs becomes very important to protect healthy people from becoming cancer patients. Chemo-surveillance is apparently functioning very well since there are far more healthy people than cancer patients. This study to report on the modest DI activity of AA as a DI, and the strong potentiation of the DI activity of AA by pregnenolone suggests that AA may be an important DI produced by the human body. AA is a normal lipid constituent of cell membrane. It can come from degraded fragments of erythrocyte membrane. AA alone is not impressive as a DI, but in the mixture with pregnenolone like that in CDA-2 preparation, it becomes a very effective agent to induce TD of cancer cells. Thus, pregnenolone is an important partner of AA to function as effective DI. Production of pregnenolone in human body is a bell shape with the peak production at the ages of 20 to 25 years old [37]. The very young and the very old age groups produce much less pregnenolone, and these are the two age groups most susceptible to cancer. Natural deficiency of surveillance DHIs should be supplemented with effective DHIs to reduce cancer incidence [24]. Equally important, the breakdown of chemosurveillance should be restored to result in effective cancer therapy [38,39].
Perpetual cell replication is the hallmark of cancer. There are multiple issues involved to make cancer cells to replicate perpetually: the breakdown of cell membrane integrity because of destruction resulted from injuries, infections, or toxic chemicals to increase membrane hyperpermeability, which is a part of the process on wound healing [26]; the propagation of PSCs to repair the damage and the appearance of CSCs if PSCs sustained a single hit to silence TET-1 enzyme, thus completely shuts off differentiation capability [1,2]; the breakdown of chemo-surveillance due to progression of membrane hyperpermeability to become systemic cachexia symptom [15]; and finally the activation of oncogenes and the inactivation of suppressor genes to manifest clinical symptom of perpetual cell replication. A perfect solution of cancer must be the one that can resolve all issues involved in the evolution of cancer. Destabilization of abnormal MEs is the strategy that can put away all issues involved in the evolution of cancer [40]. As a matter of fact, the employment of DIs and DHIs to put out abnormal MEs is the nature’s choice to prevent the occurrence of cancer [2]. If cells with abnormal MEs such as PSCs are allowed to proliferate, the chance of evolving into CSCs is very high simply to silence TET-1 enzyme, and the chance to inactivate suppressor genes is also very high simply to introduce methyl groups into the promoters of suppressor genes. Destabilization of abnormal MEs puts out the fire at the very beginning, and, therefore, is the most effective strategy for cancer prevention. By directing cancer cells to undergo TD also puts to rest the issues of oncogenes and suppressor genes.
Conclusion
After all, oncogenes and suppressor genes are cell cycle regulatory genes, they have important roles to play when cells are in cell cycle replicating, but if replicating cells exit cell cycle to undergo TD, they have no roles to play. The activation of oncogenes and the inactivation of suppressor genes often involve gene silencing or mutation, translocation, and deletion. The correction of such chromosomal abnormalities is very difficult. Even a difficult gene abnormality is miraculously solved, there may pop up another difficult gene problem. It becomes endless efforts to solve gene abnormalities. The easy way is to ignore gene abnormalities by pushing cancer cells out of cell cycle to undergo TD. There is a problem on this strategy of cancer therapy. The terminally differentiated cells may not perish, although such cells are harmless. The surviving tumor mass remains a serious concern. Surgical removal of the harmless surviving tumor mass may be a solution to eliminate concern. The endpoint of the therapy must be set differently from the cell killing strategy. The disappearance of the circulating CSCs is perhaps a valid therapeutic endpoint like the endpoint to evaluate the therapeutic efficacy of hematological cancers.
REFERENCES
- Liau MC, Kim JH, Fruehauf JP. (2019) Destabilization of abnormal methylation enzymes: Nature’s way to eradicate cancer stem cells. On J Complement Alt Med. 2019;2(5)
- Liau MC, Kim JH, Fruehauf JP. (2020) Destabilization of abnormal methylation enzymes to combat cancer: The nature’s choice to win the war on cancer. Lambert Academic Publishing 978-620-2-66889-7.
- Liau MC, Chang CF, Saunders GF, et al. S-Adenosylhomocysteine hydrolases as the primary target enzymes in androgen regulation of methylation complexes. Arch Biochem Biophys. 1981;208(1):261-72.
- Liau MC, Zhuang P, Chiou GCY. Identification of the tumor factor of abnormal methylation enzymes as the catalytic subunit of telomerase. Chin Oncol Cancer Res. 2010;7(2): 86-96.
- Liau MC, Lee SS, Burzynski SR. Modulation of cancer methylation complex isozymes as a decisive factor in the induction of terminal differentiation mediated by Antineoplaston A5. Intl J Tiss React. 1990;12: 29-36.
- Liau MC, Lee SS, Burzynski SR. Hypomethylation of nucleic acids: A key to the induction of terminal differentiation. Int J Exptl Clin Chemother. 1989;2(4): 187-99.
- Shen L, Sing CX, He C, et al. Mechanism and function of oxidation reversal of DNA and RNA methylation. Annu Rev Biochem. 2014;83: 585-14.
- Wu X, Zhang Y. TET-mediated active DNA demethylation mechanism, function and beyond. Nat Rev Genet. 2017;18(9): 517-34.
- Kudo Y, Tateishi K, Yamamoto K, et al. Loss of 5-hydroxymethylcytosine is accompanied with malignant cellular transformation. Cancer Sci. 2012;103(4): 670-74.
- Fics GM, Gibbon JG. Loss of 5-hydroxymethylcytosine in cancer: Cause or consequence? Genomics. 2014;104(5): 352-57.
- Wang KC, Kang CH, Tsai CY, et al. Ten-eleven translocation 1 dysfunction reduces 5-hydroxymethylcytosine expression levels in gastric cancer cells. Oncol Lett. 2018;15(1): 278-84.
- Liau MC, Chang CF, Becker FF. Alteration of S-adenosylmethionine synthetases during chemical hepatocarcinogenesis and in resulting carcinomas. Cancer Res. 1979;39: 2113-119.
- Prudova A, Baumann Z, Braun A, et al. S-Adenosylmethionine stabilizes β-cystathionine synthase and modulate redox capacity. Proc Natl Acad Sci USA. 2006;103: 6489-494.
- Chiba P, Wallner L, Kaizer E. S-Adenosylmethionine metabolism in HL-60 cells: Effect of cell cycle and differentiation. Biochim Biophy Acta. 1988;971: 38-45.
- Liau MC, Szopa M, Burzynski B, et al. Chemo-surveillance: A novel concept of the natural defense mechanism against cancer. Drug Exptl Clin Res. 1987;13(1): 71.
- Liau MC. Pharmaceutical composition inducing cancer cell differentiation and the use for treatment and prevention of cancer thereof. US Patent 7232578 B2. 2007.
- Woll PS, Kjallquist U, Chawdhury D, et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell. 2014;25(6): 794-08.
- Ma J. Application of differentiation induction for the treatment of malignant tumor and leukemia. CSCO Treaties on the Education of Chinese Clinical Oncology. 2007:480-86.
- Liau MC, Fruehauf JP. (2015) Destabilization of abnormal methylation enzymes as a critical mechanism for CDA-2 to reverse MDS progression. (edn), The First International Forum of Myelodysplastic Syndrome in Taizou, Jiangsu, China.
- Prassana P, Shack S, Wilson VL, et al. Phenylacetate in chemoprevention of 5-aza-2’-deoxycytidine induced carcinogenesis. Cli Cancer Res. 1995;1(18): 865-71.
- Gaudet F, Hodgson JG, Eden A, et al. Induction of tumor in mice by genomic hypomethylation. Science. 2003;300(5618): 489-92.
- Palii SS, van Emburgh BO, Sankpal UT, et al. DNA methylation inhibitor 5-aza-2’-deoxycytidine induces reversible DNA damages that is distinctly influenced by DNA methyltransferase 1 and 3B. Mol Cell Biol. 2008;28(2): 752-78.
- Kizietepe T, Hideshima T, Catley L, et al. 5-Aza-cytidine, a DNA methyltransferase inhibitor, induces ATP-mediated DNA double strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol Cancer Ther. 2007;6(6): 1718-727.
- Liau MC, Fruehauf PA, Zheng ZH, et al. Development of synthetic cell differentiation agent formulations for the prevention and therapy of cancer via targeting of cancer stem cells. Cancer Stu Ther J. 2019;4(1): 1-15.
- Liau MC, Kim JH, Fruehauf JP. In pursuance of differentiation inducers to combat cancer via targeting of abnormal methylation enzymes. J Cancer Tumor Intl. 2020;10(2): 39-47.
- Ho ATV, Palla AB, Blake MR, et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem cell strength. Proc Natl Acad Sci USA. 2017;114(26): 6675-684.
- North TE, Goessling S, Walkley CR, et al. Prostaglandin E2 repopulates vertebrate hematopoietic stem cell homeostasis. Nature. 2007;447(7147): 1007-011.
- Riciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5): 986-00.
- Kawamori T, Uchiya N, Sugimura T, et al. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24(3): 985-90.
- Payner T, Leaver HA, Knapp B, et al. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E2-dependent activation of type II protein kinase A. Mol Cancer Ther. 2006;5(7): 6817-826.
- Lalier L, Cartron PE, Pedelaborde F, et al. Increase in PGE2 biosynthesis induces a bax dependent apoptosis correlate to patient’s survival in glioblastoma multiforme. Oncogene. 2007;26(34): 4999-009.
- Yamaguchi S, Aldin G, Ito S, et al. Δ12-prostaglandin J2 as a product and ligand of human serum albumin: Formation of unusual covalent adduct at his146. J Am Chem Soc. 2010;132(2): 824-32.
- Kim EH, Surch YJ. 15-deoxy- Δ12, 14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors. Biochem Pharmacol. 2006;32(11): 1516-528.
- Strous DS, Pascual G, Li M, et al. 15-deoxy-Δ12, 14-prostaglandin J2 inhibits multisteps in the NF-kappaB signaling pathway. Proc Natl Acad Sci USA. 2000;97: 4844-849.
- Tae IH, Park EY, Dey P, et al. Novel SIRT1 inhibitor 15-deoxy-Δ12, 14-prostaglandin J2 and its derivatives exhibit anticancer activity through apoptotic or autophagic cell death pathways in SKOV3 cells. Int J Oncol. 2018;53(6):2518-530.
- Wilson JW, Potten CS. The effect of exogenous prostaglandin administration on tumor size and yield in min/+ mice. Cancer Res. 2000;60(16): 4645-653.
- Morley JE (2001) Hormone, aging, and endocrine in the elderly. In: Felig P, Forhman LA (eds) “Endocrinology and Metabolism”. (4thedn), McGrow-Hill, Inc., Medical Publishing Division, New York, pp. 1455-482.
- Liau MC, Fruehauf JP. Membrane hyperpermeability is a very important issue of cancer. Adv Complement Alt Med. 2020;5(5): 512-13.
- Liau MC, Fruehauf JP. Restoration of the chemo-surveillance capability is essential for the success of chemotherapy and radiotherapy to put cancer away. Adv Complement Alt Med. 2019;5(4): 474-75.
- Liau MC, Baker LL. Destruction promotes the proliferation of progenitor stem cells and cancer stem cells, therefore, non-destruction strategy is a better choice for cancer therapy: A commentary. J Pharmacol Pharmaceut Pharmacovig. 2020;4: 029.