Initial clinical assessment of non-traumatic acute limb ischaemia: A high degree of suspicion is pivotal in timely intervention
Received: 07-Feb-2018 Accepted Date: Feb 22, 2018; Published: 28-Feb-2018
Citation: Gunawansa N. Initial clinical assessment of non-traumatic acute limb ischaemia: A high degree of suspicion is pivotal in timely intervention. J Vas Dis Treat. 2018;2(1):004-008.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Acute limb ischaemia is both a limb-threatening and life-threatening surgical emergency. The clinical presentation of acute limb ischaemia can vary depending on the underlying aetiology as well as pathophysiology. While traumatic acute limb ischaemia becomes obvious in a trauma victim, nontraumatic ischaemia can be easily mistaken for other neurological or medical conditions. The rapidity with which acute limb ischaemia causes irreversible muscle injury signifies the importance and urgency of accurate diagnosis. A high degree of clinical suspicion and accurate bed-side assessment can be pivotal in timely diagnosis and initiation of definitive care to maximize limb salvage.
Keywords
Acute ischaemia; Embolism; Thrombosis; Arterial occlusion; Limb loss
Introduction
Acute limb ischemia (ALI) is one of the commonest vascular surgical emergencies encountered in clinical practice. It carries potentially devastating consequences including limb loss and death if timely intervention is missed. Unlike acute limb ischaemia secondary to trauma, the clinical presentation may often be shrouded leading to significant delays in diagnosis. Hence a high degree of clinical suspicion and timely intervention becomes pivotal in managing this entity with minimal morbidity to the patient.
The Trans-Atlantic InterSociety Consensus (TASC) definition of ALI describes it as “a sudden decrease in limb perfusion causing potential or established threat to limb viability” [1]. From a clinical standpoint, it is defined arbitrarily as symptoms persistent for less than 2 weeks, in order to differentiate it from chronic variety of limb ischaemia. Worldwide epidemiological data have commented on ALI as one of the commonest causes of limb loss and death in the adult population. The reported rates of major amputation and 30-day mortality after ALI are 10-15% and 15-25% respectively [2-4].
Non-traumatic occlusions account for approximately 90% of all cases of ALI [5]. The two commonest pathophysiological mechanisms of non-traumatic ALI are thrombosis (60%) and embolism (30%). However, in most cases, these two mechanisms may co-exist such as in an acute embolus that causes arterial occlusion and then leads to proximal and distal thrombosis of the affected artery.
Embolism
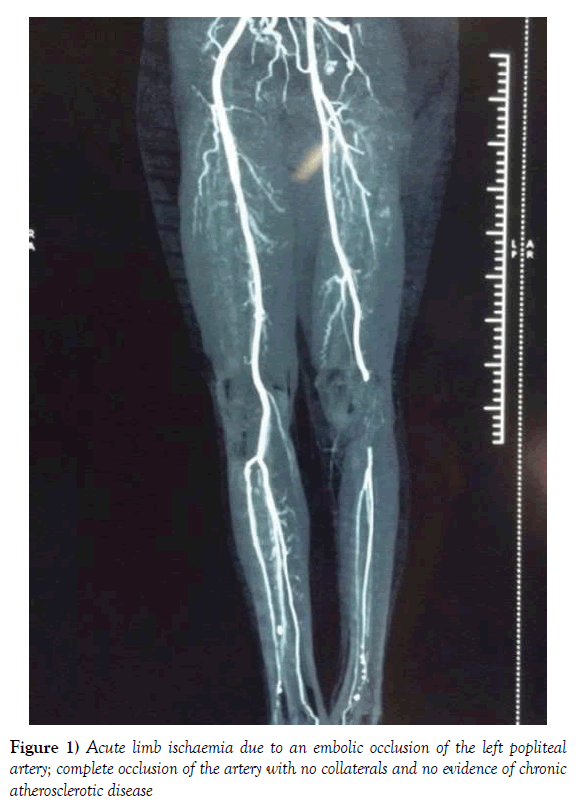
Arterial embolism occurs due to a floating embolus that gets lodged in an area of natural arterial luminal narrowing such as a point of arterial bifurcation. The commonest sites of embolic occlusion include aortic bifurcation (saddle embolus) or bifurcation points of the common iliac artery, common femoral artery, popliteal artery and brachial artery [6]. Aortic and lower extremity occlusion account for approximately 80% of all embolic ALI as opposed to upper extremity ischaemia. The affected native artery itself is healthy and gets acutely occluded by the floating embolus, leading to complete cessation of flow. The historical study looking at patients over 40 years by Abbott et al. found approximately (80-90%) of these emboli originated in the heart. Although dramatic decline in the incidence of rheumatic heart disease since that time has made it less common, cardiac emboli may still originate from degenerative heart disease, prosthetic valves, atrial fibrillation, or secondary to akinetic segments following myocardial infarction [7]. Rare cardiac tumours such as atrial myxoma can also result in cardiac origin emboli leading to ALI [8]. Alternatively, the embolus may originate from a proximal area of the artery due to aneurysmal disease such as in abdominal aortic aneurysms causing ALI of the leg or subclavian aneurysms leading to ALI of the arm. In the absence of compensatory collateral circulation, embolic ALI leads to a sudden dramatic ischaemia with rapid deterioration (Figure 1).
Thrombosis
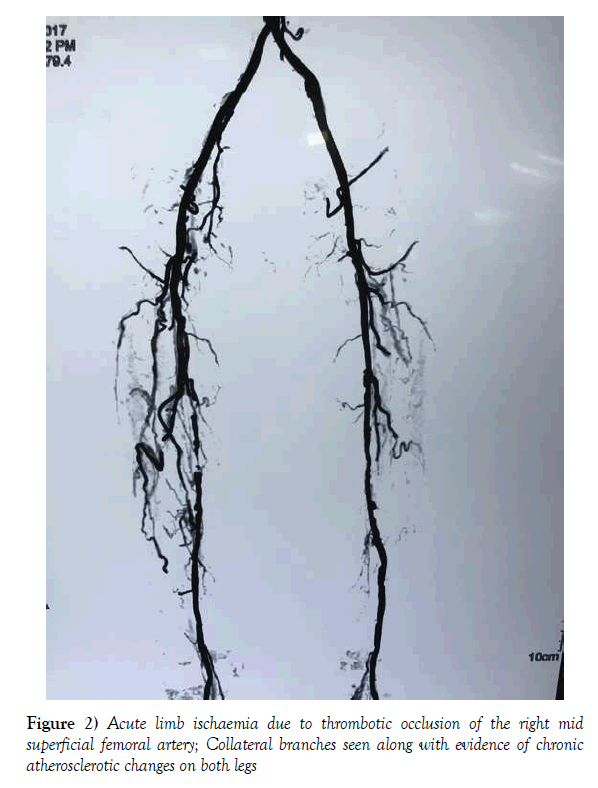
ALI due to thrombosis occurs due to in-situ occlusion of an artery that is already affected by disease. With the global epidemic of atherosclerotic peripheral vascular disease, the incidence of thrombotic ALI has shown a steady increase compared to embolic ALI [2]. Any local damage to an existing atheromatous plaque leads to in-situ thrombosis of the artery with acute exacerbation of symptoms. The clinical progression is less dramatic compared to embolic ALI and may take several hours to develop and progress to full-blown ischaemia [9,10]. The defining features of thrombotic ALI are the presence of chronic ischaemic symptoms in the past with sudden acute exacerbation, the presence of collateral circulation on imaging and angiographic evidence of background atherosclerotic disease (Figure 2). Rarely, acute dissection of an artery may lead to thrombotic ALI without the aforementioned collaterals or background atherosclerotic changes resulting in a clinical presentation which is akin to embolic ALI [11–13]. Furthermore, thrombotic ALI may occur in a pre-existing venous or prosthetic graft or stent. This should be suspected in any patient with a history of prior revascularization who presents with symptoms of ALI of the same limb.
Less commonly, arterial thrombosis can occur due to extreme hypercoagulable states. This includes instances of excessive dehydration, severe systemic hypotension, septicaemia, disseminated malignancy and hyperviscosity disorders [7]. Although rare, it is vital to consider the diagnosis of ALI in these instances as any delay in diagnosis due to the general patient condition may result in unsalvageable limb loss.
Presentation
ALI can affect both upper and lower extremities, with lower extremity ALI being approximately four times commoner than upper extremity [14]. The clinical presentation depends on several factors including the level of occlusion and presence of collateral circulation around the site. Absence of collaterals in embolic ALI or arterial dissection results in a dramatic onset and progression of symptoms compared to thrombotic ALI. Similarly, the anatomical level also determines the immediate clinical picture, with occlusion of the popliteal artery or brachial artery bifurcation often causing more pronounced ischaemia than occlusion proximally.
Clinical Assessment
A high degree of clinical suspicion is required in assessing any patient with sudden onset pain or weakness of limbs. Failure to assess limb perfusion amidst confounding clinical picture of neurological and musculoskeletal symptoms could lead to detrimental outcomes [15]. Reports of such ALI being mistaken for neurological disorders including stroke, highlights the importance of clinical assessment in these patients. A complete assessment of the affected limb, other extremities as well as the general condition of the patient is vital not only in accurate diagnosis but also prioritizing of subsequent care.
A detailed history should evaluate possible precipitating factors; atrial fibrillation, history of cardiac disease, recent myocardial infarctions, previous arterial interventions etc. Furthermore, general risk factors for atherosclerotic disease such as diabetes, hypertension, hypercholesterolemia and smoking need to be established. A history of preceding claudication or prior arterial interventions in the affected limb would suggest the possibility of thrombotic ALI.
The classical presentation of ALI has been described in terms of the “six Ps”: pain, pallor, paraesthesia, paralysis, pulse absence and poikilothermia (perishing cold). The first and most severe symptom n a conscious patient would be he ischaemic pain. However, this may be difficult to observe in the critically ill patients or those who may be unconscious, especially in the intensive care setting. As ischaemia progresses, it affects the sensory and motor nerves sequentially, with the smaller sensory nerves being more sensitive to ischaemic injury than the larger motor nerves. Hence, the patients who present early, may only have paraesthesia with intact muscle power. However, with progressive ischaemia, the motor nerves are affected causing muscle weakness and paralysis which can easily be mistaken for an acute neurological event. The presence of complete anaesthesia and paralysis may indicate a non-viable limb [7].
Examination should include assessment of the pulse status compared to the contralateral limb, temperature difference, capillary refill time, skin changes (cyanosis, skin mottling), sensory deficit and motor deficit. Absence of movement in the toes and reduced or absent flexion at the ankle along with muscle tenderness are crucial in determining limb viability. If both lower limbs are affected as in a ‘saddle embolus’, lower extremity findings should be compared with those of the upper extremity.
General examination findings may be helpful in establishing the possible pathophysiology. These include detection of pulse rhythm abnormality, presence of murmurs, proximal palpable aneurysmal disease such as abdominal aortic aneurysms or popliteal aneurysms [16].
As pointed out by Rutherford (2009) in his paper depicting the importance of clinical assessment in ALI, ccurate bed-side assessment can be done using a hand-held doppler [17]. Absence of a normal triphasic doppler signal indicates severe stenosis or proximal occlusion. The presence of a distal monophasic signal may indicate a patent outflow despite proximal occlusion while total absence of distal signal may indicate complete thrombosis of distal run off arteries. Comparison of doppler signal characteristics and ankle brachial pressure index with the contralateral limb or upper limbs is helpful in assessing the degree of ischaemia. Similarly, assessment of venous doppler signal is useful in establishing the degree of ischaemia and limb viability, whereas the absence of a venous signal signifies a poor prognostic outcome and a non-viable limb (Table 1).
| Category | Sensory impairment | Motor impairment | Arterial Doppler signal | Venous Doppler signal |
|---|---|---|---|---|
| Class I | ||||
| Viable-No immediate threat |
No | No | Audible | Audible |
| Class IIA | ||||
| Marginally threatened |
Minimal in the toes) or none | No | Often inaudible | Audible |
| Class IIB | ||||
| Immediately threatened | Involves forefoot ± Rest pain |
Mild to moderate | Usually Inaudible | Audible |
| Class III | ||||
| Irreversible | Anesthetic | Paralytic / rigor | Inaudible | Inaudible |
Table 1: Classification of Acute Limb Ischaemia (Adapted from Rutherford RB. Clinical Staging of Acute Limb Ischemia as the Basis for Choice of Revascularization Method 2009)
Classification of ALI
The earliest classification of ALI was based on aetiology and pathophysiology; traumatic, thrombotic and embolic. However, the differentiation between thrombotic and embolic ALI may not be conclusive in most cases and the outcome of treatment depends more on the degree of ischaemic tissue injury rather than aetiology. Therefore, more recent classification systems have focused on the state of the affected limb and degree of ischaemia to give a more meaningful idea regarding outcome and prognosis.
The currently accepted classification system was introduced by the Society for Vascular Surgery, based on ischaemia severity and does not depend on the aetiology or pathophysiology [17,18]. It relies on simple clinical assessment with hand-held Doppler assessment and does not require invasive imaging. Hence rapid categorization of patients can be done which help in early therapeutic decision making.
Class I; The viable limb
These patients with ALI present early and have the best chance of limb salvage. The patients may complain of acute onset pain or claudication and often have mild symptoms. They can still ‘walk in’ to the consultation or emergency department and have no evidence of sensory or motor impairment. Although the distal pulses in the affected limb may not be palpable, they continue to have audible biphasic arterial doppler signals. Majority of such patients can be successfully managed with systemic anticoagulation, anti-platelet medication and observation without immediate active intervention [19].
Class II; The threatened limb
Patients with class II ALI definitely need active intervention in order to re-establish perfusion and achieve limb salvage. Depending on the clinical assessment at presentation, these patients are subdivided in to two categories; IIa (marginally threatened) and IIb (Immediately threatened). In addition to the absence of palpable pulses and coldness of the skin, these patients will have varying degrees of paraesthesia in the extremity ranging from numbness in the toes to established rest pain in the forefoot. Motor impairment will result in varying degrees of muscle weakness compared to the contralateral limb. The arterial doppler signal is usually absent or heard only in a monophasic pattern. Venous doppler signal is still preserved.
Class II ALI is salvageable with immediate intervention and revascularization. Clinical distinction between class IIa and IIb becomes crucial in deciding the urgency of intervention. The presence of established numbness and muscle weakness help in the categorization of these patients in to class IIb where immediate revascularization is needed. Class IIa ALI allows for prompt but semi-elective revascularization, and offers time for diagnostic angiographic imaging prior to revascularization [17].
Class III; The irreversibly ischaemic limb
Stage III is the extreme of ALI where the ischaemic damage has caused irreversible damage to the limb. Apart from the absence of pulses, both arterial and venous doppler signals are inaudible. Furthermore, there is total loss of sensory and motor function, resulting in a limb which is completely anaesthetic and paralysed with varying degree of muscle rigor. Stage III ALI mandates an amputation at the level of preserved limb perfusion to prevent life threatening reperfusion or toxin induced multi-organ dysfunction [20].
Determining the Level of Occlusion
Aortic Occlusion
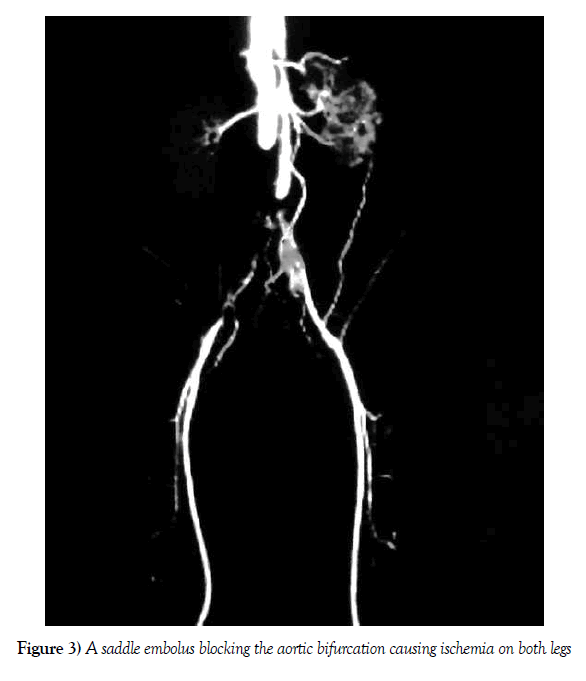
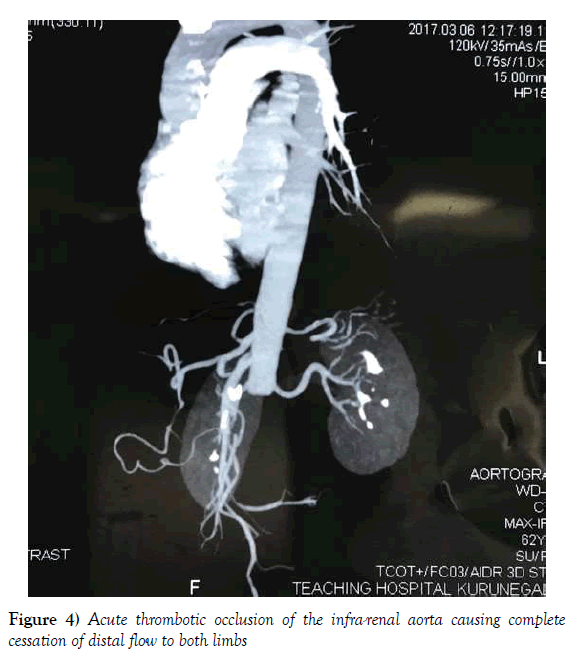
The commonest aetiology of acute aortic occlusion is a large embolus that saddles and blocks the aortic bifurcation, termed a ‘saddle embolus’ (Figure 3). Less frequently, it can occur due to a Stanford type-B aortic dissection with thrombosis [21]. This can result in varying degrees of ALI affecting both lower limbs. Less frequently, it can also occur due to acute thrombosis of a pre-existing aortic aneurysm or atherosclerotic plaque (Figure 4). The clinical presentation is characteristic in that the acute ischaemic symptoms are present in both lower limbs. Aortic dissection induced ALI will characteristically have the severe back pain as a prominent presenting symptom in addition to the ischaemic symptoms of the legs that develop subsequently. Saddle emboli causing aortic occlusion presents with sudden severe pain and paralysis of both lower limbs often being mistaken for an acute neurological event. The distinguishing feature in the acute stage is mostly the absence of extremity pulses in aortic occlusion which signifies the importance of pulse examination in all such presentations with lower limb paralysis.
Figure 3) A saddle embolus blocking the aortic bifurcation causing ischemia on both legs
Iliac Occlusion
Iliac occlusion also causes unilateral ischaemic symptoms along with unilateral neurological deficit. Again, the presentation may mimic an acute neurological event and the assessment of extremity pulses is paramount in making the clinical differentiation early. Unilateral limb ischaemia may also be caused by acute Stanford type B aortic dissection leading to thrombosis of one aortic lumen while continuing to perfuse the contralateral limb.
Femoro-popliteal Occlusion
The commonest anatomical form of ALI is occlusion of the common femoral artery bifurcation causing secondary thrombosis of the superficial femoral artery. In the event of concomitant profunda femoris artery thrombosis, the symptoms are more pronounced, and the degree of ischemia is more profound compared to instances where the profunda femoris remains patent. On clinical assessment, the common femoral pulse at the groin may still be palpable proximal to the occluding embolus or thrombus, often giving an exaggerated prominent pulse due to the ‘water hammer effect’.
Popliteal and Infra-popliteal Occlusion
Isolated popliteal and infra-popliteal occlusion is less common compared to femoral occlusion. It generally occurs due to small caliber emboli that pass through the superficial femoral artery to get lodged at the popliteal trifurcation. It can also occur due to thrombosis of the popliteal artery in the background of popliteal aneurysm, popliteal entrapment syndrome or cystic adventitial disease of the popliteal artery. As described by Robinson et al., popliteal aneurysm thrombosis causing ALI is one of the most challenging clinical presentations with an extremely high incidence of limb loss [16]. These patients will have unilateral symptoms limited to the foot and calf region with a normal palpable femoral pulse. Furthermore, in thrombosed popliteal aneurysm, a palpable firm mass may be palpable behind the knee.
Upper Limb ALI
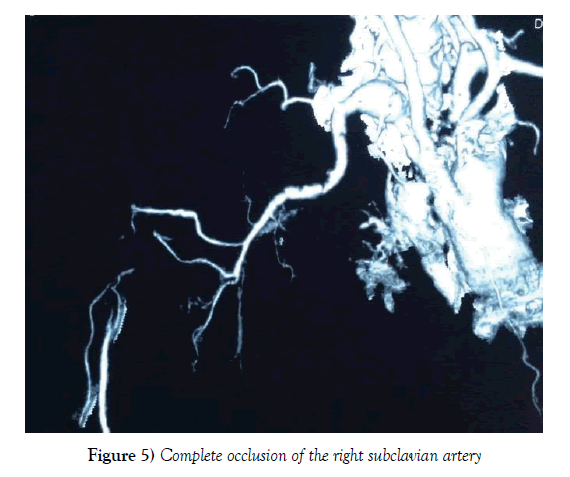
Upper limb ALI is much less common compared to the lower limbs and accounts for approximately 10-15% of all cases of ALI [14,22]. The pathophysiology is similar to the lower limbs and is most commonly embolic, thrombotic or traumatic. The clinical presentation may be less dramatic compared to the lower limbs owing to the natural collateral circulation around the scapular and cubital region. Apart from the cardiac source of emboli discussed above, possible subclavian aneurysm with thrombosis or embolism should be included in the clinical assessment (Figure 5). The ischaemic symptoms are less pronounced where the occlusion occurs in the subclavian or axillary regions, whereas occlusion of the brachial artery bifurcation at the cubital fossa can produce dramatic ischaemic symptoms similar to popliteal occlusion in the lower limbs.
Definitive Diagnostic Imaging
The diagnosis of ALI depends largely on a high degree of clinical suspicion supported by timely assessment of limb perfusion. Once a clinical diagnosis is made, further imaging is required to confirm the level of ischaemia as well as plan the necessary intervention. Further ancillary investigations may be required to assess the general condition of the patient, fitness for definitive intervention as well as to identify possible aetiological sources especially in embolic ALI. Depending on the degree of ischemia as classified above, the timing of these investigations need to be planned without causing undue delay in restoring perfusion.
An arterial duplex scan is a readily available non-invasive imaging modality to confirm clinical findings of ALI. Furthermore, it can be used to assess the degree of distal thrombosis beyond the level of occlusion, thereby defining the patency of outflow vessels before surgical intervention [23]. However, visualizing the proximal iliac and aortic occlusions may be difficult due to technical limitations of duplex imaging.
Although relevant from historical perspective, conventional angiography has only a limited place in the current diagnostic protocols of ALI. However, with the advent of mobile C-arm facilities, real-time fluoroscopy and hybrid operating theatre facilities, the on-table angiography plays a key role in definitive diagnostic imaging. Furthermore, it can also be used to guide therapeutic measures such as catheter directed thrombolysis and also to achieve completion angiogram images in establishing the success of such interventions.
Computerized Tomographic angiography (CTA) and magnetic resonance angiography (MRA) are currently the most frequently used diagnostic modalities in the assessment of class I, and IIa type ALI. It allows the use of minimal contrast medium through peripheral venous cannulation to achieve a vertical reconstruction of the anatomical arterial tree [24,25]. Nevertheless, the associated time involved in arranging CTA remains the chief limiting factor in ALI and should not be pursued in class IIb type of ALI.
Echocardiography
Echocardiography is a vital component of the investigation process in patients with ALI. It not only allows for an assessment of their background cardiac status to withstand therapeutic interventions, but also helps in determining potential cardiac sources of emboli. Trans-oesophageal rather than transthoracic echocardiography may be more sensitive detecting the possible cardiac pathology, although this may need to be delayed until after the revascularization has been performed [26]. Conditions such as mural thrombi, vegetations and myxomas can be detected on echocardiography although over 60% of patients with embolic ALI do not show any cardiac pathology on echocardiography [27]. This relatively low sensitivity of detecting cardiac pathology has prompted some clinicians to question the place of routine echocardiography in ALI. Given that it does not alter the management of these patients who are likely to be anti-coagulated for life regardless of cardiac findings, the place of invasive cardiac imaging remains debatable [26].
Initial Management
Having established the diagnosis of ALI, initial management aims at minimizing the ongoing ischaemic tissue damage and stabilizing the patient in preparation for definitive management.
Initial management involves assessing the basic biochemical status of the patient prior to therapeutic interventions. A complete blood count, serum creatinine, serum electrolytes and coagulation profile would be mandatory in the initial phase prior to angiographic imaging and operative intervention. Furthermore, blood grouping and crossmatch may be required if planning surgical revascularization.
Anticoagulation
Immediate anticoagulation is the starting point of treating ALI. It minimizes the on-going tissue ischaemia by thrombus propagation both proximally and distally. Once the clinical diagnosis is made, intravenous heparin is recommended at a bolus of 60-70 units/kg, supplemented by an infusion at 12-15 units/kg/hour [28]. Although the newer low molecular weight heparin derivatives can also be used, the ability for immediate reversal if needed with protamine sulfate and ability to titrate according to degree of thrombosis makes unfractionated heparin the drug of choice in the initial management.
Ancillary Measures Pending Definitive Revascularization
Symptomatic management of the pain often requires strong opioid analgesics. However, intramuscular or subcutaneous opiate injections are contra-indicated if therapeutic thrombolysis is likely to be considered. Alternatively, opiates should be given intravenously or with a patient controlled analgesia set up in addition to non-steroidal anti-inflammatory analgesics given orally or as suppositories.
The use of oxygen by facemask has been shown to improve capillary level perfusion amidst the ongoing ischaemia [29]. Hydration of the patients with intravenous crystalloids may be useful as majority of such patients are dehydrated due to the lack of oral intake owing to the intensity of symptoms. Furthermore, intravenous hydration also allows for the renal perfusion to be maintained thereby minimizing any potential effect of contrast on the kidneys at the time of angiographic imaging or radiographic intervention. Insertion of a urine catheter allows for easy monitoring of urine output and prevents the need to mobilize the patient for bladder emptying.
Conclusion
Non-traumatic ALI remains a common medical emergency where timely diagnosis and assessment is essential to avoid adverse patient outcomes. Amidst a combination of ischaemic, neurological and general symptoms, accurate and timely diagnosis depends on the presence of a high degree of clinical suspicion. Complete vascular clinical assessment can be completed in the bedside and remains the cornerstone of diagnosing ALI. Once the diagnosis is made, accurate classification depending on clinical assessment and bed-side Doppler assessment will allow categorization and institution of immediate treatment to maximize limb salvage.
REFERENCES
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5-67.
- Eliason JL, Wainess RM, Proctor MC, et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;3:238-382.
- Earnshaw JJ, Whitman B, Foy C, et al. National audit of thrombolysis for acute leg ischemia (natali): clinical factors associated with early outcome. J Vasc Surg. 2004;39:1018-25.
- Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24:3-15.
- Callum K, Bradbury A. ABC of arterial and venous disease: Acute limb ischaemia. BMJ 2000;320:764-7.
- Lusby RJ, Wylie EJ. Acute lower limb ischemia: Pathogenesis and management. World J Surg. 1983;7:340-6.
- O’Connell JB, Quiñones-Baldrich WJ. Proper evaluation and management of acute embolic versus thrombotic limb ischemia. Semin Vasc Surg. 2009;22:10-6.
- Dieter RA, Overly TL, Roberts MA, et al. Cardiac causes of acute and chronic limb ischemia. Crit. Limb Ischemia, Cham: Springer International Publishing. 2017;109-20.
- Peach G, Loftus IM. Acute and chronic lower limb ischaemia. Surg. 2013;31:229-35.
- Metcalfe J, Brooks M. Acute and chronic ischaemia of the lower limb. Surgery. 2010;28:277-83.
- Charlton-Ouw KM, Sritharan K, Leake SS, et al. Management of limb ischemia in acute proximal aortic dissection. J Vasc Surg. 2013;57:1023-29.
- Henke PK, Williams DM, Upchurch GR, et al. Acute limb ischemia associated with type B aortic dissection: Clinical relevance and therapy. Surgery 2006;140:532–40. doi:10.1016/j.surg.2006.06.019.
- Gargiulo M, Massoni CB, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg. 2014;3:351-67.
- Knowles M, Timaran CH. Epidemiology of acute critical limb ischemia. Crit. Limb Ischemia, Cham: Springer International Publishing. 2017;1-7.
- Holmstedt C, Chimowitz M. E-pearl: brachial artery embolus mimicking acute stroke. Neurology. 2011;76:e86-7.
- Robinson WP, Belkin M. Acute limb ischemia due to popliteal artery aneurysm: a continuing surgical challenge. Semin Vasc Surg. 2009;22:17-24.
- Rutherford RB. Clinical staging of acute limb ischemia as the basis for choice of revascularization method: when and how to intervene. Semin Vasc Surg. 2009;22:5-9.
- Sidawy AN, Gray R, Besarab A, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603-10.
- Creager MA, Kaufman JA, Conte MS. Acute limb ischemia. N Engl J Med. 2012;23366:2198-206.
- Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620-30.
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237-47.
- Wahlberg E, Olofsson P, Goldstone J. Acute upper extremity ischemia. Emerg. Vasc. Surg., Berlin, Heidelberg: Springer Berlin Heidelberg. 2007;41-4.
- Rolston DM, Saul T, Wong T, et al. Ultrasound in emergency medicine bedside ultrasound diagnosis of acute embolic femoral artery occlusion. J Emerg Med. 2013;45:897-900.
- Met R, Bipat S, Legemate DA, et al. Diagnostic performance of computed tomography angiography in peripheral arterial disease. JAMA. 2009;301:415.
- Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ. 2007;334.
- Garcia-Jacques M, Montealegre-Gallegos M, Matyal R. Acute limb ischemia and transesophageal echocardiography: making a case. J Cardiothorac Vasc Anesth. 2014;28:1164-65.
- Lewis A, Kirk G, McKinley A, et al. The role of transthoracic echocardiography in embolic acute limb ischaemia. Ir J Med Sci. 2009;178:457-9.
- Hirsh J, Warkentin T, Shaughnessy S, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinectis, dosing, monitorin, efficacy and safety. CHEST. 2001;114:489S-510S.
- Berridge D, Hopkinson B, Makin G, et al. Immediate management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2000;19:S128-29.