Engineering implants for fractured bones-metals to tissue constructs
2 Laboratory for Biomaterilas, Materials Research Centre, Indian Institute of Sciences (IISc), Bangalore, India, Email: bikramjit_basu@rediffmail.com
Received: 04-Nov-2017 Accepted Date: Nov 16, 2017; Published: 22-Nov-2017
Citation: Dutta RC, Dutta AK, Basu B. Engineering implants for fractured bones-metals to tissue constructs. J Mater Eng Appl November-2017;1(1):9-13.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Bones are essential component of spatial framework that provides visceral protection and define the shape of an organism. Similar to steel framework of a building, bone scaffold is indispensable for imparting a unique shape and imposing the body in one piece. Keeping this framework in requisite order and form post accidental fractures and breakages is a challenge. Present article discusses in brief the successive evolution in managing bone injuries and defects. It evaluates the rationale behind a shift in attention from metals to ceramics and polymer-ceramic hybrid composites as the implant material. Contemporary development of tissue engineering and bone-regeneration techniques and their need under specific context and situation is also discussed.
Keywords
Bone repair; Ceramics; Polymer; Fracture; Bone remodeling; Scaffold; RegenerationIntroduction
Fracture of bones in mammals and related morbidities is as old as their very existence. Accidental bone damage could create a fine hairline fracture or complete shattering/smashing depending upon direct or indirect impact on the bone. Smashing of bones is generally observed in osteoporotic patients where the overall quality of bones is weakened due to excessive bone porosity caused by resorption or other physiological reasons. The only way to fix a broken bone is by repositioning and supporting the dislocated bones with rods, plates, screws and pins through orthopedic surgery. Positioning the fractured bone is requisite temporarily till it heals by innate mechanisms; or permanently if the damage has created a gap not viable to be filled due to size or other clinical grounds. Supporting osteoporotic bones with metallic prosthesis is tricky and may not even feasible at times. Fixation of bones in such cases is accomplished through bone cement or glue, but that compromises the load bearing capacity of the bone and also the gait movement, more so if it is a joint.
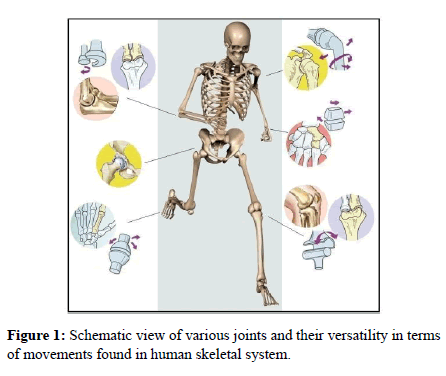
Handling bone damage in young age is much easier as it requires only a tough, bio-compatible metallic support and an expert hand to position the bone appropriately without compromising its shape and size. Juvenile bones get innately remodeled and shaped and the supportive implants could be surgically removed subsequently. Renewal and healing of bones slows down with age and also under the influence of certain diseases, like diabetes. Therefore, the possibility of bone repair in old age is scarce and requires additional care and nutritional supplements, besides the need to leave the implant permanently in the body. Here, we discuss the progress and journey of broken-bone management from metallic prosthetics through organic-inorganic composites to the exciting possibility of induced regeneration (Figure 1).
Figure 1: Schematic view of various joints and their versatility in terms of movements found in human skeletal system.
Steel to titanium
Managing bone fractures started with the use of external support as paltry as wooden clings and clutches. Later on metallic rods and plates were utilized for surgically supporting the broken bones. Stainless Steel (SS) being strong with superior corrosion resistance than Iron soon became the material of choice for creating orthopedic devices. Medical grade SS, a 316 L alloy that consists of Iron with some amount of Chromium (~18%), Nickel (~8%), Molybdenum (~2.5%), Manganese (~2%) and traces of carbon evolved as a standard for prosthesis. It is used in many devices even today. Despite having desired mechanical strength, easy manufacturability and good tolerance in most of the patients, SS implants also displayed some disadvantages; like being heavier and much stiffer than bone. They were found to be not completely resistant to corrosion in the dynamic physiological environment. Furthermore, on prolonged exposure to body fluids, elements like Ni, Co and Cr present in traces in the alloy tend to leach out causing toxicity of various kinds [1].
With the discovery of Titanium as a safe material, having better match with the bones in terms of modulus, pure Titanium and its alloys became the material of choice for orthopedic implants. Originally developed for aerospace applications, Titanium alloys intruded the biomedical implant industry due to their light weight, excellent biocompatibility and corrosion resistance. Unalloyed Titanium graded as 4 by the American Society for Testing Material’s standards (ASTM), is found mechanically strong, lighter than steel and highly resistant to corrosion. Pure Titanium due to its ductile strength that offers the required maneuverability for joints and bends is therefore recommended over its alloys for creating bone plates. Titanium alloys made with Aluminum and Vanidium (Ti-6Al-4V) or Aluminum and Niobium (Ti-6Al-7Nb) on the other hand acquire higher tensile strength and modulus compared to Titanium alone and hence preferred for making intra-medullar rods, spinal clamps and self-tapping screws. In the context of biomechanical incompatibility and mismatch leading to implant loosening, fracture and failure, Titanium based biomaterials proved superior in comparison to other metals that made them popular for orthopedic use [2]. The only major drawback observed with Titanium and its alloys is the severe frictional wear, which may at times lead to wear debris causing inflammatory reactions. Nevertheless they are still rated superior in terms of biocompatibility compared to other metal implants.
Titanium alloyed with Nickel forms another promising material Nitinol (NiTi) for implants. It exhibits super-plasticity, high damping properties besides shape memory effect, an altogether novel property [3]. The elastic modulus of porous Nitinol (38-48 GPa) is found close to cortical bone (4.4-28.8 GPa) where porosity allows bone cell penetration and better integration making it suitable for implants. Nitinol, particularly with 16% porosity exhibits excellent combination of mechanical properties. It shows high strength (1000 MPa), large compressive ductility (>7%), large recoverable strains (>6%), high-energy absorption (>30 MJ/m3) and low Young modulus (15 GPa) [4]. Excellent biocompatibility has been recorded but few studies also reported undesirable leaching of Ni, though below toxicity level from Nitinol implants [5].
Metallic implants are good for 10-17 years and if appropriately accepted/ integrated with the system, could be left in the recipient’s body without any undesired consequence.
Metals to bioceramics
Long-term stability of prosthesis depends on the fatigue strength, in-vivo compatibility and acceptance of the biomaterial. To address the problem of compromised biocompatibility due to corrosion and in turn leaching as the leading cause of toxicity associated with metallic implants; ceramic materials drew attention. Ceramics, the nonmetallic-metallic composites are tough and found much superior in terms of biocompatibility. The extraordinary physiological compatibility of ceramics is attributed to its constituents like Calcium, Potassium, Magnesium, Sodium, etc., native to the biological system that are combined with Aluminium or Titanium like metals. Plaster of Paris (CaSO4.½H2O) is the first and most elementary ceramic used for filling long bone defects [6,7]. However, traditional ceramics like porcelain, glass and other refractory materials are mostly brittle and cannot substitute metals.
Ceramics have no ductility and limited bending strength therefore brittle [8]. Ceramic wear caused by the brittleness is considered a major concern with their implants [9]. In some cases ceramic liner fracture is observed within 3-10 months of surgery [10]. Therefore, new generation of industrial ceramics that are toughened with metals like alumina, zirconia, silicon carbide or nitride are being developed.
They are found competent to metals in terms of strength. Ceramics with Alumina, Zirconia, Calcium phosphates like additives that enable and improve their biocompatibility are now termed as bio-ceramics. It is anticipated that the long term exposure to physiological conditions would not affect the inert especially the oxides or carbon compositions of bioceramics and even if some degradation occurs, it would be well taken care by the natural metabolic regulation. Most ceramics that compete with metals are harder and also possess lower density but higher strength and resistance to temperature and corrosion. Their lower density makes them lighter and better suited for implants in comparison to metals. Alumina containing ceramics are the most extensively studied ceramics that initiated immediate applications in the total hip, knee and other joint replacement surgeries. They also made their way in bone bonding (e.g., Hydroxyapatite), bone spacing (e.g., porous Alumina) and also for joining small orthopedic joints like fingers and spinal inserts.
The ceramic bone implants can be classified on the basis of their composition and resultant properties as,
Bioinert,
Bioactive,
Bioresorbable.
Bio-inert ceramics induce minimal response from the body, but otherwise exhibit high compressive strength and excellent wear resistance. Alumina, partially stabilized Zirconia and Silicon nitride compositions fall in this category. Unlike bio-inert, bioactive ceramics are capable of interacting with their surrounding either through surface functionality or inherent material porosity. Such ceramics generally have low mechanical strength and fracture toughness and are often used as a coating on metallic bone implants or as fillers in dental implants. Bioglass and Hydroxyapatite (HA) are key examples of bioactive ceramics. HA by virtue of its porous makeup allows bone in growth and facilitates osteointegration. Resorbable ceramics on the other hand are different in terms of their gradual biodegradation, in-vivo. Their degradation products must be safe and noninterfering to the physiological system. It is also imperative that they exhibit standard or predictable dissolution kinetics. Calcium phosphate ceramics especially Tri-Calcium phosphates, often used for bone repair represent resorbable ceramics.
All-ceramic hip joint with 32 mm alumina heads and alumina cups was first explored in seventies by a French surgeon Pierre Boutin [11]. The implant lasted well around 17 years until the patient died without any complications or complaints. No wear was observed in the retrieved ceramic implant, unlike the standard polyethylene cups. Thus, for the first time, hip- joint repair protocols shifted from metals to the premise of metal-nonmetal composites with a higher success rate in terms of better acceptability, less wear-tear and longevity of implants. The alumina and zirconia enriched polyethylene cups for hip-joint replacement was approved by FDA in 1990. Stryker, Wright Medical and CeramTec AG are the three companies that started marketing ceramic-on-ceramic and ceramic-polymer hip implants from 2002 [12].
So far ceramics are considered as safe material that allows osteogenesis to happen. However, extent of it is found to be dependent on particle size. It is also observed that the biological response-cascade, post-implantation of ceramics is similar to fracture healing that involves hematoma formation, inflammation, neo-vascularization, osteoclastic resorption followed by new bone formation [13]. However, these materials are reported to cause an early non-specific inflammation and bone marrow depletion if implanted intra-medullarly or close to bone marrow [14]. Other problems may arise due to modulus mismatch or micro-motions at the implant/bone interface that limits its stability. Bioresorbable ceramics are new class of ceramics that are meant to be used as temporary fillers or fusion material which eventually degrades or dissipate. The constituents as well as their metabolic degradation products should necessarily be safe and biocompatible.
The evolution of support material from steel to ceramics for bone healing and repair has been steady with the development of new metallic composites using innovative processing and sintering techniques. It is now possible to introduce desired properties through new elements and components at different stages and create novel composites. For example uniform and fine grain sizes while creating the blend can impart better strength and lower wear properties to the ceramic. Selective laser sintering and liquid phase sintering are other process improvements that have profound impact on the fracture toughness of the biomaterial. Use of Zirconia toughened alumina nano-composites are expected to create ceramic-ceramic implants with a potential life span of more than 30 years. Zirconia (2.5%), uniformly distributed among the alumina grains exceeds stress threshold as compared to individual constituents. Unlike pure Zirconia the composite does not require stabilizers, which happens to be the major source of crack generation [12]. Such developments in the last two decades have put ceramic-on-ceramics as the new benchmark in hipjoint implants.
Issues with metallic and non-metallic (bioceramic) implants
Success in achieving sufficiently lasting implants has permitted analyzing other issues associated with surgical implants and pay attention to reasons of failures causing revision surgery. Although brittleness and wear problems associated with ceramics could be handled to a large extent with the new composites there remains another issue of squeaking. Squeaking is an annoying complaint reported with not only ceramic-on-ceramic but also with metal-on-metal bearings [15-17].
It has been observed that materials possessing higher stiffness than bone cause “stress shielding effect” [18]. Such biomechanical incompatibility of implant prevents stress transfer to the adjacent bone. This results in bone resorption around the implant and its loosening as a consequence. Implant material and its adequate fixation are not the only issues that may cause the failure of implants. Acceptable osteointegration with the host that generally depends on the roughness and surface chemistry of implants also has an impact. Thrombosis, fibrosis and surface corrosion due to local body fluid reaction are other concerns that may lead to implant failure and need of revision surgery. Corrosion may cause local toxicity and/or immune reactions including chronic inflammation and pain. Recurrent surgery may also become essential if the patient has to undergo X-ray or MRI for other medical problem where the implant may interfere in the diagnosis. Another valid reason for second surgery could be when the implant surpasses its age, which is possible due to increased average life expectancy.
Prosthetic implantation is always accompanied by the risk of microbial infection, more so when the implant is introduced to fix an open-fractured bone or replace a failed implant i.e., revision surgery [19]. While biomechanical incompatibility and mismatch could lead to implant fracture and failure, the sub-optimal fatigue strength, in-vivo compatibility, acceptance/host-integration and uncontrolled microbial infections also determine the long-term success of an implant. There are two major reasons of delayed acceptance or rejection of implant by the host; one their interface does not allow bone in-growth, hindering integration and another the profound immunological activity of the host that tend to create a fibrous capsule around the implant disconnecting it all the more from the body. It is believed that this could be avoided to a large extent by maintaining the highest level of sterility over and around the implant. Thus, efficient sterilization of implant and also managing the host immunity in a localized manner for some duration before and after the implant might help. Objective should be to prevent the immune system from experiencing extreme stress so that its reactive response could be controlled or minimized. An aggressive immune response at the site of implant is not desirable to prevent the cascade of rejection. At the same time sub-optimal host immunity might also have adverse effects if it fails to counter the microbial load, inevitably present in the surroundings of the implant. Hence, the surgical environment and the balanced host immune response both are equally accountable for preventing bacteria from outgrowing and generating a biofilm around the implant. Biofilms are compact bacterial cells that co-ordinate to weave a matrix/covering in order to shield and shut-out their colonies from immediate host microenvironment. If the adhesion of bacteria occurs before bone in-growth takes place, host defense cannot prevent surface colonization of bacteria which may eventually form a biofilm. Other issues that may lead to the need of corrective surgery are thrombosis, fibrosis and sometime surface corrosion due to the reaction of local body fluids with the implant biomaterial. Surface corrosion may cause local toxicity and/or immune reactions including inflammation. Adequate osteointegration of implant generally depends on the roughness and its surface chemistry that directly comes in contact with the bone.
It has also been observed that compromised conditions during surgery may unduly increase the local bacterial load that ultimately succeeds to create a biofilm around the implant. Biofilms are difficult to be handled by the immune cells locally and often trigger an aggressive immune response leading to chronic inflammation and pain. It is already reported that bacteria in the form of Biofilm are 10-1,000 fold less susceptible to antibacterial agents compared to their planktonic (free-floating) culture [20]. A biofilm once formed over the implant is not easy to disrupt unless charged with strong antibiotics, preferably on site. Surface modifications of implants by hydrophilic polyurethanes [21], polyethylene glycol [22] and polyethylene oxide brushes [23] are found to prevent bacterial colonization but how these changes would prompt the host immunity is not yet examined. Coating and covering the implants with antibacterial agents is also attempted. Progress in inorganic and composite coatings with bactericidal activity over the metal and ceramic orthopedic implants has recently been reviewed by Simchi [24].
Besides bacterial infection and immunological causes of rejection, nonunion and loosening are recognized as other major issues associated with prosthetic implants. Loosening and non-union of implant is often considered a compelling issue for revision surgery. Poor integration of implant could be addressed by creating a nano-grooved surface for facilitated bone in-growth. Bone in-growth can strengthen the hostimplant interaction and accommodate it as an integral part of the host system. Development of nano-ceramics is expected to solve the integration issue by offering better surface adherence to the host cells for initiating integration. Improved cellular adhesion on nano-structured surface is expected to enhance osteoblast proliferation and differentiation while increasing biomineralization. Additionally, the rough surface could also be coated with metals like Silver (Ag) or some mild antibacterial agents. Silver is known for its antibacterial effect since ages and is well tolerated by the human body [25]. This would discourage even the systemic bacteria to lodge over the implant. Even the non-pathogenic bacterial colonies on the implant are undesirable as they may alter the biochemical environment through pH change.
Contemporary approaches
Bones are highly heterogenic tissue in terms of their shape, skeletal location, constitution and in turn their strength. Effective mending of bone defects therefore may involve several factors to be taken care. Thus materials suitable for supporting and healing long bones may not be equally effective or useful for the joints or ribs or joining the vertebrae of spinal cord. Keeping the positional and modulus variations and related complications other possible approaches are being worked out for managing skeletal fractures. Last few decades have witnessed more advanced and interdisciplinary efforts for addressing wider spectrum of bone related issues including immunological acceptance and nonunion of implants. Such advancements are possible through our upgraded understanding of bone morphology, its cellular arrangements and Extracellular Matrix (ECM). It is now understood that the ECM plays a major role in the remodeling of bone and cartilage. This revelation has opened another podium for intervention. Bone undergoes continual remodeling depending upon the extent and type of stress and strain it bears. This makes its ECM even more complex, disordered and heterogeneous in terms of porosity. Taking this unique fact into account attempts for creating Functionally Graded Biomaterials (FGM) are made [26]. As expected the FGM entice tissue response in a gradient manner offering a tool to manage the response as per the need [27]. Bone fixing and fusion through polymeric glue and putty are other novel approaches. They could find applications in the treatment of osteoporotic bones as well as hairline fractures which cannot possibly be helped through prosthetic implants.
Advanced approaches
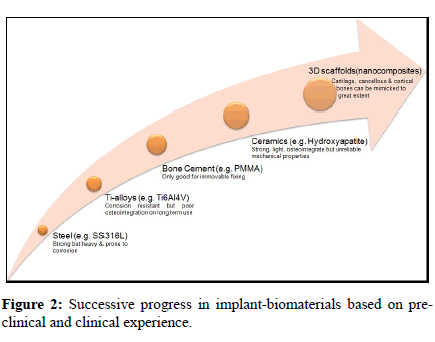
Advancement and knowledge of synthetic polymers and copolymers and possibility of having control over their physical properties has created a new hope in material research. Not just support but a complete substitution through biochemical and morphological mimicking of bone seems feasible now. The strength and elastic moduli of cancellous bone in human body ranges from 1.5 to 38 MPa and 10 to 1570 MPa respectively [28]. Such wide range of bone strength and elasticity can possibly be handled through a new advanced approach of creating porous organicinorganic nano-composites. In these composites, the organic content brings cell-interactivity while the inorganic component imparts strength to the biomaterial. The strength of the material could be controlled by changing the ratio and phases of ‘Organic-Inorganic’ crystalline medium that can cover a spectrum of material property while facilitated cell interactivity is expected to yield better host integration. Hence, such composites are expected to offer a versatile biomaterial that can be customized for different intra as well as inter-osteo- implant needs in terms of strength and compactness. This approach is inspired by biological materials that we see around. In fact natural bone, teeth, sea shells etc. represent the inorganic- organic composites only. They generally are shaped through molecular self-assembly in genetically controlled manner. An ordered assembly of biomolecules in the nano-scale allows specific structural orientation of crystalline inorganic and polymeric organic molecules, which is exceedingly tissue specific [29]. Molecular deposition makes it possible to have larger organic-inorganic interfaces that may acquire different orientations, patterns and architecture. Such ordered orientation at nano-scale level if adapted could result in unique mechanical properties in tune with that particular type of bone or cartilage. A material that can offer the versatility of altering its strength and elasticity at nano-scale, in a wider range, to appropriate the bone to be replaced may be a better option to look for (Figure 2). A sol-gel material that could be injected to the site of fracture not just to fill the gap quickly, but to integrate and induce the natural remodeling cascade would be very useful.
Challenges
One of the remarkable features of biological materials is their structural hierarchy. Bone ECM shows even more complex structural features due to continuous bone remodeling dictated by the stress and strain to which it is exposed to. The structural density and elasticity also differs from cortical, cancellous and cartilaginous bones. Not only the type but the location and its use, which is purely individualistic, also govern its strength and overall performance posing a challenge in implant development.
The dynamic use of joints in terms of load bearing varies from individual to individual and depends on different factors including personal life style, anatomy, general health and age. As the stress and strain on the bones varies, the performance of the same implant in patients may also differ widely. For example, a knee joint replacement in a young athlete may not last for the same period it may in a person of similar age but having less sporting lifestyle. Setting benchmark parameters for testing implant exvivo is therefore quite challenging.
With deeper understanding of bone fractures and issues related to bone implants, many important considerations for design and material properties have emerged. For example the material should preferably match the modulus of the bone to be repaired which could be competently achieved by hierarchical or gradient material properties, be able to withstand the positional stress and strain it might expose to once implanted, be highly resistant to corrosion under the influence of body fluids, allow osteointegration with the adjacent bone and tissues of the host for preventing loosening and detachment of the implant and appropriate measures should be taken to keep the implant and its surrounding aseptic. These issues are mostly dependent on the type and location of bones and greatly influenced by the age of the patients.
In human body, the type, flexibility and extent of movements possible in different directions vary from joint to joint (Figure 1). Considering the spectrum of human bones in terms of Young modulus, ductility and elasticity, it is imprudent to fall back upon single material for effective repair and corrections. In order to avoid revision surgery and the associated trauma, unrelenting efforts are on to develop smart biomaterials for implants that can overcome the issues of infection, integration while managing the strength at desired level [30,31] or even dynamically in gradient manner [32,33].
Acknowledgement
Authors would like to acknowledge the Department of Biotechnology (DBT), India for funding the related project. Also thank and appreciate Dr. Sundaresh, an experienced Orthopaedic for reviewing the manuscript.
Conflict of Interest
None.REFERENCES
- Wapner KL. Implications of metallic corrosion in total knee arthroplasty. Clinical Orthopaedic Related Research. 1991;271:12-20.
- Geetha M, Singh AK, Asokamani R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants-A review. Progress in Materials Science. 2009;54:397-425.
- Prymak O, Bogdanskib D, Koller M, et al. Morphological characterization and in vitro biocompatibility of a porous nickel-titanium alloy. Biomaterials. 2005;26:5801-7.
- Ryhanen J, Niemi E, Serlo W, et al. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. Journal of Biomedical Material Research. 1997;35:451-7.
- Kapanen A, Ryhanen J, Danilov A, et al. Effect of nickel-titanium shape memory. Biomaterials. 2001;2:2475-80.
- Hulbert SF, Morrison JS, Klawitter JJ. Tissue reaction to three ceramics of porous and non-porous structures. Journal of Biomedical Material Research. 1972;6:347.
- Hulbert SF, Hench LL, Forbers D. History of Bioceramics. Ceramics International. 1982;8(4):131-40.
- Hannouche D, Amine Z, Frédéric Z, et al. Thirty years of experience with alumina-on-alumina bearings in total hip arthroplasty. International Orthopaedics (SICOT). 2011;35:207-13.
- Affatato S, Francesco T, Aldo T. Microseparation and stripe wear in alumina-on alumina hip implants. Int J Artif Organs. 2011;34(6): 506-12.
- Hannouche D, Delambre J, Zadegan F, et al. Is there a risk in placing a ceramic head on a previously implanted trunion? Clin Orthop Relat Res. 2010;468:3322-7.
- Boutin P. Total hip arthroplasty with sintered alumina prosthesis. Rev Chirol Orthop. 1972;58:229-46.
- Orthopedic and Dental Industry News; Ceramics: Products and Manufacturers (Orthopedic Biomaterials 2005 report).
- Boyan BD, Hummert TW, Den DD, et al. Role of material surface in regulating bone and cartilage cell response. Biomaterials. 1996;17:137-46.
- Korkusuz F, Uluoglu O. Non-specific inflammation and bone marrow depletion due to intramedularly porous hydroxyapatite application. Bulletin Hospital for Joint Diseases (New York). 1999;58(2)86-91.
- Keurentjes JC, Kuipers RM, Wever DJ, et al. High incidence of squeaking in THAs with alumina ceramic-on-ceramic bearings. Clin Orthop Relat Res. 2008;466:1438-43.
- Schroder D, Bornstein L, Bostrom MP, et al. Ceramic-on-ceramic total hip arthroplasty: incidence of instability and noise. Clin Orthop Relat Res. 2011;469(2):437-42.
- Restrepo C, Matar WY, Parvizi J, et al. Natural history of squeaking after total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2340-5.
- Sumner DR, Turner TM, Igloria R, et al. Functional adaptation and in growth of bone vary as a function of hip implant stiffness. Journal of Biomechanics. 1998;31:909-17.
- Duan K, Wang R. Surface modifications of bone implants through wet chemistry. J Material Chemistry. 2006;16:2309-21.
- Davis D. Understanding biofilm resistance to antibacterial agents. Nature Reviews Drug Discovery. 2003;2:114-22.
- Nagel JA, Dickinson RB, Cooper SL. Bacterial adhesion to polyurethane surfaces in the presence of pre-adsorbed high molecular weight kininogen. Journal of Biomaterials Science E. 1995;7:769-80.
- Kingshott P, Wei J, Bagge-Ravn D, et al. Covalent Attachment of Poly(ethylene glycol) to Surfaces, Critical for Reducing Bacterial Adhesion. Langmuir. 2003;19:6912-21.
- Kaper HJ, Busscher HJ, Norde W. Characterization of poly (ethylene oxide) brushes on glass surfaces and adhesion of Staphylococcus epidermidis. Journal of Biomaterials Science. 2003;14:313-24.
- Simchi A, Tamjid E, Pishbin F, et al. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine: Nanotechnology. Biology and Medicine. 2011;7:22-39.
- Das K, Bose S, Bandyopadhyay A, et al. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. Journal Biomed Mater Res B. 2008;87:455-60.
- Pompe W, Worch H, Epple M, et al. Functionally graded materials for biomedical applications. Materials Science and Engineering A. 2003;362:40-60.
- Wataria F, Yokoyama A, Omori M, et al. Biocompatibility of materials and development to functionally graded implant for bio-medical application. Composites Science and Technology. 2004;64:893-908.
- Khan SN, Warkhedkar RM, Shyam AK. Human Bone strength Evaluation through different Mechanical Tests. International Journal of Current Engineering & Technology. 2014;539-43.
- Sadat-Shojai M, Khorasani MT, Khoshdargi ED, et al. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomaterialia. 2013;9:7591-621.
- Furth ME, Atala A, Dyke MEV. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials. 2007;28:5068-73.
- Sheikh Z, Najeeb S, Khurshid Z, et al. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials. 2015;8:5744-94.
- Moroni L, de Wijn J, van Blitterswijk C. 3D fiber-deposited scaffolds for tissue engineering:Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials. 2006;27:974-85.
- Sobral JM, Caridade SG, Sousa RA, et al. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomateriala. 2011;7:1009-18.