Egf regulates purine and pyrimidine metabolism
Received: 03-Oct-2017 Accepted Date: Oct 05, 2017; Published: 12-Oct-2017
Citation: Danielyan KE. Egf regulates purine and pyrimidine metabolism J Biomol Biochem October-2017;1(1):3-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
It is proposed, Phosphoribosyl Pyrophosphate (PRPP), (product of the pyrimidine biosynthesis key regulative enzyme Phosphoribosylpyrophosphate Synthase-1 (PRPS-1)), accelerates Epidermal Growth Factor (Egf) dependent cells proliferation. However, Egf or its fragments don't have direct impact on the PRPS-1. Reactive Oxygen Species, generated by purine catabolism regulative enzyme - Xanthine Oxidoreductase (XOR), might also influence on Egf mediated cells proliferation.
Keywords
Reactive oxygen species; Epidermal growth factor; Phosphoribosyl pyrophosphate, Phosphoribosylpyrophosphate synthase-1; Xanthine oxidoreductase; Cells proliferation
Introduction
Egf
Egf belongs to the group I of EGF family. The family includes also transforming growth factor-α (TGF-α), amphiregulin, betacellulin, epiregulin heparin-binding EGF (HB-EGF), epigen [1,2]. The all members of EGF family contain the same evolutionary conserved motif, which consists from 35-40 amino acids. These amino acids are separated with the conserved 6 cysteines [3,4]. The all members of the family carry the same ability to bind to the EGF receptor (EGFR, ErbB1), to activate the pathways via triggering tyrosine kinase activity, which is further stimulating the intristic intracellular mechanisms of cells proliferation, differentiation, survival, motility [5,6].
However, from the other side, EgfR (Egf Receptor) activation initiates formation of the tumors, different types of it, moreover, has a crucial role in progression of the metastasis [7]. EgfR are responsible for the triggering of the following pathways: Ras/Raf signaling cascade, which promotes cell survival and cell proliferation; phosphatidylinositol 3-kinase/Akt signaling cascade, responsible for cell growth, apoptosis resistance, cell invasion and migration; signal transducers and activators of transcription (STAT) pathway, inducing tumor progression, oncogenesis and angiogenesis; phospholipase Cγ signaling regulation of ion channels, which induces cell migration, calcium-mediated signaling; Nck/PAK signaling cascade cell, responsible for the survival and cell migration; Cbl mediated endocytosis, inducing respectfully endocytotic processes [8-13].
Thus, the most prominent signalling pathway, triggering cells proliferation after the impact or influence of Egf, is the MAPKs chain of signal transduction.
Egf, MAPK pathway and purine, pyrimidine metabolism regulation
In accordance to Sigoillot, MAPKs phosphorylate the CAD (carbamoylphosphate synthetase - aspartate carbamoyl transferase-dihydroorotase), which is the multifunctional protein, which triggers mammalian pyrimidine biosynthesis [14]. Interestingly, the activation and pyrimidine biosynthesis is elevated in S-phase of cells division, when the concentration of phosphoribosyl pyrophosphate (PRPP) is elevated. This compound is serving as the activator of CAD, whereas the UTP is the inhibitor.
Consequently, we think, it should be some additional compound activating PRPS-1 (EC: 2.7.6.1, Phosphoribosylpyrophosphate Synthase-1), which is the key regulative enzyme of the pyrimidine biosynthesis and responsible for the synthesis of PRPP. Thus, before activation of the pyrimidine biosynthesis accumulation of PRPP is the triggering factor for feed-back activation of the entire cycle and cells proliferation.
Moreover, by Zollner et al. it was proved that interchangeable dependence of purines and pyrimidies mathabolism exist, which means that by influencing on one of these chain of reactions, it is possible to change the root of the other [15].
Taking into the consideration those MAPK pathways is activated by Egf, which triggers pyrimidine biosynthesis, we proposed, that Egf or its parts, generated due to the proteolytic degradation, might directly activate PRPS-1 [16].
We made a fast docking analysis, first, to figure out which parts in Egf structure evolutionally more conserved, by comparing peptide structures from Rattus norvegicus, Homo sapiens and Danio rerio [17-20]. The most conservative sequences were the following.
| Homo sapiens | dkyacncvvgyiGERCQyrdlkwwelr |
| Rattus norvegicus | dryvcncvigyiGERCQhrdlrwwklr |
| Danio rerio | esyacncvlgymGERCQfsdlewwelq |
It was made also docking analysis of PRPS-1 and totally identical part of the Egf motif - GERQ. However, the protein structure similarity TM-score as well as interaction similarity scores were evidencing about impossible interactions of these structures.
We might conclude and propose, Egf directly don’t activate PRPP-1. However, after binding of Egf with its receptors, activation tyrosine kinase, activation of MAPKs pathway and involvement of CAD, generated PRPP accelerates by the feed-back mechanism activity of CAD, stimulating even more intensive cells proliferation.
Egf and ROS (Reactive Oxygen Species)
Egf dependent pathways might be activated by the formation of ROS in normal and pathological conditions. By Huo, it was shown that promotional effect of Egf on human corneal cell proliferation is mainly mediated by ROS signaling as well as MAPK/ERK activated pathway under disease condition [21].
By the utility of single molecule force spectroscopy, it was shown that application of the oridonin is preventing the interaction of Egf with its receptor and preventing development of the cancer, mediated by formation of the ROS [22].
Also, it was presented the mechanism by which EGF promotes prostate cancer cell progression through a ROS/STAT3/HIF-1α/TWIST1/Ncadherin signaling cascade, providing novel biomarkers and promising therapeutic targets for prostate cancer cell progression [23].
In in vitro studies it was demonstrated that ROS and especially decrease in pH generated during exposure to LEF (low electric field) are involved in EgfR ligandless activation [24].
It was demonstrated, signaling events downstream of EgfR involved PI3K- and Src-dependent activation of Rac1, are mediated by NADPHgenerated ROS responsible for MMP-2 secretion and activation [25].
The increase of the cells proliferation in the presence of Egf was detected also in conditions of Xanthine Oxidoreductase enhanced activity, which is not just the enzyme responsible for formation of uric acid but also for the generation of ROS [26].
Thus, activation of purine metabolism responsible regulative enzyme Xanthine Oxidoreductase might also trigger activation of Egf dependent cells proliferation.
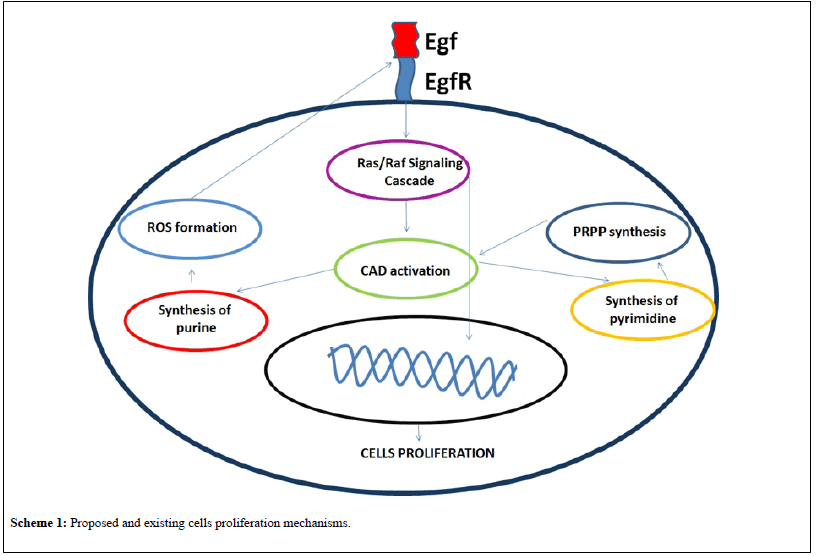
As the conclusion and graphical presentation of the proposed and existed ideas the following Scheme 1 is demonstrated.
Scheme 1:Proposed and existing cells proliferation mechanisms.
REFERENCES
- Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:4
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2-13.
- Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515-41.
- Savage CRJ, Hash JH, Cohen S. Epidermal growth factor. Location of disulfide bonds. J Biol Chem. 1973;248:7669-72.
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881-914.
- Zeineldin R, Hudson LG. Epithelial cell migration in response to epidermal growth factor. Methods Mol Biol. 2006;327:147-58.
- Lindsey S, Langhans SA. Epidermal growth factor signaling in transformed cells. Int Rev Cell Mol Biol. 2015;314:1-41.
- Gaestel M. MAPKAP kinases - MKs - two’s company, three’s a crowd. Nat Rev Mol Cell Biol. 2006;7:120-30.
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501.
- Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000-8.
- 1Patterson RL, van Rossum DB, Nikolaidis N, et al. Phospholipase C-gamma: diverse roles in receptor-mediated calcium signaling. Trends Biochem Sci. 2005;30:688-97.
- Ye DZ, Field J. PAK signaling in cancer. Cell Logist. 2012;2:105-16.
- Yu P, Fan Y, Qu X, et al. Cbl-b regulates the sensitivity of cetuximab through ubiquitin-proteasome system in human gastric cancer cells. J BUON Off J Balk Union Oncol. 2016;21:867-73.
- Sigoillot FD, Berkowski JA, Sigoillot SM, et al. Cell cycle-dependent regulation of pyrimidine biosynthesis. J Biol Chem. 2003;278:3403-9.
- Zollnerd N. Purine and pyrimidine metabolism. Proc Nuti Soc. 1982;41:329-42.
- Graves LM, Guy HI, Kozlowski P, et al. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature. 2000;403:328-32.
- Seok C, Shin WH, Lee GR, et al. Prediction of Protein Structure and Interaction by GALAXY protein modeling programs. Bio Design. 2014;2:1-11.
- Strausberg R. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci. 2003;99:16899-903.
- Vanderperre B. Direct detection of alternative open reading frames translation products in human significantly expands the proteome. PLoS ONE. 2013;8:E70698.
- Strausberg RL, Feingold EA, Grouse LH, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci. 2008;99:16899-903
- Huo YN, Chen W, Zheng XX. ROS. MAPK/ERK and PKC play distinct roles in EGF-stimulated human corneal cell proliferation and migration. Cell Mol Biol. 2015;61:6-11.
- Pi J, Jin H, Jiang J, et al. Single molecule force spectroscopy for in-situ probing oridonin inhibited ROS-mediated EGF-EGFR interactions in living KYSE-150 cells. Pharmacol Res. 2015;119:479-89.
- Cho KH, Choi MJ, Jeong KJ, et al. A ROS/STAT3/HIF-1a signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014;74:528-36.
- Wolf-Goldberg T, Barbul A, Ben-Dov N, et al. Low electric fields induce ligand-independent activation of EGF receptor and ERK via electrochemical elevation of H(+) and ROS concentrations. Biochim Biophys Acta. 2013;1833:1396-408.
- Binker MG, Binker-Cosen AA, Richards D, et al. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun. 2009;379:445-50.
- Casalino-Matsuda SM, Monzón ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581-91.