Enteric microbes from human wastes
Received: 17-Jun-2022, Manuscript No. puljmbr-22-5051; Editor assigned: 20-Jun-2022, Pre QC No. puljmbr-22-5051 (PQ); Accepted Date: Jul 15, 2022; Reviewed: 28-Jun-2022 QC No. puljmbr-22-5051 (Q); Revised: 05-Jul-2022, Manuscript No. puljmbr-22-5051 (R); Published: 22-Jul-2022, DOI: 10.37532/puljmbr.2022.5(4).39-44
Citation: Olumide AS. Enteric microbes from human wastes. J Mic Bio Rep. 2022;5(4):39-44.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Feces contain intestinal bacteria and exfoliated epithelial cells that may provide useful information concerning gastrointestinal tract health. With the gastrointestinal tract being the largest area of the body that is constantly exposed to ingested/digested food and microorganisms, it is conceivable that luminal exposure may play a significant role in the development of colorectal cancer. Campylobacter species were first recognized as a cause of abortion in cattle and sheep, and a cause of diarrhea in cattle and pigs. They were first isolated from the faeces of humans in the early 1970s. Campylobacter species are now known to be a major cause of enteritis in the developed world, and are the commonest identifiable bacterial cause of diarrhea in the UK as identified by the second Infectious Intestinal Disease study (IID2). Campylobacters are Gram-negative bacteria which are important animal and human pathogens. Campylobacter is the most commonly identified foodborne bacterial infection encountered in the world. In 2000, approximately 56,000 cases were formally recorded in UK laboratory reports, with as many as 400,000 expected to occur in total.
Keywords
Campylobacter; Salmonella; Escherichia coli O157; Human Wastes.
Introduction
Feces contain intestinal bacteria and exfoliated epithelial cells that may provide useful information concerning gastrointestinal tract health. For example, bacteria activate or metabolize potential carcinogens or can have anti-tumor effects that may have relevance to colorectal cancer, the second most common cause of cancer deaths in the USA [1-3]. With the gastrointestinal tract being the largest area of the body that is constantly exposed to ingested/digested food and microorganisms, it is conceivable that luminal exposure may play a significant role in the development of colorectal cancer [4].
Background
Campylobacter species
Campylobacter species were first recognized as a cause of abortion in cattle and sheep, and a cause of diarrhea in cattle and pigs. They were first isolated from the faeces of humans in the early 1970s. Campylobacter species are now known to be a major cause of enteritis in the developed world and are the commonest identifiable bacterial cause of diarrhea in the UK as identified by the second Infectious Intestinal Disease Study (IIDS) [5]. Campylobacters are gram-negative bacteria that are important animal and human pathogens.
Incidence
Campylobacter is the most commonly identified food-borne bacterial infection encountered in the world. In 2000, approximately 56,000 cases were formally recorded in UK laboratory reports, with as many as 400,000 expected to occur in total [6].
The number of laboratory reports has decreased slightly in recent years, but a high degree of under-reporting is still expected. Although some outbreaks have been reported, most cases occur sporadically. It is most commonly transmitted by raw poultry, raw milk and water contaminated by animal faeces.
Salmonella
These Salmonella species are ubiquitous in animal populations. Human infection is generally associated with the consumption of food of animal origin, the drinking of water contaminated by animals, or inter-personal contact. Inter-personal transmission of non-typhoid Salmonella occurs where levels of hygiene may be particularly poor, in mental healthcare units and schools. There are marked seasonal variations in the occurrence of infection with peaks of incidence during summer and autumn. Infection with Salmonella species may be associated with foreign travel, and consumption of imported foodstuffs may be associated with a higher risk of infection [7].
Incidence
Salmonellae have been some of the most frequently reported aetiological agents in fresh produce-associated outbreaks of human infections in recent years, with over 15,000 laboratory diagnoses in 2000 arising from an estimated 41,616 cases [6]. The highest incidence rates occur in patients aged less than 1 year and in individuals older than 70 years. More prudent food hygiene regulations have seen the reported cases of Salmonella enteritis PT4 (long associated with egg consumption) decline considerably. However, other PT4 strains have been slowly and steadily increasing in incidence. In 2005 there were 1771 reported cases of S. enteridis PT4 and 4868 reported cases of S. enteridis (other PTs) in the UK.
Escherichia coli O157
The genus Escherichia consists of five species, of which E. coli is the most common and clinically most important. Since its recognition in 1982, E. coli O157:H7 has been noted as one of the most dangerous pathogens, as only very small numbers of the organism may be required to cause illness. For this reason, suspect colonies must be handled with care. All tests need to be carried out in a safety cabinet, usually in a biohazard room. It is the only pathogen in this discussion classed as a ‘category 3’ risk.
Incidence
The incidence of E. coli O157 tends to fluctuate, reflecting the outbreak-specific nature of the disease. The highest levels (1087 cases) in the UK were recorded in 1997, largely associated with a highly publicized outbreak in Central Scotland [8]. On average there are approximately 600 to 800 confirmed cases each year, with 946 recorded in 2005, linked to a large outbreak in South Wales.
Literature Review
Enteric bacteria pathogens associated with diarrhea in children
Diarrhea disease is one of the leading causes of illness in young children in developing countries [9]. The public health significance of diarrheal disease cannot be overemphasized. Diarrheal diseases are the cause of almost three million deaths annually mainly among children younger than five years of age [10]. Although the extensive investigation of diarrhea has not been reported, diarrhea–specific mortality in children younger than five years of age in Africa has been estimated at 106 per 1000 [11]. Available reports in Nigeria indicate that more than 315,000 deaths of preschool-age children are recorded annually as a result of diarrhea disease [12]. The main aetiology of diarrhea is related to a wide range of bacteria, endoparasites, and viruses. The contribution of the various pathogens to diarrhea may differ substantially between regions depending on local meteorological, geographic, and socio-economic conditions [13]. Underlying reasons for the spread of diarrheal diseases are found in poor hygiene and sanitation, limited access to safe drinking water as well as in inadequate education of health care providers and recipients [14,15]. Knowledge of the pathogens associated with diarrhea is pertinent in not only allowing the optimum use of available interventions but will also direct efforts aimed at developing specific therapies and preventive vaccines. Unfortunately, due to limited resources, the microbiological diagnoses of diarrhea are not done easily in many settings in Nigeria.
Bacteria commonly associated with gastrointestinal infections
Campylobacter species
Campylobacter enteritis in the UK has marked seasonal peaks which occur in May and September. Campylobacter jejuni accounts for about 90% of reported infections and most of the remainder are caused by Campylobacter coli and Campylobacter lari; other Campylobacter species have also been isolated from cases of diarrhea [16]. The species most commonly associated with disease in humans are thermophilic, i.e., they will grow at 42°C to 43°C and 37°C, but not at 25°C. Campylobacter jejuni subspecies doylei and Campylobacter fetus (and C. fetus subspecies venerealis) do not grow at 42°C. In human hosts, diarrhea is usually brief, and sequelae are uncommon. Initial symptoms may be severe, with fever and abdominal pain suggesting appendicitis. Faeces frequently contain mucus with blood and leucocytes. Campylobacter species infection may occasionally become invasive, with consequences ranging from transient self-limiting bacteremia to fulminant Gram-negative sepsis. Occasionally an infection may produce sequelae such as reactive arthritis, bursitis, endocarditis, and neonatal sepsis. Acute post-infective demyelination may develop, affecting the peripheral nervous system (guillain barré syndrome), and/or the central nervous system and cranial nerves (eg the miller fisher syndrome (are flexia, ataxia, and cranial nerve pareses; polyneuritis cranialis). Specific serotypes are implicated in these conditions [17]. The clinical presentation of these latter conditions (affecting the brainstem and the cranial nerves) must be distinguished from that of botulism. Deep tendon reflexes are initially preserved in cases of botulism but are lost early in cases of post-infective demyelinating disease. Nerve conduction is slowed in demyelinating diseases, and the cerebrospinal fluid commonly shows an increase in protein concentration, usually without any accompanying pleocytosis [17]. Both groups of disorders may culminate in respiratory failure, requiring mechanical ventilation. Campylobacter fetus is an opportunist organism that may be isolated from blood and other body fluids of immune deficient patients and is responsible for 8% to 10% of campylobacter bacteremia cases.
Symptoms
Campylobacteriosis is an acute bacterial enteric disease ranging from asymptomatic to severe, with diarrhea, nausea, vomiting, fever, and abdominal pain, with an illness, usually lasting 2 days to 5 days.
Although Campylobacter is in itself a relatively harmless pathogen, it can cause post-infectious complications which are potentially very serious. Campylobacter is believed to be a leading cause of GBS, an autoimmune reaction that causes paralysis and kills between 5% and 10% of its victims. Approximately 1 in 1000 cases of C. jejuni develops into GBS after 7days to 21 days of infection [18]. Reitter’s syndrome, a form of reactive arthropathy, can also occur in up to 1% of campylobacteriosis patients.
Treatment
In the general population, campylobacterios is a self-limiting disease, for which antimicrobial therapy is not required [19]. However, as with many intestinal infections, infants and immunocompromised individuals are at higher risk of developing more severe infections.
Salmonella species
Non-typhoid Salmonella (Salmonella species other than Salmonella typhi, Salmonella paratyphi A, B and C) and Salmonella dublin. Gastroenteritis is the most common condition caused by Salmonella species. Symptoms include abdominal pain, diarrhea, nausea and vomiting, often accompanied by fever. Other clinical manifestations of salmonellosis include bacteremia and focal metastatic (hematogenous) infections and the organism may be isolated from other specimens such as blood and urine. A small number of patients may develop an illness that resembles enteric fever. Low numbers of Salmonella species may also be present in the faeces of healthy asymptomatic carriers. Certain underlying conditions such as malnutrition, immunosuppression, sickle-cell disease, achlorhydria, and inflammatory bowel disease may be associated with more severe infections. Salmonella infection remains a serious problem to public health significance in worldwide and causes substantial economic loss resulting from mortality, morbidity and poor growth with the hazard of transmitting food poisoning with gastroenteritis to humans and represents a serious problem for the food industry [20,21]. Human spreads Salmonella mainly through the stool. Food-borne illness among people and transmission can occur when food and water are contaminated with stool or through a direct fecal-oral route. Human stool acts as an important reservoir of Salmonella serovars that are the grouping of microorganisms based on their cell surface antigen. Species isolated from human stool are Salmonella typhi, S. paratyphi A, S. typhimurium, S. wrothington, and S. enteritidis [22]. Salmonella is a worldwide issue in the public health sector. People most at risk for serious complications due to Salmonella food poisoning include older adults, pregnant women, infants, children, and people who have compromised immune systems. Salmonellosis is manifested clinically in all hosts by one of three major syndromes, acute systemic infection, acute enteritis, or chronic enteritis [23]. Symptoms usually include headache, nausea, vomiting, fatigue, gastroenteritis, abdominal cramps and bloody diarrhea with mucus, and sometimes reactive arthritis (reiters syndrome) [24]. Following Salmonellosis dehydration with renal insufficiency and death may occur. The importance of Salmonellosis in the public health sector is a growing concern day by day throughout the world and over the last several decades there has been a significant shift in predominant Salmonella serovars associated with human infections [25]. Salmonellosis in the past has caused tremendous loss to society in many countries around the world. Two to four million cases have been reported annually and yet a significant number of cases have been unreported worldwide. Non-typhoid Salmonella is the leading cause of food-borne illness and its increasing antimicrobial resistance is associated with higher risks of hospitalization in Bangladesh (ICDDR, B). Non-typhoid Salmonella was found responsible for 66% of cases of food-borne illness in Bangladesh. The highest proportion (15%) was isolated in 1998 followed by in 1995 (13%) while it was less than 10% for other years. 36% were isolated during the summer while 28% were in the fall. There is a lack of sufficient studies emphasizing isolation and characterization of Salmonella serovers, considering human stool in Bangladesh.
Enteric fever
Although many Salmonella species are recorded to have caused invasive infections, those most regularly doing so are Salmonella typhi and Salmonella paratyphi (groups A, B, and C) - the causative organisms of enteric (typhoid) fever. Many Salmonella serotypes may be transmitted from animals to man, but S. typhi and S. paratyphi. A usually carried by humans only and transmitted via human faecal contamination of food or water. Individuals recovering from enteric fever may carry the organism for long periods. Relapsing, non-enteric Salmonella species infections may be seen in patients with HIV/AIDS. Cultures of S. typhi and S. paratyphi A, B, or C, known or suspected, must be handled at Containment Level Enteric fever is a multi-system disease characterized by:
1. Prolonged fever
2. Hypertrophy and activation of the reticuloendothelial system, particularly the intestinal and mesenteric lymphoid tissue, liver, and spleen
3. Sustained bloodstream infection without endothelial or endocardial colonization
4. Metastatic infection and immunologic complications such as immune complex deposition leading to multi-organ dysfunction
5. Rose spots
6. Association with constipation (diarrhea seldom being present until late in the disease course)
7. Reactive arthritis
8. Low white blood cell count
The causative organism of an enteric fever may not always be present in faeces. Faecal culture alone is not adequate for the laboratory investigation of enteric fever. Blood cultures should always be collected and enteric fever may be confirmed by isolating S. typhi/paratyphi from the blood (B 37- investigation of blood cultures (for organisms other than Mycobacterium species), bile (B 15 investigation of bile), bone marrow (B 38- investigation of bone marrow) or urine (B 41-investigation of urine). Chronic carrier states occur when patients recover from the acute disease (either gastroenteritis or enteric fever) but continue to shed the organism. Therefore, Salmonella species may be present in the faeces or urine of patients for one year or longer. The principal site where organisms are harbored in the biliary tract. Obstruction with gallstones or biliary scarring makes the eradication of organisms difficult. Similarly, the carriage in the urinary tract may be associated with urolithiasis, and with damage caused by urinary schistosomiasis.
Transmission
Most infections are acquired by eating contaminated poultry, eggs, or dairy products. According to some estimates, almost 3 quarters of all broiler chickens are contaminated. With Salmonella during de-feathering, slaughtering and evisceration, when faeces splatter the skin, World Health Organization (WHO) 2002. Salmonella also spreads easily from raw or undercooked poultry to innocent vegetables, fruit, or other foods via contaminated hands, knives, countertops, or cutting boards. Due to the ability of Salmonella to multiply in a wide variety of foods, it is important to be able to isolate the organisms even when present in very small numbers in the faeces.
Symptoms
The symptoms of Salmonella infection are abdominal pain, diarrhea, mild fever, chills, headache, nausea, and vomiting, developing 12 hours to 72 hours (but occasionally as long as 7 days) after infection. The discomfort generally lasts a few days. It can be dangerous for the elderly, infants, and the immunocompromised, who may become extremely ill. Salmonella is also one of the leading predictors for reactive arthritis, a painful, chronic, and potentially debilitating condition that causes joint inflammation [26].
Treatment
Salmonella infection in older children and adults is usually a self-limiting disease (presenting as acute gastroenteritis), and therapy should mainly be directed at preventing dehydration. A recent Cochrane review 18 of antibiotic treatment for Salmonella gut infections suggested that they provided no clinical benefits to otherwise healthy children and adults with non-severe cases.
Antibiotic administration may prolong Salmonella [27]. However, it is justified to use antimicrobial therapy for infants under 3 months old with Salmonella gastroenteritis, and also in immuno compromised patients and patients with septicemia. In these patients, antibiotic treatment will be most successful in the early stages of illness, and delaying treatment may result in septicemia-related dehydration and renal failure [28]. Because the early stage is often clinically difficult to determine, these patients might benefit from rapid tests that can be done quickly in the place where the patient is receiving care.
Shigella species
Infection with Shigella species manifests as a range of symptoms. At its mildest, watery diarrhea is produced, but this may progress to dysentery with frequent small volume faeces containing blood, mucus, and pus. Diarrhea may be accompanied by fever and abdominal cramps. There is often marked constitutional disturbance (in contrast to cases of dysentery caused by Entamoeba histolytica, where the patient may remain relatively well apart from gastrointestinal disturbance). There are 4 Shigella species:
1. S. dysenteriae
2. S. flexneri
3. S. boydii
4. S. sonnei
Diagnosis of bacillary dysentery is made by isolation of the infecting organism. Cultures of S. dysenteriae, known or suspected, must be handled at Containment Level 3. All 4 species are capable of causing dysentery, but Shigella dysenteriae serotype 1 causes a particularly severe form of the disease with marked constitutional disturbance.
This is due to the production of Shiga toxin, which is closely related to the toxin produced by strains of verocytotoxic E. coli O157 (VTEC). As in infection with VTEC, infection with toxigenic S. dysenteriae may result in the Hemolytic-Uraemic Syndrome (HUS). Organisms are primarily transmitted directly from person to person, and multiplication in the environment rarely occurs. Organisms are easily transferred on fingers (faecal-oral spread), via food or water, or by contaminated fomites. Shigella species are highly infective, particularly S. dysenteriae, which may require as few as 10-100 organisms for an infective dose [29]. The asymptomatic infection has been reported, particularly with strains of Shigella sonnei. Outbreaks may be associated with overcrowding in schools, prisons, mental institutions, and where there are low standards of hygiene. Deaths are more commonly seen during famine and in countries with poor socio-economic circumstances [29]. S. sonnei is the commonest species isolated in the UK. S. dysenteriae and S. boydii are rarely seen in the UK, except as a consequence of foreign travel.
E. coli VTEC (including O157) (health protection agency, 2011)
E. coli VTEC is an enterohaemorrhagic E. coli (EHEC) that produces verocytotoxins. E. coli VTEC is also known as Shiga-like ToxinProducing E. coli (STEC). Verocytotoxin is similar to the ‘Shiga’ toxin of Shigella dysenteriae, and is associated with hemorrhagic colitis and hemolytic uraemic syndrome [30,31]. There are over 300 known serotypes of VTEC, most of which are not pathogenic: the most common pathogenic serotype in the UK is E. coli VTEC O157, and this is the only VTEC for which diagnostic laboratories routinely test [32]. Low numbers of verocytotoxin producing E. coli O157 are required to cause infection. Infections vary in severity from mild to bloody diarrhea (presenting as maroon-colored stools) and may occur in any age group, although it is more common in children. Blood is not always present in faeces in verocytotoxin producing E. coli infections, and the presence of blood must not be used as the sole criterion for selecting specimens for examination for this organism. The highest incidence of verocytotoxin producing E. coli O157 infection is in children <5 years of age. There is a marked seasonal variation, with a peak incidence in the summer and early autumn. Outbreaks have been directly associated with contaminated cooked meats, milk and water, ground beef, beef burgers, and indirectly with vegetables, apple cider, and mayonnaise [33]. Outbreaks may occur in establishments such as nursing homes [33]. There have also been outbreaks of E. coli O157 infection involving visitors to open farms [34]. This SMI recommends direct plating for E. coli VTEC (including O157) of all diagnostic specimens, including those for screening asymptomatic contacts in risk groups. Culture is recommended for all diarrheal faecal samples. Suspicious isolates that have been locally confirmed by serological and biochemical tests can be identified as ‘presumptive’ E. coli (VTEC). Cultures of E. coli O157 and other VTEC, known or suspected, must be handled at Containment Level 3. There have recently been cases of infection with sorbitol fermenting VTEC O157 [35]. ‘Presumptive’ (locally confirmed) isolates should be referred to the reference laboratory for detection of verocytotoxin genes, confirmation of identity, and phage typing. Most VTEC O157 strains are motile and have the flagella antigen H7, but about 20% of strains are phenotypically non-motile. As available methods and selective agars are primarily aimed at detecting VTEC O157:H7, faeces from patients with a dysenteric syndrome for which no cause can be found by use of standard microbiological techniques should be sent to a reference laboratory for molecular testing and enhanced culture to search for both E. coli O157 and other non-O157 verotoxigenic strains of E. coli.
Symptoms
E. coli O157 can cause acute bloody diarrhea and abdominal cramps. Persons who only have diarrhea usually recover completely, without antibiotics or other specific treatment, in 5 days to 10 days. There is no evidence that antibiotics improve the course of the disease, and it is thought that treatment with some antibiotics may precipitate kidney complications. Antidiarrheal agents, such as loperamide (Imodium), should also be avoided.
Diagnosis
Vero cell cytotoxicity essays are probably the most sensitive method of detecting Shiga toxin E. coli strains such as O157:H7. However, most hospital laboratories would not routinely perform tissue culture work with Vero cell monolayers available on demand. Moreover, Vero cell assay results are generally not available for 48 hours to 72 hours. Instead, sorbitol macconkey agar is currently used to detect E. coli O157 in UK laboratories. Isolation of E. coli O157:H7 from water and other environmental samples is laborious. Culture is problematic due to the large numbers of other flora that either overgrow or mimic the non-sorbitol-fermenting E. coli O157:H7 Non-O157 strains. Although several EHEC serotypes have been associated with human infection, recent well-publicized outbreaks of infection with E. coli O157:H7 have resulted in a focus on the development of methods for the identification of the specific EHEC serotype. The number of documented infections other than O157:H7 is probably an underestimate, due to the use of serotype-specific methods. This single serotype-directed effort is in part justifiable, in that the majority of HUS cases are caused by E. coli O157:H7. However, this may lead to a failure in the assessment of the prevalence of other EHEC isolates associated with human disease and also may leave health authorities unprepared for the emergence of new clones of these organisms.
Material and Methods
Sample collection and analysis
Five fresh stool samples, obtained from a patient of the Ijebu Ode general hospital, in Ogun State, were collected in plastic containers that were immediately put on ice and taken to the laboratory for analysis. After serial dilution, 0.1 ml of each water sample was dispensed in macconkey agar and incubated at 37ºC for 24 hours.
Sterilization of materials used
Glassware such as test tubes, pipettes, conical flasks, beakers, universal bottles, and Bijou bottles was sterilized by washing them with detergents and placed in an oven at 160ºC for 3 hours. Foil paper was used to cover opened glassware before they are sterilized. Inoculating loop and needles were sterilized by flaming until red hot and allowed to cool. The culture media used were MRS Agar, nutrient agar, macconkey agar, kliger iron agar, and citrate agar and are prepared according to the manufactures prescriptions and then sterilized by Autoclaving at 121ºC for 15 minutes.
Stains used
Crystal violet solution, safranin solution, and lugol iodine. Solvents used were hydrogen peroxide, ethanol, saline water, and distilled water.
Morphological characterization
Gram Staining
The isolates were Gram-stained to determine their gram reaction.
1. A colony from supposed pure culture to be identified was emulsified in physiological saline water on a grease-free slide and allowed to air dry.
2. The dried smear was fixed by passing the slide through a Bunsen flaming for 3 minutes.
3. The dried smear was covered with crystal violet stain for 60 seconds.
4. The stain was rapidly washed off with clean water.
5. The water was tipped off and the smear was covered with lugol iodine for another 60 seconds.
6. The iodine was washed off with clean water.
7. The smear was rapidly decolorized with ethanol and immediately washed off with clean water.
8. The smear was then covered with safranin for 60 seconds
9. The stain was washed off with clear water and left to air dry on a draining rack.
10. The smear was then examined microscopically under the oil immersion of the microscope.
Biochemical Test
Catalase Test
Principle
This test is used to differentiate the bacteria that produce the enzyme catalase, from non-catalase-producing bacteria.
Procedure
1. Pour 2 ml to 3 ml of the hydrogen peroxide solution into a test tube.
2. Using a sterile wooden stick or a glass rod (not a nichrome wire loop), remove several colonies of the test organism and immerse them in the hydrogen peroxide solution.
3. Look for immediate bubbling.
Results
Active bubbling-Positive catalase test
No bubbles- Negative catalase test
Oxidase Test
Principle
A piece of filter paper is soaked with a few drops of oxidase reagent. A colony of the test organism is then smeared on the filter paper. Alternatively, an oxidase reagent strip can be used. When the organism is oxidase-producing, the phenylenediamine in the reagent will be oxidized to a deep purple color.
Citrate Test
Citrate method using simmons’s citrate agar is:
1. Prepare slopes of the medium in bijou bottles as recommended by the manufacturer (store at 2 ºC to 8ºC).
2. Using a sterile straight wire first streak the slope with a saline suspension of the test organism and then stab the butt.
3. Incubate at 35ºC for 48 hours. Look for a bright blue color in the medium.
Kligler Iron Agar Test (KIA)
KIA powder according to the manufacturer’s prescription is dissolved into the appropriate millimeters of distilled water. Boiled to dissolve the agar properly and dispense into tubes to cover 2/3 of the tubes. Pug with cotton wool at 121ºC.
Blood Agar Plates (BAP)
This is a differential medium. It is a rich, complex medium that contains 5% sheep red blood cells. BAP tests the ability of an organism to produce hemolysins, enzymes that damage/lyse red blood cells (erythrocytes).
Beta-hemolysis is complete hemolysis. It is characterized by a clear (transparent) zone surrounding the colonies.
1. Partial hemolysis is termed alpha-hemolysis. Colonies typically are surrounded by a green, opaque zone.
2. If no hemolysis occurs, this is termed gamma-hemolysis. There are no notable zones around the colonies.
3. Urease Test
Using urease agar base
1. Prepare slopes of the medium in bijou bottles as recommended by the manufacturer (store at 2°C to 8°C).
2. Using a sterile straight wire first streak the slope with a saline suspension of the test organism and then stab the butt.
3. Incubate at 35°C for 48 hours. Look for the yellow color in the medium.
Results
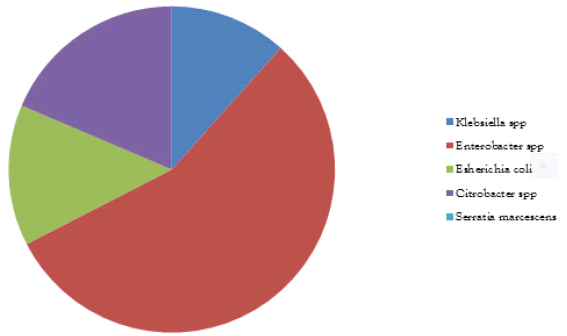
5 faecal samples were collected and 48 isolates were obtained. The microbial analysis shows that bacterial isolate was 4 Campylobacter spp (8.33%), 5 Klebsiella spp (10.42%), 24 Enterobacter spp (50%), 6 Escherichia coli (12.50%), 8 Citrobacter spp (16.68%)and 1 Serratia marcescens (2.08%) as shown in Figure 1. The biochemical analysis is shown in Table 1 below.
TABLE 1 Frequency, percentage of microorganisms isolated
| Isolated organisms | Frequencies | Percentage (%) |
|---|---|---|
| Campylobacter spp | 4 | 8.33 |
| Klebsiella spp | 5 | 10.42 |
| Enterobacter spp | 24 | 50 |
| Escherichia coli | 6 | 12.5 |
| Citrobacter spp | 8 | 16.67 |
| Serratia marcescens | 1 | 2.08 |
Discussion
Acute diarrhea due to bacterial infections is an important cause of morbidity and mortality in infants and young children in most developing countries including Nigeria [36]. Clarification of the enteropathogens involved in diarrheal disease in the country is an essential step towards the implementation of effective primary health care activities against the disease [11]. In this study, our result shows that six bacterial species (Escherichia coli, Klebsiella pneumoniae, campylobacter spp, Enterobacter spp, Citrobacter spp, and Serratia marcescens) were isolated.
Conclusion
Although there is a geographical difference in the spectrum of bacteria incriminated in human faeces, Enterobacter and Citrobacter dominate this study. However, Statistical analysis showed that Escherichia coli was significantly associated with diarrhea in children younger than 3 years (p<0.05). There appear to be conflicting reports about the association of Salmonella species with diarrhea. Bacterial isolation age-wise diminished between the ages of 25 months -36 months and agrees with past reports from Brazil, Denmark and Turkey.
REFERENCES
- Blaut M, Braune A, Wunderlich S, et al. Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria. Food chem toxicol. 2006 Nov 1;44(11):1940-7. Google Scholar Cross Ref
- Knasmüller S, Steinkellner H, Hirschl AM, et al. Impact of bacteria in dairy products and of the intestinal microflora on the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Mutat. Res./Fundam Mol Mech Mutagen. 2001; 480:129-38.Google Scholar Cross Ref
- Vanhaecke L, Van Hoof N, Van Brabandt W, et al. Metabolism of the food-associated carcinogen 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine by human intestinal microbiota. J agric food chem. 2006;54(9):3454-61. Google Scholar Cross Ref
- Fukui M, Fujino T, Tsutsui K, et al. The tumor-preventing effect of a mixture of several lactic acid bacteria on 1, 2-dimethylhydrazine-induced colon carcinogenesis in mice. Oncology reports. 2001;8(5):1073-8. Google Scholar Cross Ref
- Tam CC, O’Brien SJ, Tompkins DS, et al. Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin Infect Dis. 2012 ;54(9):1275-86. Google Scholar Cross Ref
- Adak GK, Long SM, O’brien SJ. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut. 2002;51(6):832-41. Google Scholar Cross Ref
- Iwamoto M, Ayers T, Mahon BE, et al. Epidemiology of seafood-associated infections in the United States. Clin microbial rev. 2010;23(2):399-411. Google Scholar Cross Ref
- Cowden JM, Ahmed S, Donaghy M, et al. Epidemiological investigation of the central Scotland outbreak of Escherichia coli O157 infection, November to December 1996. Epidemiol Infect. 2001;126(3):335-41. Google Scholar Cross Ref
- Parashar UD, Bresee JS, Glass RI. The global burden of diarrhoeal disease in children. Bull. World Health Organ. 2003; 81(4):236. Google Scholar
- Cho SH, Kim JH, Kim JC, et al. Surveillance of bacterial pathogens associated with acute diarrheal disease in the Republic of Korea during one year, 2003. J Microbiol. 2006;44(3):327-35. Google Scholar
- Olowe OA, Olayemi AB, Eniola KI, et al. Aetiologic agents of diarrhoea in children under five years of age in Osogbo, Osun State. Afr J Clin Exp Microbiol. 2003;4(2):62-6. Google Scholar Cross Ref
- Babaniyi OA. Oral rehydration of children with diarrhea in Nigeria, a 12 year renew of impact of morbidity and mortality from diarrhea disease and diarrhea treatment practices. J. Trop. Pediatr. 1991;37(2):16-66. Google Scholar Cross Ref
- Reither K, Ignatius R, Weitzel T, et al. Acute childhood diarrhoea in northern Ghana: epidemiological, clinical and microbiological characteristics. BMC infectious diseases. 2007;7(1):1-8. Google Scholar Cross Ref
- Curtis V, Cairncross S, Yonli R. Domestic hygiene and diarrhoea–pinpointing the problem. Trop med int health. 2000;5(1):22-32. Google Scholar Cross Ref
- Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. The Lancet. 2004;363(9409):641-53. Google Scholar Cross Ref
- Snelling WJ, Matsuda M, Moore JE, et al. Campylobacter jejuni. Letters in applied microbiology. 2005;41(4):297-302. Google Scholar Cross Ref
- Niyogi SK. Shigellosis. Journal of microbiology. 2005;43(2):133-43. Google Scholar
- Allos BM. Association between Campylobacter infection and Guillain-Barré syndrome. J Infect Dis. 1997 Dec 1;176(Supplement_2): S125-8. Google Scholar Cross Ref
- Mandal BK, Ellis ME, Dunbar EM, et al. Double-blind placebo-controlled trial of erythromycin in the treatment of clinical Campylobacter infection. J Antimicrob Chemother. 1984;13(6):619-23. Google Scholar Cross Ref
- Tabaraie B, Sharma BK, Sharma PR, et al. Evaluation of Salmonella porins as a broad spectrum vaccine candidate. Microbiol immunol. 1994;38(7):553-9. Google Scholar Cross Ref
- Khan AA, Melvin CD, Dagdag EB. Identification and molecular characterization of Salmonella spp. from unpasteurized orange juices and identification of new serotype Salmonella strain S. enterica serovar Tempe. Food microbiology. 2007;24(5):539-43. Google Scholar Cross Ref
- Kumar Y, Sharma A, Sehgal R, et al. Distribution trends of Salmonella serovars in India (2001–2005). Trans R Soc Trop Med. Hyg. 2009;103(4):390-4.Google Scholar Cross Ref
- Merchand IA, Packer RA. Veterinary bacteriology and virology. Vet bacterial\ virol. 1967:286-306. Google Scholar
- Dworkin MS, Shoemaker PC, Goldoft MJ, et al. Reactive arthritis and Reiter's syndrome following an outbreak of gastroenteritis caused by Salmonella enteritidis. Clin. Infect. Dis. 2001;33(7):1010-14. Google Scholar Cross Ref
- Foley SL, Nayak R, Hanning IB, et al. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl environ microbiol. 2011;77(13):4273-9. Google Scholar Cross Ref
- Gaston JH, Lillicrap MS. Arthritis associated with enteric infection. Best pract res Clin rheumatol. 2003;17(2):219-39. Google Scholar Cross Ref
- Wiström J, Norrby SR. Fluoroquinolones and bacterial enteritis, when and for whom?. J. Antimicrob Chemother. 1995;36(1):23-39. Google Scholar Cross Ref
- Versalovic J, editor. Manual of clinical microbiology. Am Soc Microbiol Press; 2011 :480–481. Google Scholar
- Gillespie SH, Hawkey PM, editors. Principles and practice of clinical bacteriology. John Wiley & Sons; 2006: 389-98. Google Scholar
- Morandi E, Grassi C, Cellerino P, et al. Verocytotoxin-producing Escherichia coli EH 0157: H7 colitis. J clin gastroenterol. 2003;36(1):44-6. Google Scholar Cross Ref
- Siegler R, Oakes R. Hemolytic uremic syndrome; pathogenesis, treatment, and outcome. Curr opin pediatr. 2005;17(2):200-4. Google Scholar Cross Ref
- GI Programme Board VTEC Working Group. Operational guidance for HPA staff dealing with cases and incidents of VTEC infection. Health Protection Agency. 2011. Google Scholar
- Afza M, Hawker J, Thurston H, et al. An outbreak of Escherichia coli O157 gastroenteritis in a care home for the elderly. Epidemiol Infect. 2006 Dec;134(6):1276-81. Google Scholar Cross Ref
- Payne CJ, Petrovic M, Roberts RJ, et al. Vero cytotoxin–producing Escherichia coli O157 gastroenteritis in farm visitors, North Wales. Emerg Infect Dis. 2003;9(5):526. Google Scholar Cross Ref
- Weekly CD. CDR. Sorbitol-fermenting Vero cytotoxin-producing E. coli (VTEC 0157). CDR. 2006. Google Scholar Cross Ref
- Adegunloye DV. Carrier rate of enteric bacteria associated with diarrhoea in children and pupils in Akure, Ondo State, Nigeria. Afr J Biotechnol. 2006;5(2):162-4. Google Scholar