Intracranial Embryonal tumor with Multiple Rosettes: Case report and overview of literature
Received: 02-Nov-2021 Accepted Date: Nov 16, 2021; Published: 23-Nov-2021
Citation: Ankita Singh. Intracranial Embryonal tumor with Multiple Rosettes: Case report and overview of literature. J Neuropathol 2021;1(2):1-6.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
There are three known variations in histology in the family of Embryonal tumour with multiple rosettes. This family incorporates embryonal tumor with plenteous neuropil and true rosettes (ETANTR), ependymoblastoma and medulloepithelioma. We report a case of 6-year-old child showing with highlight of expanded intracranial weight and found to have a cleared out parieto-occipital injury in brain. The patient experienced a sub-total extraction of the tumor and histopathology and immunohistochemistry was suggestive of ETANTR. Craniospinal irradiation (CSI) was arranged and amid treatment, patient developed numerous episodes of seizures and grade IV haematological toxicity amid radiotherapy and thus patient lapsed with overall survival of 5 month. We moreover performed a comprehensive outline in terms of clinical, pathological, molecular and management results of this uncommon entity of paediatric brain tumor.
Keywords
Primitive Neuroectodermal Tumour, Embryonal tumour with Abundant Neuropil and true Rosettes; LIN28A gene; Cranio Spinal Irradiation.
Introduction
Embryonal tumor of central nervous system (CNS) generally occurs in paediatric group and account for around 1-3% of all brain tumors. Embryonal tumor with abundant neuropil and true rosettes (ETANTR) was to begin with portrayed by Eberhart et al in 2000 [1]. This could be a particular subtype of embryonal tumors, which has not been classified independently in WHO 2007 classification of brain tumors but are considered to be a part of family of CNS primitive neuro-ectodermal tumors (PNET) ETANTR is characterised by presence of undifferentiated neuroepithelial cells, wide zones of well differentiated neuropil, and ependymoblastic rosettes. The median age of presentation is 2.5 years (0.5-6), female are two time more inclined to develop than male (F: M=2:1) and generally in supratentorial region. Most of the patients present with partial convulsive seizure, altered general status, highlight of increased intra cranial weight like migraine, sickness and heaving, seizure, and shifted indications as per area of tumor. Histopathological examination are characterised by the presence of Homer Wright rosettes, foci of neurocyte, ganglionic cells maturation, strong synaptophysin expression and MYC/MYCN intensification. On immunohistochemistry, Glial Fibrillary Acid Protein (GFAP) are positive, with morphology steady with reactive astrocytes. GFAP) staining is basically seen in neuropil like areas in stellate scattered cells but not in undifferentiated areas. Synaptophysin stained neuropil zones and component with variable intensity. Recently expression of LIN28A is a highly specific marker and has found more prominent and intense in multilayered rosettes and poorly differentiated small cell tumors [2].
Treatment of this uncommon tumor is still not standard, as this clinicopathological entity is distinct from CNS PNET and considered as an aggressive paediatric brain tumors [3-5]. Five-year event free survival (EFS) rates of 60% have been accomplished within the last few years by distinctive strategies, including high-dose chemotherapy (HDCT), hyper-fractionated accelerated radiotherapy (HART), or concomitant once per day administration of carboplatin amid craniospinal irradiation (CSI) in combination with a variety of pre- and post-CSI chemotherapy regimens. Gandola et al had detailed 5-year EFS, progression free survival (PFS) and overall survival 70%, 72% and 73% in 33 patients treated with chemotherapy followed by HART. Gessi et al had reported median overall survival of 9 month. The foremost acceptable treatment of these forceful tumors may be chemotherapy based on HIT-SKK2000 protocol. Pattern of disease progression are exceedingly variable and most encounter local recurrence. In any case, a small number of subset develop leptomeningeal spread and have PFS of 8 month [6].
Case History
A 6 years old male child presented with chief complaints of fever, headache and vomiting since 3 months. He consulted to local doctor and received symptomatic treatment but symptoms reappeared after 10 days [7].
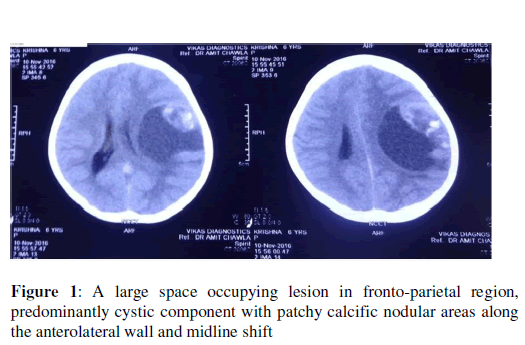
Contrast enhanced computed tomography (CECT) suggestive of large intra axial space occupying lesion is seen involving left fronto-parietal lobe measuring ~ 6.5X4.5X5.5 cm in size, composed predominantly of large cystic component with patchy nodular calcific areas along its antero- lateral portion with minimal to non-enhancing hyper dense areas with compression over left lateral ventricle with midline shift of ~ 0.9 cm towards right side. Possibly of neoplastic etiology like ganglioglioma/ desmoplastic neuroectodermal tumour or primitive neuro-ectodermal tumour (Figure 1).
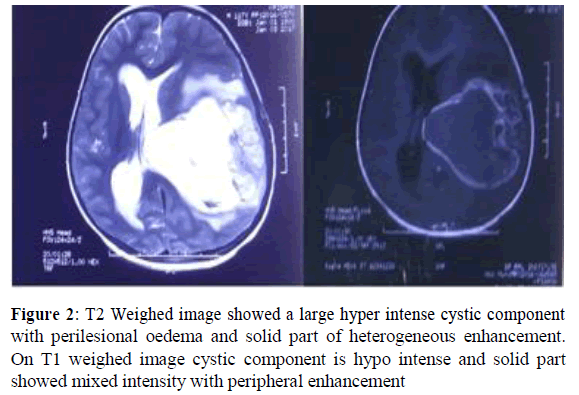
Magnetic resonance imaging (MRI) of brain was suggestive of a large mixed intensity solid and cystic lesion measuring ~ 8.0X7.1X7.2 cm in size noted in left parieto-temporal region with mild perilesional oedema. Adjacent sulci and gyri are effaced. Multiple areas of blooming were noted within was suggestive of intratumoral haemorrhage [8]. No restriction was seen on diffusion weighed images. Solid part of lesion shows heterogeneous enhancement and cystic part shows peripheral enhancement. The mass was compressing ipsilateral lateral and 3rd ventricle with midline shift of ~1.6 cm towards right side. Medially it was compressing body of corpus callosum. Inferiorly mass was compressing the midbrain with mild uncal herniation towards right side [9]. Right lateral ventricle was prominent with mild periventricular ooze suggested residual disease (Figure 2).
MRI of whole spine showed no metastatic deposits
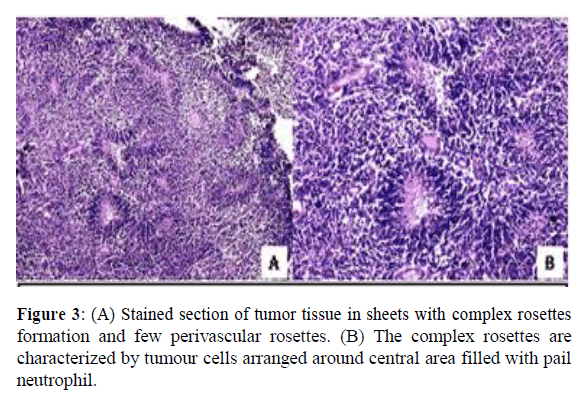
He underwent craniotomy and removal of cystic space occupying lesion under general anaesthesia. Post-operative histopathological examination was suggestive of astrocytoma grade III (WHO grading). After 2 weeks of asymptomatic period, symptoms reappeared and he underwent re-aspiration of cystic component two times. He presented to us with chief complaints of headache, vomiting and irritant behaviour. On examination, he was well oriented to time, place or person. Higher function, all cranial nerve, sensory and motor functions were intact. On work up, haematological investigation was within normal limit. Chest X-Ray and Ultrasonography of abdomen were within normal limit. Slide and block reviewed at our centre revealed tumour deposited in sheets with stratified complex rosettes characterized by tumor cells around central area filled with pale neuropil along with few perivascular rosettes. The tumor cells were small round with round hyperchromatic nuclei and scant cytoplasm. Mitosis, necrosis or endothelial proliferation was not evident (Figure 3).
On immunohistochemistry, vimentin and CD99 was positive. Synaptophysin was positive in intervening neuropil and GFAP had framework positivity (negative in tumor cells) while Pan CK, S100, EMA, and Chromogranin were negative and suggested Embryonal Tumors with Multi-layered Rosettes-NOS (WHO Grade- IV). Cerebrospinal spinal fluid cytology suggestive of no malignant cells was found. We planned for craniospinal irradiation (CSI) followed by boost to primary. We started CSI 36 Gy @ 1.8 Gy/# followed by boost to primary lesion up to 54 Gy. During radiotherapy he developed recurrent seizures for which he was under four antiepileptic drugs. He had completed CSI and during boost phase, developed grade IV haematological toxicity. Radiation was stopped and put on G-CSF and symptomatic. He doesn’t respond to medication and despite of all effort he declared dead.
Discussion
Embryonal tumors of CNS in paediatric bunch are uncommon and considered as a profoundly malignant tumor. Most of the studies unequivocally recommend incorporating four molecular medulloblastoma subgroups into the classification as separate entities. Most common location is supratentorial and predominantly in frontal lobe, it may be seen in the pineal region, in posterior fossa. Our case is also having lesion in supratentorial locale (parieto-occipital). Most of the patients show up under 4 years and predominantly in female. In contrast our reported case is 6-yearold male patients, Andrey Korshunov et al also reported male female proportion as 1.1:1 with median age 2.3 year (0.5-6 years). Radiologically, ETANTR appeared in different features when compared with other embryonal tumors. The lesions are in most cases are well differentiated and hyper intense on contrast improved T2 weighed images, in contrast to most CNS PNET, generally enhances focally or not. In our case MRI also suggested hyper intense lesion for both strong and cystic component. Judkins and Ellison proposed withdrawing the determination of EPBL from the classification of CNS tumors since ETANTR and EPBL are having single biological entity. On immunohistochemistry, GFAP and synaptophysin stained neuropil like areas in elongated or dispersed cells is seen but not in undifferentiated areas while MIB1 index was high in undifferentiated areas. In our case GFAP and synaptophysin are strongly positive for neuropil areas. The prognosis of ETANTR is very poor. Gessi M et al described the data of 25 patients and they had received post-operative chemotherapy alone or in combination with radiotherapy. Chemotherapy regimen was not consistent but most of the patients had received cisplatin, vincristine and cyclophosphamide. They reported 76% of mortality due to disease with a median survival of 9 month only. Other studies and case series also reported median overall survival 9-16 months. Further, aggressive treatment used for high risk embryonal tumor by Carsten Friedrich et al called HIT 2000 protocol. In which cisplatin, lomustine and vincristine chemotherapy regimen was used for 8 cycles as a maintenance therapy after post-operative radiotherapy. They had also used sandwich protocol with SKK chemotherapy protocol. They reported 3 year progression free survival and overall survival were 68 ± 12 and 66 ± 13 % respectively. We found in our case, the disease was highly progressive in nature. Three times aspiration of cystic component done due to reappearance of symptoms within a period of 2 month. During radiotherapy he developed recurrent episode of seizures, it may be due to disease progression, for which combination of four antiepileptic drugs was prescribed. At the end of CSI patient had developed grade IV haematological toxicity. This toxicity may be due to synergistic effect of radiotherapy and antiepileptic drugs induced. Radiation was stopped and put on G-CSF and symptomatic. He doesn’t respond to medication and despite of all effort he declared dead.
On conclusion, ETMR is a distinct entity of paediatric embryonic brain tumors that included ETANTR, EBL and MEPL. It may be considered as subtype of CNS PNET. This is mostly occurring in less than 4 years preferably female. These tumors may be presented with feature of increased intracranial pressure, seizure and symptom according to location of tumor. On clinicopathological consideration, it is highly aggressive tumor and may be incorporated in future WHO classification under paediatric brain tumors. Recently developed all biological markers are helpful for making a proper diagnosis especially expression of C19MC and LIN28 including GFAP, synaptophysin. It is useful to know the biological significance of prototype molecular knowledge of ETMR which may provide new targeted therapeutic approach for these type highly malignant tumors. Hopefully, the extensive molecular knowledge may be translated not only into a proper diagnosis, but also improving the more efficacious management.
REFERENCES
- Ñiguez BF, Martínez-Lage JF, Almagro MJ, Fuster JL, Serrano C, Torroba MA, Sola J. Embryonal tumor with abundant neuropil and true rosettes (ETANTR): a new distinctive variety of pediatric PNET: a case-based update. Child's Nervous System. 2010; 26: 1003-1008.
- Fuller C, Fouladi M, Gajjar A, Dalton J, Sanford RA, Helton KJ. Chromosome 17 abnormalities in pediatric neuroblastic tumor with abundant neuropil and true rosettes. Am J Clin Pathol.. 2006; 126: 277-283.
- Dunham C, Sugo E, Tobias V, Wills E, Perry A. Embryonal tumor with abundant neuropil and true rosettes (ETANTR): report of a case with prominent neurocytic differentiation. Journal of neuro-oncology. 2007; 84: 91-98
- Wang B, Gogia B, Fuller GN, Ketonen LM. Embryonal Tumor with Multilayered Rosettes, C19MC‐Altered: Clinical, Pathological, and Neuroimaging Findings. Journal of Neuroimaging; 28: 483-489.
- Korshunov A, Ryzhova M, Jones DT, Northcott PA, Van Sluis P, Volckmann R, Koster J, Versteeg R, Cowdrey C, Perry A, Picard D. LIN28A immunoreactivity is a potent diagnostic marker of embryonal tumor with multilayered rosettes (ETMR). Acta neuropathologica. 2012; 124: 875-881
- Spence T, Perotti C, Sin-Chan P, Picard D, Wu W, Singh A, Anderson C, Blough MD, Cairncross JG, Lafay-Cousin L, Strother D. A novel C19MC amplified cell line links Lin28/let-7 to mTOR signaling in embryonal tumor with multilayered rosettes. Neuro-oncology. 2014; 16: 62-71
- Bouali S, Zehani A, Mahmoud M, Said IB, Kallel J, Jemel H. Embryonal tumor with multilayered rosettes: illustrative case and review of the literature. Child's Nervous System. 2018 ; 34: 2361-2369.
- Govindan A, Vp M, Alapatt JP. Embryonal tumor with multilayered rosettes in a 3-year-old girl: case report. Turk Neurosurg. 2017; 8: 1.
- Wang Y, Chu SG, Xiong J, Cheng HX, Chen H, Yao XH. Embryonal tumor with abundant neuropil and true rosettes (ETANTR) with a focal amplification at chromosome 19q13. 42 locus: further evidence of two new instances in China. Neuropathology. 2011; 31: 639-647.
- Ferman LA, Daigle P, Weil A, Ellezam B, Hamel P, Perreault S. Embryonal tumor with multilayered rosettes presenting with intermittent third nerve palsy. Canadian Journal of Neurological Sciences. 2018; 45: 483-484.