Real-time polymerase chain reaction and serological markers in the diagnosis and monitoring of hepatitis-B infected patients in southeast Nigeria
2 Gastroenterology Unit, Department of Medicine NAU/NAUTH Nnewi, Nigeria
3 Department of Obstetrics and Gynecology, Nnamdi Azikiwe University Teaching Hospital, PMB 5025, Nnewi, Anambra State, Nigeria
4 Medical Microbiology Department, Nnamdi Azikiwe University Teaching Hospital, PMB 5025, Nnewi, Anambra State, Nigeria
5 Community Medicine, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria
6 Department of Laboratory Accreditation Service, Medical Laboratory Science Council of Nigeria, Plot 1166 Mohammed Umar Lane, Durumi 2, Garki 2, Abuja, Nigeria
Received: 09-Nov-2021 Accepted Date: Nov 23, 2021; Published: 30-Nov-2021
Citation: Kalu SO, Chukwurah SN, Ugwunze OE, et al. Real-time polymerase chain reaction and serological markers in the diagnosis and monitoring of hepatitis-B infected patients in southeast Nigeria. J Hepato Gastroenterology. 2021; 5(2):10-14.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Keywords
HBV; Real-time PCR; Serological markers
Introduction
Hepatitis B virus (HBV) infection is a severe public health problem. It is the single most common cause of viral hepatitis in developing and underdeveloped settings that causes chronic liver disease, cirrhosis. It is linked to primary Hepato Cellular Carcinoma (HCC) [1]. Serological markers for HBV infection include Hepatitis B Surface Antigen (HBsAg), Hepatitis B Surface Antibody (HBsAb), Hepatitis-B envelops Antigen (HBeAg), and Hepatitis B envelops Antibody (HBeAb), and Hepatitis B core Antibody (HBcAb). The identification of serological markers allows the detection of individuals that are infected with HBV. It is also essential to elucidate the natural course of Chronic Hepatitis B (CHB) to assess the clinical phases of HBV infection and monitor antiviral therapy [2]. In the past, HBeAg and HBeAb were used to determine infectivity and viral replication. Still, now this purpose has been replaced by HBV DeoxyriboNucleic Acid (DNA) PCR assays. The seroconversion from HBeAg to anti-HBe is related to the remission of hepatic disease [3]. However, active viral replication is sustained in some individuals with seroconversion due to mutations in the pre-core and core regions preventing or reducing HBeAg production and expression [4]. PCR technology is used to directly measure HBV DNA viral load, which reveals the replication activity of the virus.
When serological HBsAg and HBeAg are negative (occult HBV) infections, a real-time PCR assay can detect occult HBV, thereby increasing awareness in clinical medicine [5]. The detection of HBV DNA through PCR technology is a reliable marker of replication activity. Higher HBV DNA viral load is a sign of rapid disease progression and higher incidence of Hepato Cellular Carcinoma (HCC) [6]. Additionally, PCR HBV DNA testing is critical in routine clinical settings to determine patients who need antiviral therapy and monitor patients on antiretroviral treatment for effectiveness or suitability of treatment [7]. The Roche real-time PCR technology can detect a wide dynamic range of viral load (lower range, 20 IU/mL to 170,000,000 upper ranges IU/mL). The Roche CobasAmplirep (CAP)/CobasTaqMan (CTM)/ is fully automated and does not generate carry-over contamination.
Materials and Methods
Study design and site
This prospective cohort study was performed at the PCR Laboratory of the Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. The laboratory is a reference laboratory for Early Infant Diagnosis (EID) of HIV among HIV exposed babies, HIV viral load for HIV positive patients on Anti-Retroviral Treatment (ART), HCV DNA, and hepatitis b viral load. The laboratory receives plasma samples from several southeastern states of Nigeria for HBV serological markers and DNA quantification tests. The NAUTH PCR laboratory implemented quality management and was accredited by the African Society for Laboratory Medicine (ASLM) in 2017.
Study population/study period
HBV positive patients sent to our laboratory for confirmation and quantitation tests within the period of study between May 2018 to April 2020.
Sample collection, procession, and storage
Ten ml of whole blood sample was collected into EDTA anticoagulant Vacutainer tube and gently mixed by inversion. The blood was centrifuged at 1,800 RPM for 20 minutes. The plasma was aliquot into 2 ml cryovial tubes and stored at -86°C until enough samples were pooled for testing.
5-panel testing
After sample separation, the rapid panel cassettes were placed on a plain table. Using the inclusion plastic pipette, dispensed two drops of the ample unto each of the five sample pots (wells) of the cassette. Two drops of the buffer were also added to the samples and allowed for 15-20 minutes.
The principle of the test
Hepatitis markers were analyzed by the colloidal gold and membrane chromatography technology. HBsAg, HBsAb, and HBeAg were measured in plasma with the dual-antibody sandwich method. In contrast, HBeAb and HBcAb were measured by the competitive neutralization method (Biosino Biotech Company, China).
Quantitation of hepatitis B virus DNA in plasma
Each patient’s HBV viral load was measured by real-time PCR using the COBAS AmpliPrep/COBAS TaqMan HBV test (Roche Molecular Systems, Inc., Branchburg, NJ, USA). The frozen plasma was placed at room temperature (15-30°C) until completely thawed before use. The Roche controls (High Positive, Low Positive, and Negative controls) were removed from 2-8°C storage to room temperature before use. All reagent cassettes were removed from 2-8°C storage, immediately loaded onto the COBAS AmpliPrep Instrument, and allowed to equilibrate to room temperature for at least 30 minutes before the first specimen procession. The appropriate number of reagent cassette racks, sample racks with Input S-tubes, SPU racks, K-tip racks, K-tube racks, and K-carriers on K-carrier racks was loaded accordingly onto the COBAS AmpliPrep Instrument. The sample rack was prepared by attaching a barcode label clip to where a specimen (S-tube) was placed. Barcodes label clips for the controls were also attached to the S-tubes accordingly in their position. The Amplilink software was used to create the specimen orders for each specimen and control them in the Orders window. The sample folder and HBV test definition file were selected and saved. The samples and controls were vortexed for 5 seconds, and 650 μL of the sample and controls were dispensed to the S-tubes. The S-tubes were then inserted in the sample rack and loaded to the AmpliPrep. The Amplilink software was used to start the COBAS Ampliprep.
The specimen preparation and simultaneous Polymerase Chain Reaction (PCR) amplification of target DNA were performed. The cleaved duallabeled oligonucleotide detection specific to the target was detected. After the COBAS TaqMan Analyzer run, the results were validated. The Roche CAP/CTM automated amplification and detection assay have a limit of detection of 20-10,000,000 IU/mL. HBV viral load levels exceeding 2,000 IU/mL were considered high-level HBV replication. In comparison, levels below 2,000 IU/mL were deemed low-level replication [8]. An undetectable viral load (target not detected-TND) was defined as HBV DNA below the level of sensitivity of the assay (that is, a viral load <20 iu/ml.
Results
Of the 280 HBsAg positive HBV samples received, 15 were negative with the HBV panel test methods. The 15 samples that were HBsAg panel negative showed target not detected with real-time PCR. More males, 163/280 (58.3%), were infected with HBV than their female counterparts 117/280 (41.7%). More males, 27(73.0%), were associated with higher viral load results (>20,000 IU/ml) than their female counterparts, 10 (27.0%). HBeAg positive patients were associated with higher HBV DNA viral load 2.28 log 10 to 8.28 log 10 compared to those with HBeAb negative (1.3 log 10 to 6.45 log 10).
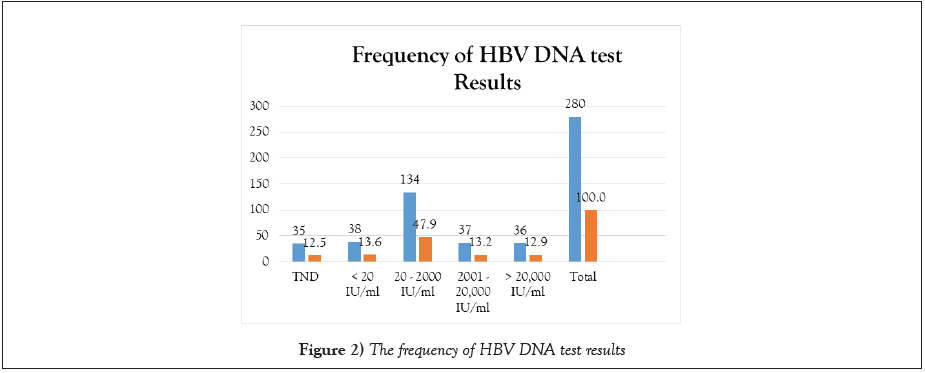
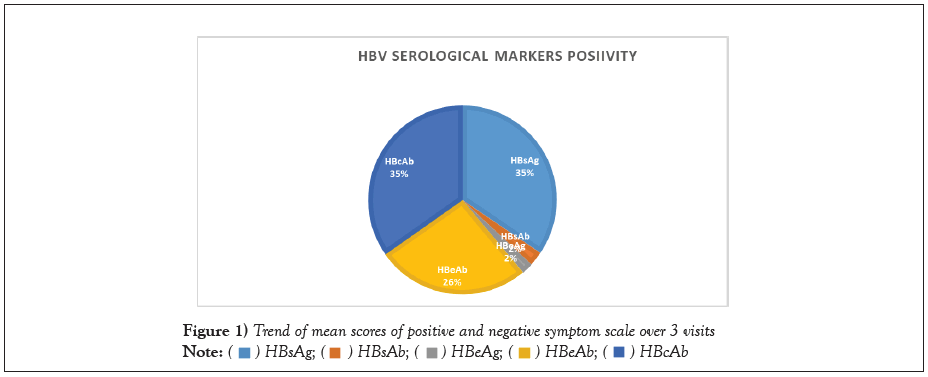
Figure 1 shows the percentage of HBV markers’ positivity and Figure 2 shows the frequency of HBV DNA test results.
Among the 280 samples tested, 35/280 (12.5%) showed target not detected while the range of detectable viral load were as follows 38/280(13.6%) <20 iU/mL, (134/280 (47.9%) (20-2000 i/ml, 37/280 (13.2%), 2001-20,000 iU/ mL, and 36/280 12.9%) was greater than 20,000 iU/mL.
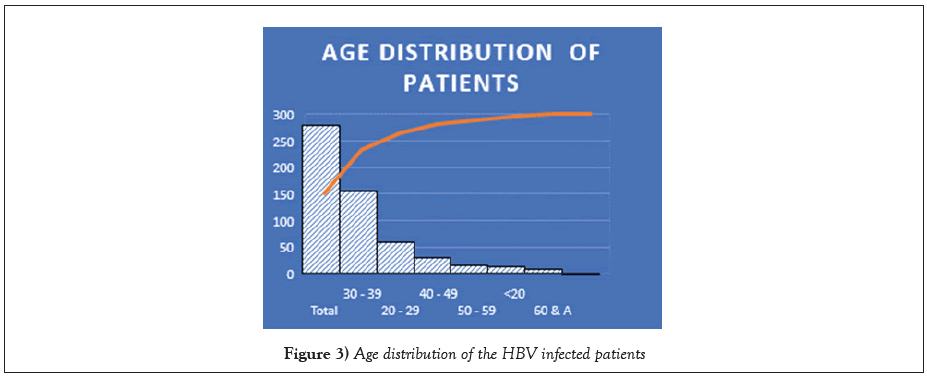
The patients between 30 to 39 years of age were frequently infected with HBV, followed by those between 20-29 years, while the least infected were 60 years and above. Of the 280 plasma samples tested for HBV serological markers, 265 were HBeAg negative (94.6%). Fifteen out of 265 patients were HBeAg positive (explicative disease stage), while 255/265 were were non-replicative. Two hundred and thirty (86.8%) had a detectable viral load, while 35/265 (13.2%) had an undetectable viral load. Out of the 230 plasma samples with detectable viral load, 171 (74.3%) had a viral load of less than 1000 IU/mL, 46/230 (20%) had viral load between 2000-9,999 IU/mL, while 13/230 (5.7%) had a viral load of ≥ 1.000.000 iu/ml.
Discussion
The presence of HBsAg is the most common marker of HBV infection. In contrast, HBeAg is a marker of active HBV infection. It is associated with a high viral load and progressive liver disease. Again, HBV DNA, HBeAg, and anti-HBeAg in patients’ plasma are also clinically useful for assessing patients with HBV infection. HBsAg is a standard diagnostic marker for HBV infection but does not provide information about active virus replication. The panel serological tests are better diagnostic tests because they can detect HBV infection; give an idea of those with active disease (the presence of HBeAg), those who have come in contact with HBV (the presence of HBcAb) as well as those that have received HBV vaccination (HBsAb). However, the PCR techniques have higher sensitivity and the potential to fill in this gap of low sensitivity as well as detecting occult cases [9].
The combination of these laboratory tests is not cost-effective for the patients. The test combinations are crucial for accurate diagnosis, clinical staging, and monitoring of treatment. It also helps to allay anxiety in the patients, especially for cases of confirmed negative tests.
Fifteen of the 280 plasma samples submitted for panel and quantitation tests were negative for serological marker and HBV DNA quantitation tests. We suspect this is due to differences in detection level, sensitivity, specificity of test methods, and false-positive test results.
We grouped our patients into replicative HBV disease by the presence of HBeAg and non-replicative by the absence of HBeAg. The number of replicative HBeAg was 15/265 and non-replicative 255/265. In this study, all the 15 HBeAg-positive patients (100%) had a detectable HBV DNA viral load by real-time PCR. This was similar to the 93% ((117/126) that was reported by Baker et al. among chronic hepatitis patients with replicative HBV disease by amplification PCR method [8]. Our finding also shows that HBeAg positive patients were associated with higher HBV DNA, more severe liver disease, and more infectious than negative results. HBeAg vivacious indicates viral replication and the reason for the high HBV DNA viraemia and increased risk of liver disease.
Most HBeAb positive patients showed different detectable HBV DNA levels with our study’s real-time PCR test method. The issue with seroconversion from HBeAg to anti-HBe has been explained to be due to mutation in the pre-C region that prohibits HBeAg synthesis and secretion [10,11]. This is probably the reason why some HBV-infected individuals are potentially infectious yet remain HBeAg negative. The reason for the non-detection of HBeAg in patients’ plasma could be due to low viraemia or low sensitivity of the test methods. PCR-based technology has the advantage of detecting HBV DNA in patients with low viraemia and among those with non-replicative HBeAg HBV disease.
Liver cancer is more common among men than females despite ethnicity or race [12]. More males were frequently infected with HBV in our study. They were also associated with higher viral load results (>20,000 IU/ml) than their female counterparts. Other reports suggest that the possible factors contributing to the male predominance with HBV disease could be the higher proportion of male tobacco use and heavy alcohol drinking, significantly tied to liver disease risk. Men who are current drinkers are eight times at higher risk of developing liver disease. In comparison, women who are current drinkers are four times as likely. Smoking also increases the risk of liver disease for both men and women. However, men smoke more than women and are twice more likely to develop liver disease than women, who are six percent more likely [12,13].
Conversely, the study of Nkrumah et al. [14] revealed a higher burden of HBV disease in females than males. The finding [15] also agreed with Nkrumah et al. [14] that females were highly associated with HBV infections. Still, HCV was higher among the male population. The authors reported the reason for female preponderance for the high acquisition of HBV than their male counterparts due to early onset of sexual activity, considering the wide vaginal surface, more extended semen contact and the possibility of iatrogenic acquisition in healthcare institutions where women more often work, admitted during pregnancy, delivery, and the care of family members than men.
The finding of Januszkiewicz et al. [16] shows that HBV was associated with Hepatocellular Carcinoma (HCC), which develops more often in men than in women, with a female/male ratio ranging from 1:4 to 1:7 [16]. Again, female HBV carriers have lower viral loads than male carriers [17,18]. Some studies have attributed the high serum testosterone levels in males to account for the associated increased risk of HCC development among male carriers of HBV [19].
Another potential reason for the gender disparity is the impact of sex hormones on the release of inflammatory cytokines in the opposite way. The female estrogens induce pro-inflammatory cytokines while male androgens suppress pro-inflammatory responses. Suppose inflammatory cytokine release occurs in the HCC microenvironment. In that case, it can contribute to the epigenetic changes responsible for malignant transformation in opposite ways between males and females. The studies by [20,21] have shown that androgens exert an inhibitory effect on the differentiation of the Th1 arm of the immune system, which causes a reduction in the production of IFN-γ that may explain the reason for the higher susceptibility to viral infections in males compared to the females [22].
Besides, men consistently are two times as likely as women to develop cirrhosis or liver cancer. A study conducted by scientists in China reports unusual liver proteins found only in males, which they attribute as the reason for the long-standing mystery behind the Hepatitis B Virus (HBV) sexually discriminates – the reason why HBV infection is hitting men harder than women [23].
Interestingly, scientists who experimented with laboratory mice found abnormal forms of Apolipoprotein A-I (Apo A-I). This protein is useful in fighting inflammation in the livers of infected male mice but not in infected female mice. The abnormal forms of these Apo A-I proteins were also found in the blood of men infected with HBV, but not in women. The researchers suggest that the proteins may provide significant markers for tracking HBV progression besides other gender differences [24,25].
The males and females between 30-39 years of age in our study were more frequently infected by the Hepatitis B virus, followed by those between the ages of 21-29 years, while the patients 40 years and above showed a notable decline HBV infection. Our finding is consistent with the reports of [26] that reported higher HBV infection among 20-29 and 30-39 years of age (26.33% and 21.67%) respectively and lowest among those aged 50 years and above. The reports of [27] is in tandem with our finding that there is a higher prevalence of HBV in both young and middle-aged adults.
Similarly, the reports of Alam et al. [28]; and Castolo et al. [29] are in agreement with our finding that the prevalence of HBV infection is significantly higher in persons with age between 21-40 years and lower in 41-60 years ago [30].
The findings of [31] from their meta-analysis study across all the regions and age group in Turkey is in keeping with our report that the highest age prevalence of HBV infection is among the young and middle-aged individuals.
Our finding was close to the reports by LEARM [32] that women between 25-34 years of age had a higher rate of HBV transmission. The report of the study conducted in Cameroon [33] and studies from Sudan (Rashaet al., [34] and Nigeria (Habiba and Memon, [31]; also confirms that HBV seroprevalence is higher among young and middle-aged individuals. The high transmission rate among individuals in this age-group correlates with the peak age of high sexual activity, confirming the role of sexual intercourse as one of the routes of HBV transmission [32].
In our study, patients with detectable serological HBsAg and HBeAg were associated with higher viral load (between 2.28 to 8.28 logs 10). This is not surprising because HBeAg is a core antigen secreted by the viral DNA, a marker of active viral replication with wild-type viruses [35,36]. Individuals with HBeAg positive usually have a higher risk of transmitting the infection to their households and sexual partners. Mother–to–child transmission of HBV may be greater than 90% when positive for HBeAg with high HBV DNA viral load compared to HBsAg-positive/HBeAg-negative mothers (15%-20%).
Our findings reveal that the panel serological markers test was more accurate than the HBsAg mono-screening test in confirming the presence of HBsAg in plasma samples and being important in the clinical staging of HBV disease.
Although over two-thirds of the patients were HBeAg-negative, most of them had a high HBV DNA viral load with a PCR test [37-40]. The distinction between chronic inactive HBV carriers and patients with HBeAg-negative disease is important in making clinical decisions for HBV-infected patients. The active HBV replication in the latter stage of the HBV disease represents the strongest single predictive biomarker associated with disease progression. Active disease replication is also attributed to HBV viral mutations that replicate aggressively without generating the HBe antigen [41,42]. Importantly, patients with HBeAg-negative disease require treatment evaluation and may require antiviral treatment for disease control.
Sex disparity in immune responses to viral infections
Research findings have shown that sex dimorphism occurs in humans and animals concerning immune responses and the transmission of viral infections [43]. Males are more susceptible to viral infections because females mount a more efficient and prolonged immune response for innate, humoral, and cell-mediated [44,45]. Besides, expression of Toll-Like Receptors (TLR7) and several monocytes, macrophages, and dendrite cells (innate immune response players) are significantly higher in females than in males, thereby accomplishing a more intense inflammatory response in females than in males [46-49]. Males have a lower antiviral response during homeostasis, contributing to their increased risk of persistent viral infections than females. Also, females have a higher number of CD4+T cells activated by viral antigens’ engagement. At the same time, there is a lower antiviral response in males contributing to the increased risk of undergoing persistent viral infections than the females [50-54].
Strengths and limitations
Strengths: The standard Roche molecular platform used for the HBV DNA detection in the study is susceptible and specific. It has heightened the validity of our findings. Also, the implementation of a quality management system and international accreditation of the NAUTH PCR laboratory enhances the confidence of our data.
Limitations: The study was only a quantitative study. We could not access risk factors that predisposed the patients to HBV infection. Moreover, we did not do IgM and IgG Quantitation tests to classify the patients into active and chronic HBV patients. Finally, we carried out liver function tests on the patients to know the biochemical effects of HBV infection.
Implication for health policy
HBV-infected individuals should not be denied PCR test investigation and treatment based on HBeAg negative laboratory results because the pre-C/core stop cordon mutation prohibits HBeAg synthesis and expression, leading to a negative HBeAg result. The implication for our findings is the need for policymakers to scale up HBV screening and vaccination intervention for the vulnerable group (infants and younger adults).
That panel test should be used for all screening tests instead of the HBsAg mono test because of its specificity, ability to reveal those who have received the vaccine, and the individuals with occult HBV infection, especially for blood bank services.
The young and middle-aged adult population (20 to 39 years of age) had greater exposure to HBV, probably due to high-risk behaviors common to the group. They should be specific targets for health programming.
An awareness program should familiarize them with HBV screening and vaccination through health campaigns, especially on World Hepatitis Day. The government of Nigeria should take aggressive steps towards HBV vaccination awareness programs involving the media to decrease the future burden of HBV in the population (Figures 1-3).
Conclusion
Our findings have shown that most HBeAg negative individuals may have significant detectable HBV DNA viral load probably due to mutation at the pre-core and core mutation, which hinders or reduce the rate of HBeAg synthesis and expression.
The advent of Real-time PCR assays is valuable in confirming the accuracy of other HBV test methods because of their high sensitivity over other test methods. We conclude that real-time PCR-based tests serve as an essential tool in several clinical settings, particularly in detecting low levels of viraemia in non-replicative HBV disease and patients with past HBV infection. Realtime PCR tools are also essential in diagnosing chronic hepatitis infection; they help establish HBV prognosis, most significantly, their application in guiding HBV treatment decisions and assessing response to antiretroviral drugs.
REFERENCES
- Pazgan-Simon M, Simon KA, Jarowicz E, et al. Hepatitis B virus treatment in hepatocellular carcinoma patients prolongs survival and reduces the risk of cancer recurrence. Clin Exp Hepatol. 2018; 4(3):210.
- Control CfD, Prevention. Epidemiology and prevention of vaccine-preventable diseases. Washington, DC: Public Health Foundation, 2011; 12.
- Deny P, Zoulim F. Hepatitis B virus: from diagnosis to treatment. Pathol Biol. 2010; 58(4):245-53.
- Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol. Hepatol. 2008; 2(4):553-62.
- Datta S, Chatterjee S, Veer V. Recent advances in molecular diagnostics of hepatitis B virus. World J Gastroenterol. 2014; 20(40):14615.
- Chen CJ, Yang HI, Su JU, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006; 295(1):65-73.
- Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol. 2008; 22(6):1031-48.
- Mbamalu C, Ekejindu I, Enweani I, et al. Hepatitis B virus precore/core region mutations and genotypes among hepatitis B virus chronic carriers in South-Eastern, Nigeria. Int J Health Sci. 202; 15(2):26.
- Randhawa PS. Application of PCR to the diagnosis of infectious diseases affecting the gastrointestinal tract and liver. PCR-based diagnostics in infectious disease. 1st edition, Garth D. Ehrlich. Greenberg SJ, eds. (Blackwell scientific publications, Inc); 1994: 244 -245.
- Carman WF, Karayiannis P, Waters J, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990; 336(8711):325-9
- Lai CL, Gregory PB, Wu PC, et al. Hepatocellular carcinoma in Chinese males and females. Possible causes for the male predominance. Cancer. 1987; 60(5):1107-10.
- Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991; 13(3):398-406.
- Nkrumah B, Owusu M, Frempong HO, et al. Hepatitis B and C viral infections among blood donors from rural Ghana. Ghana Med J. 2011; 45(3).
- Alomatu HO. HIV, HBV, HCV and Syphilis Infections among Blood Donors in Koforidua, Ghana (Doctoral dissertation, University of Ghana).
- Januszkiewicz-Lewandowska D, Wysocki J, Rembowska J, et al. Transmission of HCV infection among long-term hospitalized onco-haematological patients. J Hosp Infec. 2003; 53(2):120-3.
- Ruggieri A, Barbati C, Malorni W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. International journal of cancer. 2010; 127(3):499-504.
- Farza H, Salmon AM, Hadchouel M, et al. Hepatitis B surface antigen gene expression is regulated by sex steroids and glucocorticoids in transgenic mice. Proceedings of the National Academy of Sciences. 1987; 84(5):1187-91.
- Breidbart S, Burk RD, Saenger P. Hormonal regulation of hepatitis B virus gene expression: influence of androgen receptor. Pediatr Res.1993; 34(3):300-2.
- Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res. 1993; 53(4):790-4.
- Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. 2015; 294(2):87-94.
- Kissick HT, Sanda MG, Dunn LK, et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci. 2014; 111(27):9887-92.
- Kumar NA, Shan LX, Hardy MP, et al. Mechanism of androgen-induced thymolysis in rats. Endocrinology. 1995; 136(11):4887-93.
- Drexel University (2017) “Male hepatitis B patients suffer worse liver ailments, regardless of lifestyle.” ScienceDaily. ScienceDaily, 25 July 2017.
- Yang F, Yin Y, Wang F, et al. An altered pattern of liver apolipoprotein AI isoforms is implicated in male chronic hepatitis B progression. J. Proteome Res. 2010; 9(1):134-43.
- Kolou M, Katawa G, Salou M, et al. High prevalence of hepatitis B virus infection in the age range of 20-39 years old individuals in Lome. Open Virol J. 2017; 11:1.
- Lesi OA, Audu RA, Okwuraiwe AP, et al. Serological and virological markers of nigerian patients with hepatitis B infection. Niger J Clin Pract. 2019; 22:534-8.
- Alam MM, Zaidi SZ, Malik SA, et al. Molecular epidemiology of Hepatitis B virus genotypes in Pakistan. BMC Infect Dis. 2007; 7(1):1-6.
- Cisneros-Castolo MA, Ibarra-Robles IE, Escobedo-De La Peña J. Prevalence of hepatitis B virus infection and related risk factors in a rural community of Mexico. Am J Trop Med Hyg. 2001; 65(6):759-763
- Khan F, Shams S, Qureshi ID, et al. Hepatitis B virus infection among different sex and age groups in Pakistani Punjab. Virology journal. 2011; 8(1):1-5.
- Toy M, Önder FO, Wörmann T, et al. Age-and region-specific hepatitis B prevalence in Turkey estimated using generalized linear mixed models: a systematic review. BMC infectious diseases. 2011;11(1):1-2.
- Lem Edith Abongwa, and Penn Kenneth. Assessing Prevalence And Risk Factors Of Hepatitis B Surface Antigen Amongst Pregnant Women Attending Antenatal Clinic In The Northwest Region Of Cameroon. J Res Med Sci. 2016; 4 (1).
- Ducancelle A, Abgueguen P, Birguel J, et al. High endemicity and low molecular diversity of hepatitis B virus infections in pregnant women in a rural district of North Cameroon. PLoS One. 2013;8(11):e80346.
- Elsheikh RM, Daak AA, Elsheikh MA, et al. Hepatitis B virus and hepatitis C virus in pregnant Sudanese women. Virol J. 2007;4(1):1-3.
- Habiba, S, Memon, M. Prevalence of Hepatitis B infection in pregnant women in a tertiary care hospital. Inf Dis J Pak. 2007;35-38.
- Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol. 2008; 2(4):553-562.
- World Health Organization .Guidelines for the prevention, care, and treatment of persons with chronic hepatitis B infection. 2015.
- Andersson MI, Rajbhandari R, Kew MC, et al. Mother-to-child transmission of hepatitis B virus in sub-Saharan Africa: time to act. Lancet Glob Health. 2015; 3(7):e358-9.
- Jardi R, Buti M, Rodriguez-Frias F, et al. The value of quantiative detection of HBV-DNA amplified by PCR in the study of hepatitis B infection. J Hepatol. 1996;24(6):680-685.
- Merican I, Guan R, Amarapuka D, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15(12):1356-1361.
- Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen–negative chronic hepatitis B. Hepatology. 2001;34(4):617-624.
- Yang H, Lu S, Liaw Y, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174.
- Iregbu KC, Nwajiobi‑Princewill PI. Viral load pattern among hepatitis B surface antigen‑positive patients: Laboratory perspective and implications for therapy. Ann Med Health Sci Res. 2016; 6(2):95-99.
- World Health Organization (WHO). Global Hepatitis report 2017.
- Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen–negative chronic hepatitis B. Hepatology. 2001; 34(4):617-624.
- Ghosh S, Klein RS. Sex drives dimorphic immune responses to viral infections. J Immunol. 2017;198(5):1782-1790.
- Giefing‐Kröll C, Berger P, Lepperdinger G, et al. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging cell. 2015; 14(3):309-321.
- Ruggieri A, Anticoli S, D’Ambrosio A, et al. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016; 52(2):198-204.
- Melgert BN, Oriss TB, Qi Z, et al. Macrophages: regulators of sex differences in asthma?. American journal of respiratory cell and molecular biology. 2010;42(5):595-603.
- Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153(6):2544-2550.
- Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34(12):1050-1059.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hy. 2015; 109(1):9-15.
- Donnelly CA, Bartley LM, Ghani AC, et al. Gender difference in HIV‐1 RNA viral loads. HIV Med. 2005;6(3):170-178.
- Hsieh AR, Fann CS, Yeh CT, et al. Effects of sex and generation on hepatitis B viral load in families with hepatocellular carcinoma. World J Gastroenterol. 2017; 23(5):876.
- Park HJ, Choi JM. Sex-specific regulation of immune responses by PPARs. Experimental & molecular medicine. 2017; 49(8):e364.




 HBcAb
HBcAb