Protective effect of ginger extract against alterations of rat thyroid structure induced by cypermethrin administration
Received: 27-Jan-2019 Accepted Date: Feb 13, 2019; Published: 20-Feb-2019
Citation: Yousef DM, Abd El-Fatah SS, Hegazy AA. Protective effect of ginger extract against alterations of rat thyroid structure induced by cypermethrin administration. J Exp Med Biol. 2019;1(1):19-25.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Cypermethrin (CYP) a type of insecticides is in common use. It has been found to be accumulated in the different body tissues leading to organ dysfunction. This study aimed to investigate the toxicity of CYP on thyroid gland structure and the possible ameliorating effect of ginger. Fifty adult male albino rats were used and equally divided into 5 groups (10 rats/group). Group I received only balanced diet and tap water. Group II rats were given corn oil (solvent of CYP). Group III (ginger extract group) received 750 mg/Kg body weight (BW) by gavage. Group IV (CYPtreated group) rats were given CYP (20 mg/kg BW) dissolved in corn oil by gavage. Group V (CYP and ginger extract- treated group) received CYP followed with ginger extract by the same manner mentioned above. After 14 days, venous blood samples were collected for assaying serum T3, T4 and TSH levels. Rats were anaesthetized then sacrificed. The thyroid gland was harvest for light and electron microscope examinations. The CYP-treated group showed histopathological and ultrastructure changes of thyroid follicles that appeared distended with flattened lining epithelium. Follicular cells showed vacuolation in their cytoplasm. Other follicles were lined by multiple layers of follicular cells (stratification). Many electron empty zones devoid of organelles and disrupted dilated cisternae of rough endoplasmic reticulum were noticed. Absence of microvilli congested dilated blood vessels and many collagen fibers were also detected. Follicular cells had apoptotic nuclei. Such alterations were associated with a highly significant increase in TSH (p<0.001) concomitant with decrease of T3 and T4. On the other hand, coadministration of ginger extract was noticed to ameliorate the damaging effects of CYP on thyroid tissues in animals of group V. It is suggested that co-administration of ginger could alleviate or minimize the possible toxicity resulting from CYP exposure.
Keywords
Thyroid; Microanatomy; Cypermethrin; Ginger; Ameliorating toxicity
Introduction
Pyrethroid pesticides have been used as worldwide insecticides since the past decade. They are a group of man-made products used in pest control in households and agriculture. Such pyrethroids have been classified as endocrine-disrupting compounds (EDCs) as they possess hormonal activities forming a potentially threat to human and wildlife [1]. EDCs have different mechanisms to decrease the concentration of natural hormones. First one (agonist action), binds to and activates the various hormone receptors and then mimic the natural hormone’s action. Second one (antagonist action) binds to these receptors without activating them and blocks the receptors and inhibits their action. Third mechanism may interfere with the synthesis, transport, metabolism and elimination of hormones [2]. Research on the endocrine disrupting effects of pyrethroid should be therefore given more concern.
The thyroid gland is considered as the main goal of endocrine disrupting chemicals (EDCs). Disruption may have severe drawback as thyroid hormones play an important role in the maintenance of normal body physiological status [3].
Cypermethrin (CYP), type II pyrethroid insecticides, is being extensively used for pest management and a chemotherapeutic agent against ectoparasite infestation in animals. It can be found in trace amounts or at higher concentrations in soil and air [4]. Usage of CYP has arrived at a frightening rate and causes a number of undesirable effects on non-target organisms including human beings by causing abnormal generation of reactive oxygen species (ROS) and significant damage to cell structure, lipids, proteins, carbohydrates and nucleic acids. Consistent with its lipophilic nature, it has been found to accumulate in body fat, skin, liver, kidneys, adrenal glands, ovaries and brains leading to oxidative damage as a result of increase in ROS causing organ dysfunction [5]. Administration of CYP to rats leads to disturbance of thyroid hormones, which are essential for some physiological processes as a controller of metabolic activity, including bone remodeling, cardiovascular activities, and abnormal behaviour [6].
Nowadays, people tend to use complementary and alternative medicine, especially herbal drugs, because of fewer side effects and lower cost. Medicinal plants have attracted the attentions due to the inherent antioxidant potential of the phytochemicals that reduce the free-radical induced oxidative damage. Consumption of plants parts that have high amounts of total phenolic and antioxidant compounds is important in protecting against oxidative damage [7]. Ginger is one among the plants which has been used for thousands of years in Asian and other Eastern cultures as an anti-vomiting, diabetes mellitus, and cancer treatment. Ginger (Zingiber officinale), belongs to the family Zingiberaceae, and contains flavonoids and polyphenolic constituents that have antioxidant, anti-inflammatory, antidiabetic and analgesic properties [8]. Therefore, the present work aimed to investigate the changes of thyroid structure induced by administration of CYP; and to highlight the possible protecting role of ginger.
Materials and Methods
Chemicals
Cypermethrin: CYP 98% purity liquid produced by Jiangsu Yangnong Chemical Group Co L.T.D., China. 2-Corn oil: was brought from commercial sources.
Preparation of ethanolic ginger extract
Ginger (Z. officinale Roscoe) rhizomes were purchased from the Zagazig markets. One kilogram fresh ginger rhizome was cleaned, washed under running tap water, cut into small pieces, air dried and powdered. 100 g of this powder were macerated in 1000 ml of ethanol for 12 h at room temperature and then filtered. The extract concentration was 750 mg/ml. Each animal was orally given 1 ml of the final aqueous extract [9].
Experimental animals
This study was carried out on fifty adult male albino rats each weighing 150-250 gm. They were obtained from the animal house of Faculty of Medicine, Zagazig University.
Experimental design
All animals were kept under hygienic conditions. Standard food and water ad-libitum were administrated. They were kept in animal house in hygienic cages. Temperature was maintained at 23°C ± 2°C. They were accommodated to the laboratory conditions for 15 days before being experimented. All rats were handled in accordance to the standard guide for the care and use of laboratory animals. The animals were equally divided into 5 groups (10 rats/each group) as the following:
Group I (Negative control group)
The animals received only regular diet and tap water to measure the basic parameters.
Group II (Positive control group)
Rats were given (1 ml/kg/day) of corn oil (solvent of CYP).
Group III (Ginger extract group)
Rats were gavaged orally with ginger extract (750 mg/kg body weight), once daily for 14 days [10].
Group IV (CYP treated group)
Rats were gavaged orally with CYP (20 mg/kg body weight) dissolved in 1 ml of corn oil, once daily for 14 days [11].
Group V (CYP and ginger extract-treated group)
Each animal was given CYP followed with ginger extract by the same manner mentioned above.
At the end of the experiment, venous blood samples were collected from retro orbital plexuses for assaying serum thyroid stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4) levels. Then, the animals were anaesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal injection) then sacrificed. The skin of the neck was incised and the trachea was exposed and dissected out. The thyroid gland was removed for light and electron microscope examinations.
Biochemical investigations
Blood samples were left for serum separation, and then centrifugation was done at 400 x g (times gravity) for 5 min and serum was kept at -20°C for further analysis. The T3 and T4 levels were determined using a radioimmunoassay (RIA) kit while TSH levels by enzyme-linked immunosorbent assay (ELISA), performed at Clinical Pathology Lab, Faculty of Veterinary Medicine, Zagazig University.
Light microscope (LM) study
After thyroid removal, it was immediately cut up into small pieces for microscope examination half of specimens for LM and the other for EM). The specimens for LM were immediately immersed in 10% buffered formaldehyde for 48 hours at 4ºC. After that, the specimens were processed for preparation of paraffin sections. These sections were stained by Hematoxylin and Eosin (H&E) [12]. Stained slides were then examined by light microscope at the Anatomy Department, Faculty of Medicine, Zagazig University.
Electron microscope (EM) study
Thyroid gland specimens from the control and treated rats were immediately fixed in 3% gluatraldehyde buffer at pH 7.4. The tissues were removed; and further fixed in 1.3% osmium tetroxide in phosphate buffer (pH 7.4) for 1 h. The samples were then processed and embedded in epoxy resin mixture. Semi-thin sections (1 ml) of thyroid gland tissue were achieved using an LKB ultra-microtome, then stained with toluidine blue and examined by a light microscope. Ultrathin sections (70-90 nm) were prepared and stained with uranyl acetate and lead citrate [13]. Stained grids were then examined by a JEOL electron microscope 1010 at the Regional Centre of Mycology and Biotechnology, Al-Azher University.
Statistical analysis
Data analysis was done using SPSS version 23 for windows. The means and Standard Deviation (SD) values of variables were calculated as well as Pearson correlation. One-way ANOVA test were used for comparison between different groups. The p-value ≤ 0.05 was considered statistically significant. P-values of serum TSH, T3 and T4 levels of adult male albino rats in each pairs of groups using independent t-test were calculated. Correlation was a bivariate analysis that measured the strength of association between two variables and the direction of the relationship. In terms of the strength of relationship, the value of the correlation coefficient varied between +1 and -1 [14].
Results
LM results
Group I (negative control group)
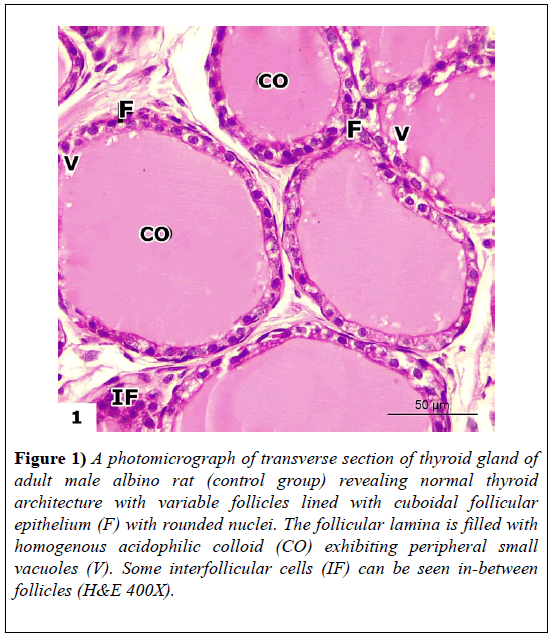
Examination of H&E-stained sections in the thyroid glands of control rats showed that thyroid parenchyma was composed of multiple follicles of variable sizes and shapes. The follicles were lined by cuboidal follicular epithelium with rounded nuclei. The follicular lumen was filled with homogenous acidophilic colloids with peripheral vacuoles. A few interfollicular cells appeared in between follicles (Figure 1).
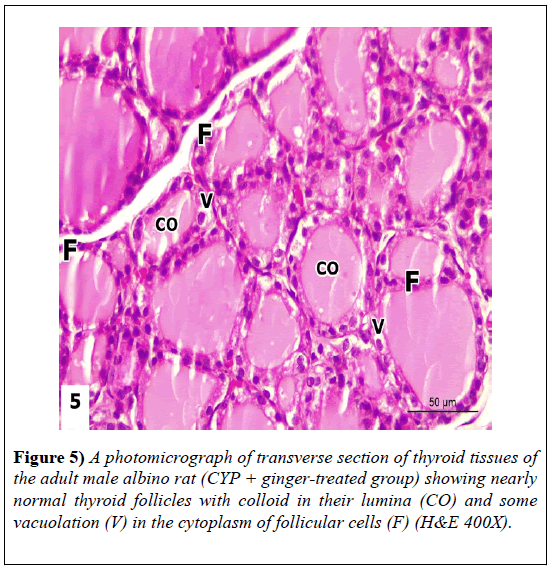
Figure 1: A photomicrograph of transverse section of thyroid gland of adult male albino rat (control group) revealing normal thyroid architecture with variable follicles lined with cuboidal follicular epithelium (F) with rounded nuclei. The follicular lamina is filled with homogenous acidophilic colloid (CO) exhibiting peripheral small vacuoles (V). Some interfollicular cells (IF) can be seen in-between follicles (H&E 400X).
Groups II (positive control) & III (ginger extract group)
Examination showed the normal thyroid tissues as control group.
Group IV (CYP-treated group)
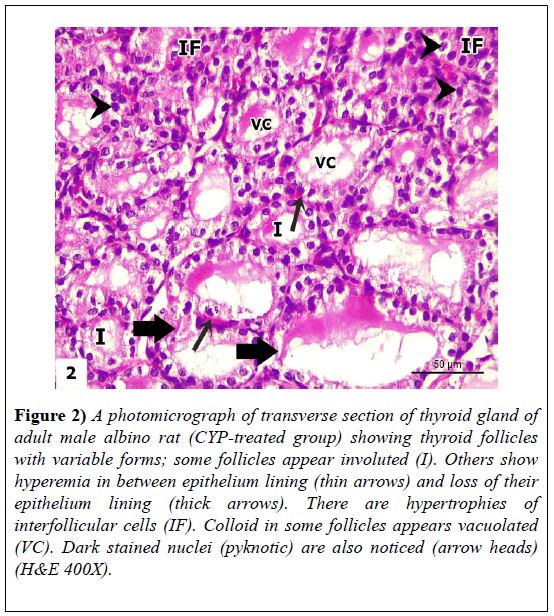
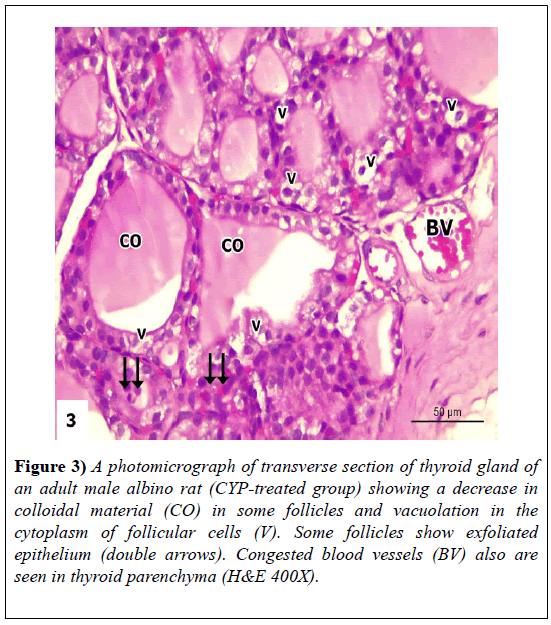
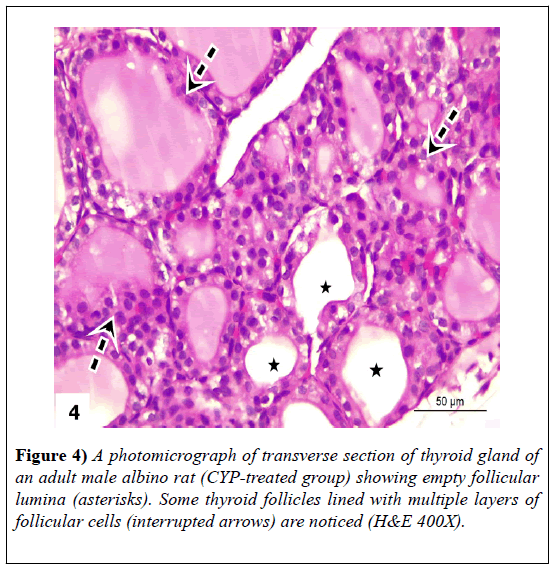
The sections revealed loss of normal thyroid architecture. Thyroid follicles appeared to have variable forms, where some follicles appeared shrunken and atrophied. Others showed hyperemia in-between epithelium lining and loss of their epithelium lining. There were hypertrophies of inter-follicular cells. Dark stained nuclei (pyknotic) were also noticed. The lining epithelia of some follicles appeared with hyperplasia (lined with multiple layers of follicular cells). Other follicular cells showed vacuolation in their cytoplasm or exfoliated cells in follicular lumen. Lumen of large number of follicles contained few and vacuolated colloid; while other follicles were devoid of colloid. Thyroid parenchyma showed congested blood vessels (Figures 2-4).
Figure 2: A photomicrograph of transverse section of thyroid gland of adult male albino rat (CYP-treated group) showing thyroid follicles with variable forms; some follicles appear involuted (I). Others show hyperemia in between epithelium lining (thin arrows) and loss of their epithelium lining (thick arrows). There are hypertrophies of interfollicular cells (IF). Colloid in some follicles appears vacuolated (VC). Dark stained nuclei (pyknotic) are also noticed (arrow heads) (H&E 400X).
Figure 3: A photomicrograph of transverse section of thyroid gland of an adult male albino rat (CYP-treated group) showing a decrease in colloidal material (CO) in some follicles and vacuolation in the cytoplasm of follicular cells (V). Some follicles show exfoliated epithelium (double arrows). Congested blood vessels (BV) also are seen in thyroid parenchyma (H&E 400X).
Group V (CYP and ginger extract-treated group)
Examination of sections revealed marked improvement of thyroid structure. It nearly retained its normal architecture. The colloid material filled the follicle lumen; and the follicular cells exhibited normal shape and arrangement except some vacuolation at follicular lining epithelium (Figure 5).
EM results
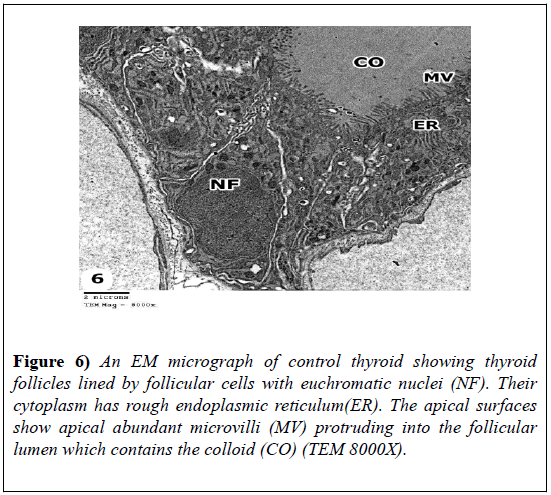
The follicular cells contained euchromatic nuclei. Their cytoplasm had rough endoplasmic reticulum. The apical surfaces showed apical abundant microvilli that protruded into the follicular lumen (Figure 6).
Figure 6: An EM micrograph of control thyroid showing thyroid follicles lined by follicular cells with euchromatic nuclei (NF). Their cytoplasm has rough endoplasmic reticulum(ER). The apical surfaces show apical abundant microvilli (MV) protruding into the follicular lumen which contains the colloid (CO) (TEM 8000X).
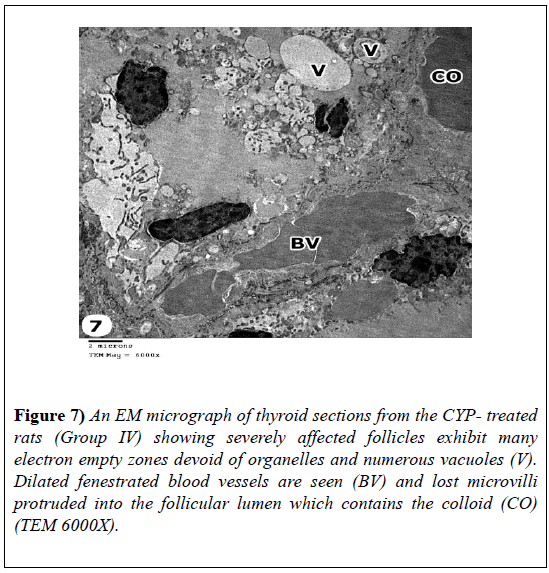
The CYP-treated rats revealed severely affected follicles exhibiting loss of normal ultrastructure of thyroid tissue. Their follicular cells exhibited loss of their nuclei and their cytoplasm showed numerous vacuoles with absence of microvilli that protruded into the follicular lumen. Dilated fenestrated blood vessels were seen (Figure 7).
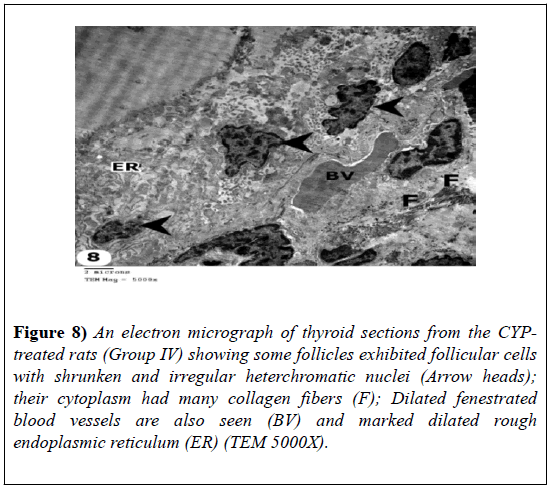
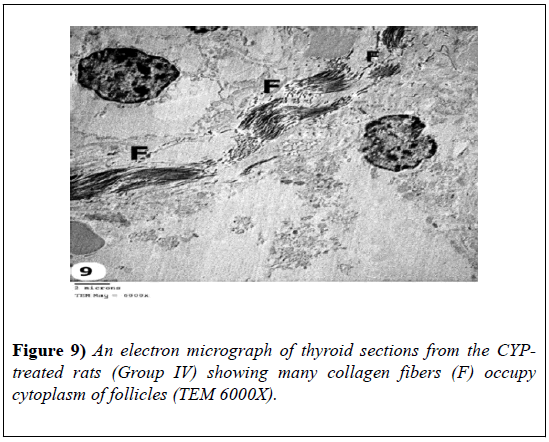
Some follicles showed follicular cells with shrunken and irregular heterochromatic nuclei; their cytoplasm had marked dilated rough endoplasmic reticulum and many collagen fibers. Dilated blood vessels were observed (Figures 7-9).
Figure 7: An EM micrograph of thyroid sections from the CYP- treated rats (Group IV) showing severely affected follicles exhibit many electron empty zones devoid of organelles and numerous vacuoles (V). Dilated fenestrated blood vessels are seen (BV) and lost microvilli protruded into the follicular lumen which contains the colloid (CO) (TEM 6000X).
Figure 8: An electron micrograph of thyroid sections from the CYPtreated rats (Group IV) showing some follicles exhibited follicular cells with shrunken and irregular heterchromatic nuclei (Arrow heads); their cytoplasm had many collagen fibers (F); Dilated fenestrated blood vessels are also seen (BV) and marked dilated rough endoplasmic reticulum (ER) (TEM 5000X).
Figure 9: An electron micrograph of thyroid sections from the CYPtreated rats (Group IV) showing many collagen fibers (F) occupy cytoplasm of follicles (TEM 6000X).
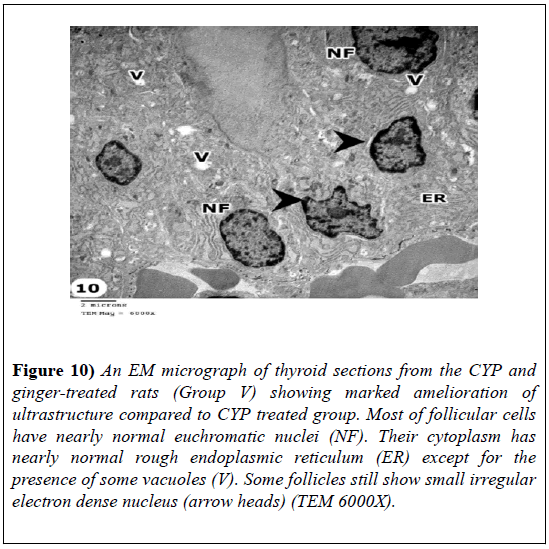
Ultra-structurally, thyroid follicles of CYP and ginger treated rats showed marked amelioration of the ultrastructure compared to CYP-treated group. The most of follicular cells had nearly normal euchromatic nuclei. Their cytoplasm had nearly normal rough endoplasmic reticulum except for the presence of some vacuoles. Cells of some follicles still show small electron dense nuclei (Figure 10).
Figure 10: An EM micrograph of thyroid sections from the CYP and ginger-treated rats (Group V) showing marked amelioration of ultrastructure compared to CYP treated group. Most of follicular cells have nearly normal euchromatic nuclei (NF). Their cytoplasm has nearly normal rough endoplasmic reticulum (ER) except for the presence of some vacuoles (V). Some follicles still show small irregular electron dense nucleus (arrow heads) (TEM 6000X).
Biochemical results
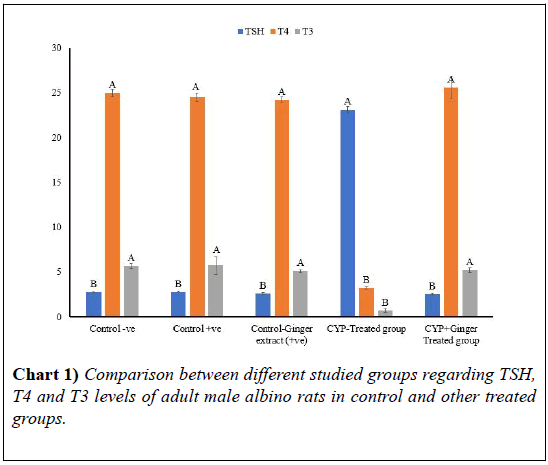
In the present work, no statistical difference was found among the first three groups. A significant decrease in the levels of T3 and T4 has been recorded after 14 days of CYP administration when compared with both the control and the CYP and Ginger treated groups. On the contrary, the serum level of TSH was significantly increased in the CYP treated group (Table 1 and Chart 1).
| Parameters | TSH ± SD (95%Cl, SE) | T4 ± SD (95%Cl, SE) | T3 ± SD (95%Cl, SE) |
|---|---|---|---|
| Control –ve group | 2.74 ± 0.21B (0.26, 0.09) | 25.00 ± 0.89A (1.1, 0.4) | 5.67 ± 0.67A (0.83, 0.3) |
| Control +ve group | 2.77 ± 0.16B (0.2, 0.07) | 24.50 ± 1.00A (1.24, 0.45) | 5.72 ± 2.25A (2.79, 1.01) |
| Control-Ginger extract group | 2.61 ± 0.18B (0.22, 0.08) | 24.20 ± 0.76A (0.94, 0.34) | 5.11 ± 0.33A (0.41, 0.15) |
| CYP-Treated group | 23.1 ± 0.88A (1.09, 0.39) | 3.19 ± 0.33B (0.41, 0.15) | 0.69 ± 0.4B (0.5, 0.18) |
| CYP &Ginger-Treated group | 2.52 ± 0.27B (0.33, 0.12) | 25.60 ± 2.62A (3.25, 1.17) | 5.2 ± 0.61A (0.76, 0.27) |
| p-values | <0.0001 | <0.0001 | <0.0001 |
| LSD at 0.05 | 0.573 | 1.801 | 1.46 |
• T3: Tri-iodothyronin, T4: Tetra-iodothyronin, TSH: Thyroid stimulating hormone
• p-value <0.0001 =very highly significant difference
• Means with the same letter are not significantly different from each other
• N=5 in each group
TABLE 1: Means’ comparison and p-values between each pairs of groups of TSH, T4 and T3 levels of adult male albino rats in control and other treated groups (± SD).
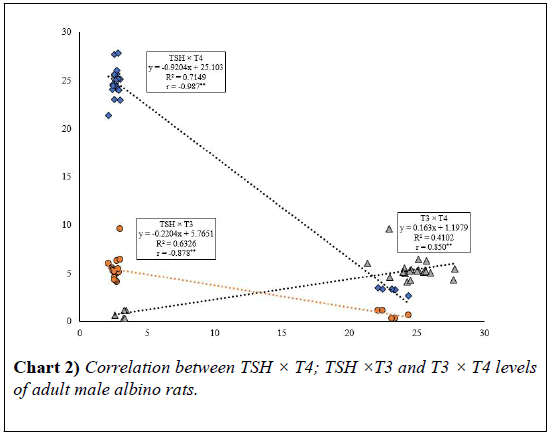
When the correlation coefficient (r) between two variable (T3 × T4) equal 0,850 (positive), it means when T3 gets larger the T4 gets larger. While, When the correlation coefficient (r) between two variable (TSH × T4) and (TSH × T3) equal (-0.987) (negative) and (-0.878) respectively. It means that when TSH gets larger, the other variable (T3 and T4) get smaller (inverse correlation) (Table 2 and Chart 2).
| Parameter | TSH | T4 | T3 |
|---|---|---|---|
| TSH (µIU/ml) | 1 | ||
| T4 (µg/dl) | -0.987** | 1 | |
| T3 (ng/ml) | -0.878** | 0.850** | 1 |
•** Correlation is significant at the 0.01 level (2-tailed) that measures the strength of association between two variables and the direction of the relationship.
TABLE 2: Correlation between TSH, T4 and T3 levels of adult male albino rats.
Discussion
Cypermethrin (CYP) is a pesticide widely used in killing household insects as cockroaches, fleas, and termites. It persists in the air, on walls and furniture for about three months after use. In countries like Egypt where agriculture is labor intensive, CYP used to kill insects on cotton and lettuce. Therefore, it represents a big challenge to the public health in the developing countries, including Egypt. The present study examined the possible toxic effect of CYP on rat thyroid structure by histopathological investigation and biochemical parameters. Zoeller et al. [15] supported the current study design as the structure of any organ closely reflects the state of its function; histological examination of the thyroid gland provides a sensitive early indicator of the glandular activity than serum T3 and T4 levels.
Concerning light microscopic examination of CYP treated rats; there was loss of the normal thyroid architecture. Thyroid follicles appeared to have variable forms, where some follicles were involuted and atrophied with vacuolated colloid. Follicular cells showed vacuolation in their cytoplasm or exfoliated cells in follicular lumina. Follicles exhibited loss of their normal ultrastructure. Follicular cells exhibited loss of their nuclei and their cytoplasm showed many electron empty zones devoid of organelles, disrupted cisternae of rough endoplasmic reticulum. These findings are in consistence with the study of Ruwhof and Drexhang, that attributed the presence of vacuolization to the markedly swollen mitochondria or markedly dilated rough endoplasmic reticulum (RER) [16]. Dabrowska et al. [17] added that RER reacted rapidly to the action of toxic compound so that its rough component undergoes vacuolization (vacuolar degeneration). The dilation of RER might be a compensatory mechanism as injured cells required oxidative enzymes for detoxification. Abdul- Hamid and Salah attributed the dilation of RER to retention of aberrant protein within the cisternae [18].
The lining epithelia of some follicles appeared with hyperplasia; lined by multiple layers of follicular cells. Thyroid parenchyma showed a hypertrophy of inter-follicular cells. Chiamolera and Wondisford explained that prolonged stimulation of the pituitary by decreasing levels of thyroid hormones so that the feedback inhibition of TSH is attenuated and more TSH is secreted by the thyrotrophs which may lead to thyroid gland neoplasia manifested as hypertrophy as well as hyperplasia of follicular cells and shrinkage of colloid area [19].
As regard the present LM examination, dark stained nuclei (pyknotic) were also noticed. It was confirmed by EM examination by presence of follicular cells with shrunken and irregular heterochromatic nuclei. These findings are compatible with the study of Abdul-Hamid and Salah, who observed degenerated dark follicular cells in some thyroid follicles with indistinct organelles after deltamethrin exposure [18]. Yu et al. [20] reported also degeneration and apoptosis of follicular cells might be owed to oxidative damage caused by chlorpyrifos with the generation of reactive oxygen species (ROS) and lipid peroxidation. Also, Zhang et al. [21] mentioned that nuclear changes are a sign of cell necrosis and apoptosis.
Soliman et al. [22] is in general agreement with present study that CYP administration could cause significant degeneration in the histological structure of liver tissues (hyperplasia, congestion, lymphocytic infiltration, pyknosis, necrosis, and vacuolation). Kassab and El-Aasr reported also that chlorpyrifos-treated rats showed some follicles of the thyroid gland with pyknotic nuclei and vacuolated [23]. Moreover, congestion and hemorrhage are the main components of the histopathological alteration.
In the present work, CYP-treated group showed dilated fenestrated blood vessels noticed by light and electron microscopes. On the same line, Forshaw and Bradbury added that deltamethrin has a role in increasing catecholamine release, resulting in some cardiovascular effects such as increased mean arterial pressure which raises the question of its direct role in heavy congestion and hemorrhages [24]. Shady and Noor El-den reported that oxidative stress and lipid peroxidation cause dilation and congestion of blood vessels by inducing lesion of their walls [25].
In the current work, ultrastructure examination of thyroid gland of CYPtreated rats revealed absence of microvilli that protruded into the follicular lumen. Nakazawa et al. [26] reported that poor thyroid hormones synthesis may be the main cause of lack of microvilli which disturb the transport of colloid between follicular lumen and follicular cells. Furthermore, ultra-structurally CYP-treated thyroid gland showed many collagen fibers in follicular cytoplasm. This is in general agreement with observation of Hashem et al. [27] in prostate who reported that CYPtreated group shows an increase in area percentage of the collagen fibers content, congested blood vessels and cellular infiltrations in prostate stroma.
Khaki et al. [28] mentioned that ginger has a strong antioxidant effect and also prevent generation of free radicals. Rodrigues et al. [29] added explanation that how ginger acts as potent antioxidant. Ginger contains phenolic compounds called gingerols which reduce lipid peroxidation and nitrosative stress and also increase activity of glutathione (GSH) and superoxide dismutase (SOD). Light microscope and ultra-structural results of CYP and ginger extract-treated rats in the current work revealed marked improvement of thyroid structure. It nearly retained its normal architecture except some vacuolation at follicular lining epithelium. These results are in accordance with Ansari et al. [30] who emphasized that ethanolic extract of ginger alleviates isoproterenol-induced myocardial necrosis in rats. Furthermore, Sakr and Al-Amoudi showed that ginger extract elucidate the structural alterations in testes caused by deltamethrin of albino rats [31]. Moreover, it decreases the expression of p53 and bax so that decreases the apoptosis.
In the current investigation, biochemical assessment of thyroid hormones and TSH level in the serum revealed that no statistical difference was found among the first three groups and CYP+ginger treated group. A significant decrease in the levels of T3 and T4 was recorded after 14 days of CYP administration when compared with both the control and the CYP + ginger groups. On the contrary, the serum level of TSH was significantly increased in the CYP treated group. Scanlan et al. [32] reported that the damage of follicular cells in the thyroid seems to be a reason for impaired thyroid hormones; and oxidative stress might be another reason. This result explained by that thyroid hormone liberation begins with endocytosis of small amounts of colloid into vesicles transported inside the follicular cells. Lysosomes then fuse with these vesicles and release T4/T3.
It has been found in previous studies that mixed pesticide exposures affected thyroid hormone levels (in both directions) depending upon the agents used, the exposure level, and the season. It was also found that TSH was elevated, total T3 suppressed, and T4 marginally decreased in pesticide formulator [33]. Biochemical results of CYP+ginger treated group of the current study are in line with those observed by Sakr and Shalaby, who reported that aqueous extract of ginger improved the histological as well as the histochemical alterations induced by metalaxyl may be attributed to its antioxidant properties [34].
It is concluded that CYP adversely affect the structure of thyroid gland with subsequent decrease of thyroid hormones. Therefore, CYP usage should be extremely limited; and must be under strict precautions. On the other hand, changes in thyroid gland were ameliorated with coadministration of ginger extract. It might be suggested as a useful method to protect against hazards occurring at the thyroid structure caused by CYP exposure. More future researches with new biomarkers and methods are recommended to ascertain the current findings; and to explore the safety and importance of ginger use in other organs and in humans.
Acknowledgements
The authors thank Dr. Assmaa Othman, professor of histology Zagazig University for her great support dedicating her effort, time and scientific experience in this work.
Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
REFERENCES
- Han Y, Xia Y, Han J, et al. The relationship of 3-PBA pyrethroids metabolite and male reproductive hormones among non-occupational exposure males. Chemosphere. 2008;785-90.
- Mnif W, Hassine AI, Bouaziz A, et al. Effect of endocrine disruptor pesticides: A review. Int J Environ Res Public Health. 2011;2265303.
- Tebourbi O, Hallegue D, Yacoubi MT, et al. Subacute toxicity of p,p'-DDT on rat thyroid: Hormonal and histopathological changes. Environ Toxicol Pharmacol. 2010;271-9.
- Wang XZ, Liu SS, Sun Y, et al. β-Cypermethrin impairs reproductive function in male mice by inducing oxidative stress. Theriogenology. 2009;599-11.
- Rini G, Choudhury CS. Alteration in estrous cycle, lipid peroxidation and antioxidant status in female rat after exposure to lambda cyhalothrin and its attenuation by taurine. Int J Bioassays. 2015;4526-32.
- Azubuike US, David O, Ibrahim RP, et al. Thyroid Pathology of Cypermethrin and Its Reproductive Implications in Yankasa Rams, Int J Biomed Materials Res. 2016;43-8.
- Rostamichijan M, Mosavat SH, Ghahramani L, et al. Anorectal diseases in Avicenna’s “canon of medicine”. Acta Med Hist Adriat. 2015;103-14.
- Daily JW, Yang M, Kim DS, et al. Efficacy of ginger for treating Type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J Ethnic Foods. 2015;36-43.
- Kamtchouing P1, Mbongue Fandio GY, Dimo T, et al. Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian J Androl. 2002;299-01.
- Aljahawey MHO, Soeharto S, Sujuti H. The effect of ginger extract (zingiber officinale roscoe) on male leydig cell and testosterone Level in carbofuran induced rat. Int J Pharm Tech Res. 2015;879-88.
- Grewal KK, Sandhu GS, Kaur R, et al. Toxic impacts of cypermethrin on behavior and histology of certain tissues of albino rats. Toxicol Int. 2010;94-8.
- Hegazy R, Hegazy A. Hegazy’ Simplified Method of Tissue Processing (Consuming Less Time and Chemicals), Ann Int Med Den Res. 2015;57-61.
- Gluert AM, Lewis PR. Biological specimen preparation for transmission electron microscopy, Princeton University Press, 1998.
- Dawson B, Trapp RG. Basic & Clinical Biostatistics, 4th Edition, McGraw-Hill, 2004.
- Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;11-53.
- Ruwhof C, Drexhang HA. Iodine and thyroid autoimmune disease in animal models. Thyroid, 2001;427-36.
- Dabrowska A, Jacewicz D, Lapinska A, et al. Pivotal participation of nitrogen dioxide in L-arginine induced acute necrotizing pancreatitis: protective role of superoxide scavenger 4-OH-TEMPO. Biochem Biophys Res Commun. 2005;313-20.
- Abdul-Hamid M, Salah M. Lycopene reduces deltamethrin effects induced thyroid toxicity and DNA damage in albino rats. J Basic Appl Zool. 2013;155-63.
- Chiamolera MI, Wondisford FE. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;1091-96.
- Yu F, Wang Z, Ju B, et al. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp Toxicol Pathol. 2008;415-23.
- Zhang C, Niu W, Wang Z, et al. The effect of gonadotropin on glucose transport and apoptosis in rat ovary. PloS One. 2012;e42406.
- Soliman MM, Attia HF, El-Ella GA. Genetic and histopathological alterations induced by cypermethrin in rat kidney and liver: Protection by sesame oil. Int J Immunopathol Pharmacol. 2015;508-20.
- Kassab A, El-Aasr M. Effect of Avocado pulp extract on chlorpyrifos-induced thyroid gland injury in rats: A histological and morphometric study. Egypt J Hist. 2018;83-92.
- Forshaw PJ, Bradburry JE. Pharmacological effects of prethroids on the cardiovascular system of the rat. Eur. J. Pharmacol. 1983;207-13.
- Shady AM, Noor El-deen F. Effect of chlorpyrifos on thyroid gland of adult male albino rats. Egypt J Histol. 2010;441-50.
- Nakazawa T, Murata S, Kondo T, et al. Histopathology of the thyroid in amiodaroneinduced hypothyroidism. Pathol Int. 2008;55-8.
- Hashem HE, Abd El-Haleem MR, Abass MA. Epithelial and stromal alterations in prostate after cypermethrin administration in adult albino rats (histological and biochemical study). Tissue Cell. 2015;366-72.
- Khaki A, Fathiazad F, Nouri M, et al. The effects of ginger on spermatogenesis and sperm parameters of rat. Iran J Reprod Med. 2009;7-12.
- Rodrigues SM, Ximenes CF, de Batista PR, et al. Tributyltin contributes in reducing the vascular reactivity to phenylephrine in isolated aortic rings from female rats. Toxicol Lett. 2014;378-85.
- Ansari MN, Bhandari U, Pillai KK. Ethanolic Zingiber officinale R. extract pretreatment alleviates isoproterenol-induced oxidative myocardial necrosis in rats. Indian J Exp Biol. 2006;892-97.
- Sakr SA, Al-Amoudi WM. Effect of ginger extract on deltamethrin induced histomorphological and immunohistochemical changes in Testes of Albino Rats. Life Science J. 2012;771-78.
- Scanlan TS, Suchland KL, Hart ME, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;638-42.
- Zaidi SS, Bhatnagar VK, Gandhi SJ, et al. Assessment of thyroid function in pesticide formulators. Hum Exp Toxicol. 2000;497-01.
- Sakr SA, Shalaby YS. Ginger extract protects metalaxyl-induced histomorphological and histochemical alterations in testes of albino mice. J Applied Pharmaceut Sci. 2011;36-42.