Epidemiology of contagious caprine pleuropneumonia in Ethiopia
2 Department of Veterinary Clinical Science and Laboratory Technology, School of Veterinary Medicine, Wollega University Nekemte, Oromia Region, Ethiopia
3 Department of Animal Health, Oromia Regional State, Girja District Agricultural Sector, Sidama, Ethiopia
Received: 21-May-2023, Manuscript No. PULJVRP-23-6449; Editor assigned: 23-May-2023, Pre QC No. PULJVRP-23-6449 (PQ); Reviewed: 05-Jun-2023 QC No. PULJVRP-23-6449; Revised: 06-Jan-2025, Manuscript No. PULJVRP-23-6449 (R); Published: 13-Jan-2025
Citation: Woldesenbet YB, Layo GD, Boru DA. Epidemiology of contagious caprine pleuropneumonia in Ethiopia. J Vet Res Med. 2025;7(1):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Contagious Caprine Pleuropneumonia (CCPP) is a disease of major economic importance in Africa and Asia, posing a major constraint to goat production. It has been reported from almost all regions of Ethiopia and it is more prevalent in the arid and semi-arid lowland areas of Rift Valley, Borana rangelands, South Omo, Afar and other pastoral areas. The disease is caused by members of the Mycoplasma genus, usually Mycoplasma capricolum subspecies capricolum but occasionally also Mycoplasma mycoides subspecies capri or Mycoplasma mycoides subspecies mycoides. It is known to be devastating disease of goats, which is characterized by 100% morbidity and 60 to 100% mortality. The main clinical signs are fever, coughing, dyspnea and death. Contagious caprine pleuropneumonia is a contagious disease and it is transmitted during close contact by the inhalation of respiratory droplets. The occurrence of CCPP is underpinned by risk factors related to environment; production system and immune status of the host population and the exact mechanism of pathogenesis are still unclear. Mycoplasma capricolum subspecies capripneumoniae causes lesions specifically in thoracic cavity and fibrinous pleuropneumonia is the typical gross pathological lesion observed at necropsy. The pulmonary pleurae become thickened, sometimes covered by deposit of fibrin and pleural adhesion to the thorax observed. Diagnosis is one of the most important and challenging aspects of the disease as it influences prophylactic and therapeutic regimens and the control strategies for the prevention of global spread. Confirmatory diagnosis is mainly based on isolation and identification of the causative agent. Administration of long-acting oxytetracycline stopped morbidity and mortality, and controlled further CCPP spread, vaccination is the most cost effective technique in the control of CCPP than any other control measures. As a general, the contagiousness nature of the disease should be taken into consideration to prevent and control focusing on sanitary prophylaxis, medical prophylaxis, treatment and vaccination. Professionals at any country should contribute their best for the proper diagnosis of the disease to reduce the threat to other countries.
Keywords
CCPP; Diagnosis; Goat; Mycoplasma mycoides; Vaccination
Introduction
Contagious Caprine Pleuropneumonia (CCPP) is a severe and devastating respiratory disease with high morbidity and mortality in goats [1,2]. The disease is caused by Mycoplasma capricolum subspecies capripneumoniae (Mccp) and it’s included in the list of modifiable diseases of the World Organization for animal health as it threatens a significant number of goat populations throughout the world. Contagious caprine pleuropneumonia affects goats in more than 40 countries of the world thereby posing a serious threat to goat farming around the globe. It occurs in many countries in Asia, Middle East and Africa and is a classical transboundary animal disease.
Clinically the disease are characterized by extreme fever (41°C-43°C), and high morbidity and mortality in susceptible herds affecting all ages. Associated common clinical signs are anorexia, weakness, emaciation, dullness, exercise intolerance, and respiratory signs such as dyspnea, coughing, and nasal discharges. Further, abortion and high mortality rates have been reported. Transmission of CCPP from diseased animals to susceptible animals occurs through aerosol droplets produced during coughing, when animals are in close contact [3].
To diagnose CCPP commonly used serological tests are indirect hemaglutination test, complement fixation, and Latex Agglutination (LAT) to detect the antibody response of goats to MCCP. Recently, a Competitive Enzyme-Linked Immune Assay (CELISA) for CCPP has been developed and found highly specific. The introduction of the CELISA for CCPP permits the implementation of serological studies on a large scale. In addition to serological tests, molecular detection of Mccp directly in clinical samples was found highly sensitive and specific and mostly used for diagnosis of CCPP, especially in outbreaks to confirm the disease for rapid control. Therefore, in this study a Competitive Enzyme-Linked Immunoassay (CELISA) will be used for serological studies [4-6].
Prevention and control of CCPP is undertaken through vaccination, quarantine, movement controls, slaughter of infected and exposed animals and cleaning and disinfection of premises. Movement restrictions and slaughtering of infected and contact animals are recommended for newly infected countries or regions. Strict control on animal movements and a prohibition on the importation of live animals from infected regions. In other words herd biosecurity is essential to prevent CCPP distribution. Slaughtering infected animals is recommended for countries which are newly infected by the disease.
CCPP is a major cause of economic losses in the goat industry globally as it is known to cause up to 100% morbidity and 60-80% mortality rates, reduced milk yield cost of treatment and vaccination of the disease and indirect loss (hard currency) due to the imposition of trade restrictions. For instance, it is estimated that the total yearly cost of CCPP is about US$507 million in endemic areas thus involving major economic losses. Outbreaks of CCPP have been reported from almost all regions of the country, especially from the lowlands areas which are known for their goat rearing practices [7]. However there was no adequate information is organized on the disease causative agents, transmission modes, its clinical signs, diagnosis methods, its prevention and control methods. Hence, the objectives of this seminar paper were:
• To review the current study on the disease’s history, diagnosis, epidemiology and transmission.
• To review the current status of contagious caprine pleuropneumonia in Ethiopia.
Literature Review
Contagious caprine pleuropneumonia
Historical perspectives of contagious caprine pleuropneumonia: Contagious caprine pleuropneumonia was first described in 1873 in Algeria. It was not initially recognized as contagious because the disease was endemic in most of the regions examined; climate conditions were instead blamed for disease outbreaks. In 1881, there was a major outbreak in South Africa following the introduction of diseased goats coming from Turkey, and this led to the conclusion that CCPP was highly infectious. The burden and distribution of this disease, however, remains largely unknown. Contagious caprine pleuropneumonia is extremely contagious and frequently fatal; in some naive flocks, the morbidity and mortality may reach 100% and causes major economic losses in Africa, Asia and the Middle East, where goat husbandry plays significant role in the livelihood of the community [8].
Etiology: Mycoplasma capricolum subspecies capripneumoniae (Mccp) is causative agent of CCPP. The emergence of Mycoplasma mycoides clusters was dated back to about 10,000 years. The emergence and spread of the cluster has been suggested to coincide with the domestication of small ruminants. The organism; as a causal agent of CCPP was first isolated in 1976 in Kenya and was shown to cause CCPP and later, it was isolated in many countries of Africa and Asia. It was given its species name “M. capricolum subspecies capripneumoniae” in 1993. Strain F38 is the type strain of Mccp described after its identification in Kenya as a cause of CCPP. Different strains were isolated from different countries in Africa and Asia. Molecular characterization of strains revealed valuable information used to classify them into many groups and help in understanding the epidemiology of the diseases. The taxonomic status of F38 was unclear until in 1993 when it became a subspecies of M. capricolum and was named M. capricolum subspecies capripneumoniae.
Epidemiology of CCPP
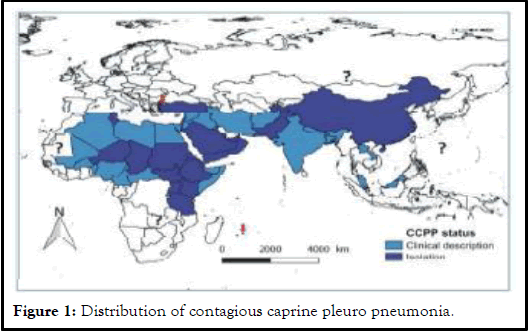
Geographical distribution: CCPP affects goats in more than 40 countries of the world thereby posing a serious threat to goat farming around the globe. Although a precise description of the distribution of CCPP is not available, the clinical disease has been reported in 30 countries mainly in Africa, Asia and Middle East. When considered the contagious nature of the disease and the movements of pastoral goat flocks, it may be suggested a much wider distribution of CCPP from the known geographical area (Figure 1) [9].
Transmission: Contagious caprine pleuropneumonia is a contagious disease. The disease is transmitted during close contact by the inhalation of respiratory droplets. Chronic carriers may exist; but this remains unproven. Some outbreaks have occurred in endemic areas when apparently healthy goats were introduced into flocks. Outbreaks of the disease often occur after heavy rains (e.g. after the monsoons in India), after cold spells or after transportation over long distances. This may be because recovered carrier animals shed the infectious agent after the stress of sudden climatic or environmental changes. Sources of the agent could be infectious aerosols and carrier state is likely but not proven. Inhalation of infected aerosols is the main route of transmission. The main source of contamination is direct contact with affected animals. Under cold, moist and overcrowded environment pathogen can persist longer and may lead to severe outbreaks. Shorter survival time (3-14 days) in external environment limits transmission of Mycoplasma capricolum subspecies capripneumoniae.
Potential risk factors: Livestock mobility and presence of naïve populations in an infected area are major predisposing factors. The presence of chronically infected animals in close proximity with naive animals is also an important factor. In regions where CCPP already occurs, the severity of the disease may depend on the following factors: The proportion of immune animals, as an animal which has survived a previous infection is thought to be protected, the presence of co-existing viral infections like PPR which may favors the development of CCPP, poor climatic conditions, such as a large temperature difference between day and night or an abrupt change of climate, especially during the period between the dry and rainy seasons and stress due to movement over long distances are main factors. Movement of animal due to internal insecurity, informal trade, transhumance, watering, grazing and marketing accompanied by porous borders are key point for CCPP spreading. In addition to these, infected goats remain carriers even when treated with the available antibiotics and are a potential risk to the healthy goats in the flock and those they come in contact at feeding areas, watering points and markets.
Pathogenesis: Tigga, et al., reviewed that the exact mechanism of pathogenesis is still unclear. The mycoplasmas are extracellular pathogens that adhere to epithelial cell surfaces. Thus, adherence proteins are one of the major virulence factors. The adherence protein has been identified as a 168 kD Protein (P1). The P1 adhesion localizes at tips of the bacterial cells and binds to Salic acid residues on host epithelial cells. Colonization of the respiratory tract results in the cessation of ciliary movement. The normal clearance mechanisms of the respiratory tract do not function, resulting in contamination of the respiratory tract and the development of a dry cough. The intimate association of the mycoplasma and the host cells provides an environment in which toxic metabolic products accumulate and damage host tissues. Both hydrogen peroxide and superoxide which are products of mycoplasma metabolism have been implicated in pathogenesis. Furthermore, the mycoplasmas have been shown to inhibit host cell catalase thereby increasing the peroxide concentrations.
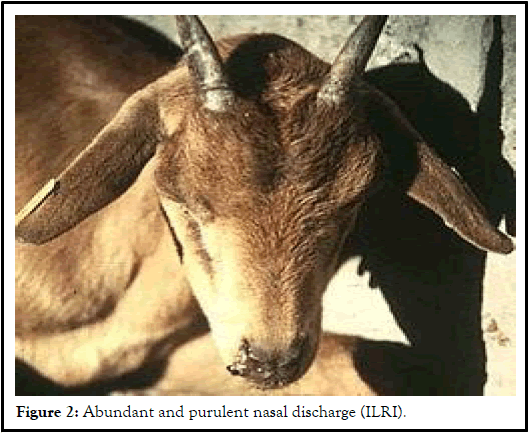
Clinical sign: Typical cases of CCPP are characterized by extreme fever (41°C-43°C), and high morbidity and mortality in susceptible herds affecting all ages. Anorexia, weakness, emaciation, dullness, exercise intolerance, and respiratory signs including dyspnea, polypnea, coughing, and nasal discharges are common signs. Coughing is irregular and nasal discharge is often absent initially, however, frothy nasal discharge and stirringly salivation often seen shortly before death. In some goats abortion can occur. The acute disease is more noticeable in naive populations in newly affected areas with high mortality and morbidity. The incubation period generally lasts on average 10 days but may vary between 2 and 28 days. The first signs of CCPP are reluctance to walk and the onset of fever. Respiration accelerates and becomes painful with violent bouts of coughing. Affected animals stand with limbs abducted and neck extended. In the terminal stages, the goats are unable to move, mouth-breathing, tongue protrusion and frothy salivation and death follows quickly. In sub-acute or chronic forms, signs are milder with coughing usually noticeable only following exercise. High mortality can be seen in kids, where death is usually the result of septicemia [10].
Gross pathological lesions: Macroscopic lesions of pleuropneumonia are often unilateral with rare cases involving both lungs and an entire lobe may become consolidated. Lesions in classical CCPP are confined to the thoracic cavity. Pea-sized yellowish nodules are seen in the lungs in early cases, whereas in more established cases there is marked congestion around the nodules. The pulmonary pleurae become thickened, sometimes covered by deposit of fibrin and pleural adhesion to the thorax observed. A varying degree of lung consolidation or necrosis with marble appearance is common. In addition bronchial and mediastinal lymph nodes are swollen, edematous with areas of congestion in acute cases. In some circumstances pericardial sacs are filled with a sero-haemorrhagic fluid. The liver and kidneys are enlarged with haemorrhages and diffused necrotic foci. In severe and advanced cases tracheal congestion and in some cases, hepatisation and abscessation of lungs. Pulmonary fibrosis, peri-bronchiolar mononuclear cuffing has also been observed. Interlobular oedema is more prominent, but interlobular oedema has also occasionally been reported [11]. Peri-bronchial and peri-bronchiolar lymphoid hyperplasia with mononuclear cell infiltration is also described (Figure 2).
Diagnosis: Tentative diagnosis is based on clinical signs and post-mortem findings. But confirmation of the disease based on clinical signs is difficult for reasons that, from clinical point of view, CCPP cannot be differentiated from a number of diseases presenting similar respiratory signs in small ruminants, such as peste des petits ruminants and pasteurellosis, thus laboratory confirmation is required for differential diagnosis with other diseases.
Confirmatory diagnosis is mainly based on isolation and identification of the causative agent. However, being fastidious organisms it is very difficult to isolate Mycoplasmas on ordinary media in in vitro culture. In fact, it has been observed that a negative bacteriological result does not indicate the absence of infection. Whole genome sequencing has become the gold standard for high resolution typing method, which supersedes all previous phenotypic or genotypic methods, which could be applied for major public health pathogens. The preferred samples for diagnosis are the pleural fluid and sections of hepatised lung, particularly from the interface between consolidated and unconsolidated area.
Identification of the causative agent: After cloning and purification, it can be isolated by several biochemical’s, immunological and molecular tests. Isolating the causative agent is a very difficult task for the mycoplasma diagnostic laboratory because of its highly fastidious nature, grows slowly in broth media, and produces only minute colonies on solid media. It has been successfully grown and isolated from infected lungs through culturing on Hayflick medium (H25P) reported by Balikci et al., Cetinkaya et al. and Noah et al. Similarly modified Hayflicks media have been used for the growth and isolation of Mccp organisms.
Serological tests: A number of serological tests currently exist, but most are difficult to use in situ, due to lack of specificity, or require resources unavailable in many countries affected by the disease. There are quite a few serological tests that are available to be used in the field for the confirmatory diagnosis of CCPP. These serological tests include: Complement Fixation Test (CFT), Latex Agglutination Test (LAT), Enzyme- Linked Immunosorbent Assay (ELISA) and Growth Inhibition Test (GIT):
Molecular diagnostic tests: Due to antigenic and genetic similarities among the member of the mycoides cluster, the best and most accurate diagnostic method for identification of Mccp is molecular typing. Recently a fieldapplicable recombinase polymerase amplification assay for rapid detection of Mccp has been developed. All members of the M. mycoides cluster have two rRNA operons and there are differences in the sequence of 16S rRNA genes of the two operons. Many of the members of Mycoplasma share genomic and antigenic structures that often cause immunological crossreactions. However, different species can be distinguished from each other by using different molecular techniques. Molecular techniques have been reported to greatly improve detection of Mccp.
Polymerase chain reaction method has radically improved the detection and identification of microorganisms which do not grow easily in vitro. The tests have been described and shown to be specific, sensitive and can be applied directly to clinical material, such as lung and pleural fluid, dried sample on filter paper and culture material. Due to the difficulty of isolating Mccp, polymerase chain reactions are the technique of choice for the diagnosis of CCPP. However, isolation of Mccp remains the confirmatory test. In recent years, with the improvement of sequencing technologies, several complete mycoplasma genomes have become publicly available. The first complete genome sequence of Mccp strain M1601, isolated from clinically infected animals in China, was described by Chu et al. Recently, three additional complete and annotated Mccp genome sequences have been published: Mccp strain 9231-Abomsa, the type strain F38 and the field strain ILRI181, isolated during outbreaks in Kenya [12].
Discussion
Prevention and control
Treatment: As a result, good quality vaccines are almost never used in regions where CCPP is highly prevalent, for technical as well as financial reasons and goat owners resort frequently to antibiotic treatments in case of an outbreak. Various therapeutic trials were reported by different authors who used different antibiotics of variable efficacy like streptomycin and long-acting oxytetracycline. Streptomycin treated goats suffering from natural and experimental CCPP recovered on the third day of treatment and became completely immune to re-infection with Mccp.
Administration of long-acting oxytetracycline stopped morbidity and mortality, and controlled further CCPP spread. Some antibiotics, such as tetracyclines, fluoroquinolones (e.g., danofloxacin) and the macrolide family, can be effective if given early. Among them tylosin in naturally infected goats and danofloxacin in experimentally infected goats showed successful results [13]. The goats treated with tylosin show positive response within 2-3 days and show recovery from clinical disease after 3rd day. Danofloxacin was found to be highly effective in the treatment of clinical CCPP in goat.
Vaccination: Vaccination is the most cost effective technique in the control of CCPP than any other control measures. Inactivated mycoplasma protein based vaccine obtained by centrifugation has been in use since many years. An inactivated vaccine with saponin as an adjuvant was produced in Kenya following a series of trials; the vaccine was proved to be effective in the field and protects animals for approximately one year. According to Gelagay et al., inactivated CCPP vaccine adjuvated with saponin currently study focusing on evaluation of the safety and immunogenicity of inactivated whole culture of CCPP vaccine developed at National Veterinary Institute (NVI) showed that it is as immunogenic as the protein based vaccine, which could be used for mass vaccination if field immunogenicity trials show good results [14,15].
Quarantine: Apply quarantine measures as laboratory confirmation is awaited. Once CCPP is confirmed apply full quarantine in the identified area. Movement restrictions and slaughtering of infected and contact animals are recommended for newly infected countries or regions. Strict control on animal movements and a prohibition on the importation of live animals from infected regions. In other words herd biosecurity is essential to prevent CCPP distribution. Slaughtering infected animals is recommended for countries which are newly infected by the disease.
Economic losses
Small ruminants particularly goats (also known as poor man’s cow or the rural bank), contribute significantly to the nutrition and cash income of small farmers in Africa and Asia, the two regions occupied with the largest concentration (about 72.9%) of the poor peoples in the world [16,17]. Africa, the Middle East and Asia regions those are home to over 80% of the world’s sheep and goat population and for more than 330 million of the world’s poorest people play an important role in the livelihoods and food security.
Prevalence of CCPP in Ethiopia
In Ethiopia, the circulation of CCPP has been suspected for a long period, especially in remote regions those are bordering to the known infected countries with Mycoplasma capricolum subspecies capripneumoniae like Kenya and Sudan In 1990 outbreak of CCPP occurred in Ogaden in eastern Ethiopia and in east Shoa province. The causative agents were recovered in pure culture but Mycoplasma capricolum subspecies capripneumoniae isolated and characterized in the same year from the pleura fluid that was collected during the outbreaks in Gojam in 1982 [18]. Since then the disease has been known to be endemic in different regions of the country [19]. Clinical diseases have been identified by various investigators especially in the pastoral areas of the country, Borana and Afar. A meta-analysis of CCPP conducted by Asmare, et al., using more than twelve published articles indicated that the seroprevalence of the disease is 25.7% [20]. Different scholars reported seroprevalence of CCPP from different districts with various proportions. In different years outbreaks of CCPP have been reported from different regions (Table 1).
| Year | Study area | Study animal | Sample size | Prevalence |
|---|---|---|---|---|
| 2006 | Afar | Goats | 1183 | 29.1 |
| 2007 | Oromia | Goats | 169 | 20.1 |
| 2008 | SNNPR | Goats | 679 | 15.5 |
| 2009 | Tigray | Goats | 280 | 43.9 |
| 2010 | Afar | Goats | 329 | 22.5 |
| 2011 | Oromia | Goats | 900 | 13.2 |
| 2012 | Dire Dawa | Goats | 244 | 4.9 |
| 2014 | Oromia | Goats | 510 | 31.6 |
| 2015 | Gambella | Goats | 1152 | 18.1 |
| 2019 | Oromia | Goats | 789 | 31.2 |
Table 1: Results of sero-prevalence studies done on CCPP in different parts of Ethiopia.
Conclusion
It has been noted that every year outbreaks of respiratory diseases occur in the goat population with an alarming rate of morbidity and mortality but lack of advanced techniques is a big hindrance in the proper diagnosis of CCPP. Diagnosis of the disease is one of the challenging aspects as it influences prophylactic and therapeutic regimen and the control strategies for the prevention of global spread. However, determination of seroprevalence of the disease and potential risk factors could pave the way in devising control and prevention strategies. From the current review the endemicity of CCPP and the epidemiological nature of the disease should be taken into consideration as it could affect the country from different corners of losses and threat of spread. It is impossible to rule out the presence of CCPP in many parts of the country due to possible underreporting of cases and lack of adequate laboratory support to correctly diagnose the disease. Therefore:
• The exact epidemiological picture of CCPP in Ethiopia has to be well established through continuous gap-filling works to exactly put the updated status of the disease.
• Professionals should contribute their best to reveal the truth behind the disease in the country for the benefits of inhabitants.
• Advanced technology should implement for diagnosis of the disease.
References
- Atim SA, Ayebazibwe C, Mwiine FN, et al. A Survey for contagious caprine pleuropneumonia in Agago and Otuke districts in Northern Uganda. Open J Vet Med. 2016;6(01):9.
- Matios Lakew TS, Ayelet G, Eshetu E, et al. Seroprevalence of contagious caprine pleuropneumonia and field performance of inactivated vaccine in Borana pastoral area, southern Ethiopia. 2013.
- AU-IBAR. Standard methods and procedures (SMPs): For contagious caprine pleuro pneumonia (CCPP) in the Greater Horn of Africa, Nairobi. 2015.
- Gelagay A, Teshale S, Amsalu W, et al. Prevalence of contagious caprine pleuropneumonia in the Borana pastoral areas of Ethiopia. Small Ruminant Res. 2007;70(2-3):131-5.
- Balikci E, Kizil O, Karapinar T, et al. Efficacy of marbofloxacin for naturally occurring contagious caprine pleuropneumonia. Small Ruminant Research. 2008;77(1):75-9.
- Bates PG. External parasites of small ruminants a practical guide to their prevention and control. CABI; 2012.
- Bekele T, Asfaw Y, Gebre-Egziabeher B, et al. Seroprevalence of contagious caprine pleuropneumonia in Borana and Guji lowlands, Southern Ethiopia. Ethiopian Vet J. 2011;15(2).
- Cetinkaya B, Kalin RE, Karahan MU, et al. Detection of contagious caprine pleuropneumonia in East Turkey. Rev Sci Tech. 2009;28(3).
[Crossref] [Google Scholar] [PubMed]
- Chaber AL, Lignereux L, Al Qassimi M, et al. Fatal transmission of contagious caprine pleuropneumonia to an Arabian oryx (Oryx leucoryx). Vet Microbiol. 2014;173(1-2):156-9.
[Crossref] [Google Scholar] [PubMed]
- Chakraborty S, Kumar A, Tiwari R, et al. Advances in diagnosis of respiratory diseases of small ruminants. Vet Med Int. 2014;2014(1):508304.
[Crossref] [Google Scholar] [PubMed]
- Chu Y, Gao P, Zhao P, et al. Genome sequence of Mycoplasma capricolum subsp. capripneumoniae strain M1601. J Bacteriol. 2011.
[Crossref] [Google Scholar] [PubMed]
- CSA R. The federal democratic republic of Ethiopia central statistical agency report on area and production of major. Statistical Bulletin. 2016.
- Dupuy V, Thiaucourt F. Complete genome sequence of Mycoplasma capricolum subsp. capripneumoniae strain 9231-Abomsa. Genome Announc. 2014;2(5):10-128.
[Crossref] [Google Scholar] [PubMed]
- Saeed EM, Osman SA. Clinical and laboratory diagnosis of contagious caprine pleuropneumonia in Qassim region, Saudi Arabia: A comparative study. Trop Biomed. 2018:35(1): 67-75.
[Google Scholar] [PubMed]
- Eshetu L, Yigezu L, Asfaw Y. A study on Contagious Caprine Pleuropneumonia (CCPP) in goats at an export oriented abattoir, Debrezeit, Ethiopia. Trop Anim Health Prod. 2007;39:427-32.
[Crossref] [Google Scholar] [PubMed]
- Falquet L, Liljander A, Schieck E, et al. Complete genome sequences of virulent Mycoplasma capricolum subsp. capripneumoniae strains F38 and ILRI181. Genome Announc. 2014;2(5):10-128.
[Crossref] [Google Scholar] [PubMed]
- Giadinis ND, Petridou EJ, Sofianidis G, et al. Mortality in adult goats attributed to Mycoplasma capricolum subspecies capricolum. Vet Rec. 2008;163(9):278.
[Crossref] [Google Scholar] [PubMed]
- Hadush B, Eshetu L, Mengistu W, et al. Seroprevalence of contagious caprine pleuropneumonia in Kefta Humera, Alamata (Tigray) and Aba-‘ala (Afar), Northern Ethiopia. Trop Anim Health Prod. 2009;41:803-6.
[Crossref] [Google Scholar] [PubMed]
- Hussain R, Auon M, Khan A, et al. Contagious caprine pleuropneumonia in Beetal goats. Trop Anim Health Prod. 2012;44:477-81.
[Crossref] [Google Scholar] [PubMed]
- Yatoo MI, Parray OR, Bashir ST, et al. Contagious caprine pleuropneumonia-a comprehensive review. Vet Q. 2019;39(1):1.
[Crossref] [Google Scholar] [PubMed]