Review: Interactions between nanoparticles of platelets
, Manuscript No. PULJNN-23-6492; Editor assigned: 05-Jun-2023, Pre QC No. PULJNN-23-6492 (PQ); Reviewed: 19-Jun-2023 QC No. PULJNN-23-6492; Revised: 01-Jan-2025, Manuscript No. PULJNN-23-6492 (R); Published: 08-Jan-2025
Citation: Lahir YK. Review: Interactions between nanoparticles of platelets. J Nanosci Nanomed. 2025;9(1):1-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Pristine and engineered nano-materials are administered in human body or other bio-systems (a test model) either directly or via release form the pharmaceutical products, implants, and the products used for day to day life. The components of blood vascular system like blood plasma, leukocytes, erythrocytes and blood platelets encounter these nano-materials when these are within a bio-system. Among humans and other vertebrates the blood platelets have higher chances of interacting with administered nanoparticles because of their numbers, structural and cellular physiology and proliferative rate. Platelets are one of the active components that participate in hemostasis, plasma coagulation, tumor metastasis, and inflammatory process, interactions with leukocytes, antimicrobial, and immune response or host defence system. Activation of platelets is of great therapeutic significance and the parameters affecting their inactive state play significant role during the treatment of arterial thrombotic disorders. Fresh inflammation and increased healing are among the primary objects during the bioactive surgical clinical research. Recent developments on healing process reflect on the significant role of platelets in healing of hard tissues that involves a wide range of intra and extracellular orchestrated events under the specific control of signaling proteins. Any factor disturbing the inactive state of platelets needs careful investigation. Thus, knowledge about structural and functionality of platelets seems to be of great significance in the biomedical field. Some of the most/preferred nanomaterials in biomedical field are metal and metal oxide nanoparticles, various forms carbon nanoparticles, quantum dots, and dendrimers. The dependence and varied applications of nano-materials in biomedical science is also of great importance because the nano-materials applied in biomedical field are used as carriers, imaging agents and for detection of biomolecules. The interactions between platelets and these nano-materials become the focal point in understanding the mechanism of these interactions. Thus, it is thought imperative to evaluate the interactions between platelet and nano-materials.
Keywords
Carbon nanoparticles; Dendrimers; Metal nanoparticles; Nanotoxicity; Nano safety index; Platelets; Quantum dots
Introduction
Platelets are enucleated, small, and present in large numbers with limited life period. Their role during thrombosis and hemostasis is well established. This blood component also plays vital role in many pathophysiological processes [1]. The multiple genes concerning normal hemostasis, like, genes for encoding receptors, and signaling or secretory proteins influence platelets numbers and their degree of responsiveness. Because of the high level of secretory responsiveness of platelets these effectively influence ambient environment via releasing growth factors, chemokines, coagulating factors, species of RNA and extracellular vesicles. The platelets also have capacity to undergo changes in accordance to the fluctuating ambient environment. These blood components reach the site of the affected tissues that face pathogenesis. Such tissues include inflamed vascular wall, their interaction with leukocytes, system of coagulation and during interactions of thermo-inflammation. This concept offers an open field for further investigation concerning their responsiveness, numbers, and interactions [2].
Nanotechnology and nano-science play an eminent role in the field of sensing system and improved analytical performance. The nano-materials have enhanced the possibilities of new routes and degree of detection of analytes and target molecules [3]. Manufactured and natural nano-materials possess specific physicochemical features and these plays significant interactive roles with bio-components. These features are their size, shape, surface nature and charge, surface modifications and formulations, hydrophobicity, and hydrophilicity. The steric properties due to surface modifications, biocompatibility and non-compatibility of nanoparticles also play significant role during such interactions. These physicochemical characteristics and mode of administration of nano-materials have their functional impact during the interactions between nano-materials and biological cells [4]. These interactions include their up-take, dissolution at bio-nano-interface, inflammatory response, biodegradation, immunemodulatory interactions, and as distributors and carriers for pharmaceutical drugs. Nano-materials are likely to induce structural and functional fluctuations in a bio-cell depending in the dose, duration, physiological nature of the bio-system and the cell, and nature of the nanomaterials [5,6].
Natural and man-made nano-materials are of common occurrence in our ecosystem. The man-made nano-materials are copiously used in industries, medical field, food technology, biosensor, investigatory procedures, pharmaceuticals, and energy systems, [7-11]. Blood and its components are the first one to have direct impact of administered nanomaterials during biomedical processes and from ecosystem. The administered nanomaterials rapidly interact with biomolecules like proteins and form protein corona i.e., these biomolecules envelop these nanoparticles. Formation of protein corona is an important phenomenon that occurs and physiologically interferes in blood of bio-system.
The physicochemical features of developed and functionalized nanomaterials may affect their overall properties and also influence the biophysiological phenomena in a relevant bio-system. A geed deal of advancement in the nano-engineering, surface chemistry and reductionist functionalization has taken place but natural complex interface cannot be matched. This gives rise to a concept to enclose polymeric and other nanoparticles in naturally occurring cell membrane; the membrane of platelet appears to be very suitable for such purposes. Some of the most preferred applied nano-materials in biomedical field are metal and metal oxide nanoparticles, various forms carbon nanoparticles, quantum dots, and dendrimers.
Using membrane of platelets to enclose nanoparticles carrying significant cargos enhances safe bio-distribution/bio-dispersion of biomolecules or pharmaceutical products. Further, chances of escaping immune related responses in the recipient and elevating the degree of adherence with diseaserelevant molecules become favorable. Such membrane coated nanoparticles are suitably functionalized in accordance to immunomodulation and adhesive antigens present on the platelets. This aspect can influence cellular uptake and complement activation specifically in human plasma due to the mimicking features of the plasma membrane of platelets. The mimicking features like selective adhesion to damaged vasculation, and pathogens like bacteria. There is a possibility of therapeutic enhancement and disease targeted distribution of functionalized nanoparticles. Dendrimers are one of the most suitable carriers for molecular cargo like drugs, gene, therapeutics, and contrast agents. These nano-materials are mostly mono-dispersible, biocompatible, non-immunogenic and with enormous area for loading the biomedical cargo. This association involves very specific mechanism while interacting with cells, cell membrane, blood components and may exhibit cytotoxicity and hemolysis.
Literature Review
Interaction between metallic nanoparticles and platelets
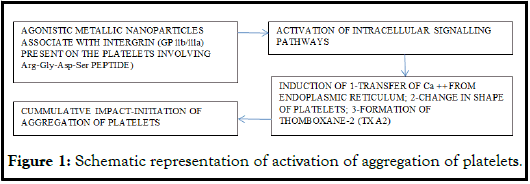
Generally, metallic nanoparticles induce aggregation of platelets. This process gets affected when ADP pathway gets interrupted due to the appropriate dose of either clopidogrel or apyrase. This condition promotes the second swing of aggregation of platelets and this depends on the extent of release of the platelet granules. The glycoproteins IIb/IIIa (GPIIb/IIIa, also known as integrin αIIb β3) are the major receptors on the platelets and play significant role during the interaction between metallic nanoparticles involving Arg-Gly-Asp-Ser, (a tetra-peptide); it helps with inhibition of activation without affecting GPIIb/IIIa [12]. The overall mechanism involves during interaction of metallic nanoparticles and platelets relates with the intracellular signalling pathways resulting in sequential physiological process. In short, after agonist associates with the respective receptor the orchestrated signalling sequence starts and it activates other signal pathway and affects the transfer of Ca++ ions form endoplasmic reticulum. The release of platelet granules, change in the shape of platelet and the formation of Thromboxane A2 (TXA2) starts in a specific sequence. These processes cumulatively activate the fibrinogen receptor (glycoprotein llb/llla) and primary phase of aggregation begins while the other resting platelets get activated to cause secondary phase of aggregation because of the released granules contents including ADP, ATP, and TXA2 (thromboxane2) (Figure 1). During this process there is a complex interplay of integrin receptors, second messengers, kinases, phosphatases, and mobilization of Ca ++ along with glycoproteins.
The circulating platelets along with blood exhibit ability to adhere when come in contact with administered material. This adherence involves cascade of signals resulting in cross linking of fibrin that results in the production of a clot. This mechanism is helpful in maintaining specific physiological hemostatic state, checks hemorrhages and also in the formation of harmful thrombi that affect physiological cerebrovascular anomalies. Nanomaterials are one of the prime favorable components involved in nanobiomedical applications and also are potential agents that influence the interaction with platelets. The gold nanoparticles impact the process of activation of platelets, and this influence is size dependant of these nanoparticles. The physicochemical nature of the nanoparticle along with size of SiO2, enhances the protein expression for the molecules within the cell which in turn induces intercellular adhesion among vascular cells. The SiO2 nanoparticles creep into plasma membrane of endothelial cells and stimulate the release of nitric oxide during in vitro experiments. These interactions also decline the ratio between the concentrations of NO and ONNO- [13]. Gold, silver, and quantum dots like CdTe and CdSe enhance the activation of platelets. The metal nanoparticles coated with carboxyl, amino, phosphate or hydroxyl groups also activate the platelets. The nanoparticles having negative charge on their surface aggravate thrombotic events and this interaction results in the physiological coagulation. The nanoparticles having fibrinogen adsorbed (fibrinogen protein corona) on their surface, interacts with platelets and enhance the degree of adhesion and also induce thrombosis. The protein corona formed on PEGylated nanoparticles does not affect hemocompatibility. The nanoparticles having positive charge interact with platelets (negative charge on its surface) and ensure the aggregation of platelets while gold nanoparticles with opposite charge than platelets do not affect aggregation of platelets. Zinc oxide nanoparticles exhibit anti-cancer, anti-bacterial impacts because these induce apoptosis, formation of reactive oxygen species, anti-viral and as an adjuvant for vaccine. The zinc oxide functionalized with quantum dots enhances its anti-bacterial and anti-cancer potential.
There are specific techniques used for the detection of effect of metallic nanoparticles and the physicochemical features of the media used for their bio-distribution. For example, the use of “Quartz Crystal Microbalance with Dissipation” (QCM-D), this technique shows that metallic nanoparticles influence the functionality of platelets during investigation under flow conditions. This technique is based on the ‘ring down technique’ and is applied to study interface acoustic sensitivity [14]. Commonly, this technique is used to study the thickness of film in liquid environment i.e., to measure the thickness adsorbed layer of protein. The Quartz Crystal Microbalance with dissipation reflects on the degree of analytical sensitivity of the technique over the traditional technique i.e., LTA, The LTA technique is ‘Lateral Transition Analysis’. This is a statistical technique and involves combination of cross-sectional measurement of categorical latent variables and longitudinal description of change, It involves three aspects i.e., a person-centred approach, lateral categorical variable, and longitudinal design]. The comparative results of these two techniques, QCM-D technique provide better and more sensitive observations related to interactions and compatibility of platelets.
Gold nanoparticles: Gold nanoparticles are safe, nontoxic, and have better cellular permeability and bio-dispersibility. Hence, these are suitable for drug delivery and applications in biomedical studies. There are some references that report on its toxic behavior basically caused due to their physical size, shapes, and surface chemistry. As a result, there exists an ambiguity regarding the compatibility of gold nanoparticles with reference to blood components. Their size exhibits different impacts on platelets [15]. Bigger gold nanoparticles (60 nm and above) show no influence on platelets while smaller ones affect the activation process of platelets. In some cases, the gold nanoparticles affect molecular mechanisms, specifically, during the canalicular system of platelets and intracellular signalling mechanism activation. These mechanisms involve tyrosine phosphorylation, release of α- granules and translocation of P-selectin. The spherical gold nanoparticles having approximately 30 nm size do not cause aggregation of platelets; probably, due to the protein corona formation on the spherical gold nanoparticles. The PEGylated gold nanoparticles enhance the degree of compatibility of platelets. In the case of equine species the conjugated gold nanoparticles with Polyethyleneimine (PEI) and Polyvinyleprrolidone (PVP) facilitate formation of filopodia and degranulation in platelets. This process relates to the degree of adsorption of fibrinogen; this process gets more elaborated when gold nanoparticles are conjugated with Polyacrylic Acid (PAA) interact with platelets. The PAA after binding with fibrinogen induces conformational changes in it. The changed conformated fibrinogen impacts the hemocompatibility of such nanoparticles.
Silver nanoparticles: There are conflicting reports on the impact of interactions between uncoated and coated silver nanoparticles and platelets. Silver nanoparticles cause a rise in the degree of aggregation of platelets. This increase is due to the elevated levels of intraplatelet Ca++, secretion of GPIIb/IIIa, P-selectin and serotonin, during the interaction between platelets and spherical silver nanoparticles having 10-100 nm size, the adhesion of platelets increases but no further impact on aggregation of platelets. Silver nanoparticles coated with polyethylene glycol (PEG), or (PEGylated) affect the levels of collagen, ADP, thrombin and arachidonic acid an as a result of this impact, there is an increased degree of adhesion of platelets and this effect is dose dependent. The monodispersed spherical silver nanoparticles having size 10-15 nm exhibit antiplatelet features. The probable variations in the observations might be due to the nature of dispersing medium and the type of technique involved. Most common technique adopted is ‘Light Transmission Aggregometry’ (LTA) to evaluate degree of aggregation of platelets. This technique determines the percentage of aggregation of platelets in platelet-rich plasma. The measure of the increase in emission of light against the amount of platelet agonist used in the given platelet suspension. This technique has limitations like, its level of sensitivity for the measure of the NP-induced platelet aggregation. The silver nanoparticle exhibit significant light absorbance features at 10 μg concentration; if the threshold of silver nanoparticle is not maintained then the observations are affected to a greater extent. The LTA technique may not detect increased platelets aggregation in ex-vivo experimentation at 10 μg/mL concentration of commercial oral colloidal silver nanoparticles.
Iron oxide nanoparticles are very suitable as contrast agents during biomedical and investigatory studies, specifically, during scanning electron microscopy. These are useful to demarcate healthy and diseased cells and to evaluate the impacts of cytotoxic drugs. There are inconsistent reports on the effects of functionalized and non-functionalized iron oxide nanoparticles on platelets. Carbon coated iron carbide magnetic nanoparticles influence the up-regulation of GPIIb and P-Selectin but PEGylated iron nanoparticles reverse this effect [16]. During induction of hyperthermia using dextran-stabilized iron oxide nanoparticles neither supported this process nor the functionalities of platelets. In an another study, the starch-stabilized iron oxide nanoparticles do not affect the functionalities of platelets but those stabilized with citric acid prevent aggregation of platelets. The use of labelled platelets in the studies of survival, preparation of various concentrations of platelets, and to differentiate donor and recipient or impaired platelets for investigations, is of great utility; quantum dots can be very feasible option for such experimentations. This view may have limitations specifically when serum albumin is used for functionalization of platelets.
Nickel nanoparticles cause cytotoxic impacts along with changes in the structure of platelets. There is a greater need for further investigations in this aspect of interaction between nickel nanoparticles and platelets.
Zinc oxide nanoparticles having solution of citrate, glucose as medium of dispersion activates the process of platelets activation, specifically, in case of species of horses. The zinc oxide nanoparticles do not cause the formation of thrombus. There seems to be scanty reports in this field and hence there a need to ponder and delve in these aspects. ZnO nanoparticles are relatively safe, nontoxic and biocompatible but being photosensitive are likely to generate Reactive Oxygen Species (ROS) in bio-system. This makes the ZnO nanoparticles unsafe to some extent (Table 1). Mesosilica nanoparticles reduce the hemolytic effect while interacting with red blood cells, iron oxide nanoparticles affect the pH of red blood cells elevate the osmotic fragility causes change in the morphology of red blood cell.
Copper, iron and cadmium sulfide nanoparticles exhibit ADP dependent pro-aggregatory activity while interacting with platelets. The pro-aggregatory impact involves the low affinity Purinergic receptor (P2Y12) under the influence of ADP. The Purinergic receptor (P2Y12) and the ADP are the potential target for the wide range of nanoparticles. The degree of proaggregatory effect relates with the material and the shape of the nanoparticle.
Titanium dioxide nanoparticles are known for their photocatalytic properties and generation of varied reactive oxygen species; hence, these can be a potential cellular toxic factor. These nanoparticles induce activation of platelets in dogs but the degree of activation gets reduced in case of human platelets. Titanium dioxide rutile nanoparticles cause murine platelets aggregation but this impact is not seen with TiO2 anatase nanoparticles Titanium dioxide rutile nanoparticles do not induce activation of platelets in mice (Table 1 and Figure 2).
| No. | Nanoparticles | Physical and specific conditions | Nature and impact on platelets |
|---|---|---|---|
| 1 | Gold | Safe, mostly nontoxic, improve permeability and biodispersibility. | |
| 30 nm spherical | Do not cause aggregation of platelets. | ||
| 60 nm and above | Affect molecular mechanism in canalicular system of platelets. | ||
| Coated with polyethylene glycol | Enhance compatibility of platelets. | ||
| Coated with polyvinyleprrolidone | Facilitate formation of filopodia and degranulation in platelets. | ||
| Conjugated with polyacrylic acid | Induces conformational changes and affect hemocompatibility. | ||
| 2 | Silver | Contradictory reports: over all enhance the degree of aggregation of platelets | |
| 10-100 nm spherical | Elevate intraplatelet Ca++, secretion of GPIIb/IIIa, P-selectin and serotonin. | ||
| coated with polyethylene glycol | Dose dependent effect; affect levels of collagen, ADP, thrombin and arachidonic acid, increase degree of adhesion of platelets. | ||
| 3 | Iron oxide | Inconsistent observations. | |
| Carbon coated iron oxide | Up-regulate GPIIb and P-selectin. | ||
| Coated with polyethylene glycol | Down-regulate GPIIb and P-selectin. | ||
| Stabilized with citric acid | Prevent aggregation of platelets. | ||
| 4 | Nickel | Cause cytotoxic impacts and changes the structure of platelets. | |
| 5 | Zinc oxide | Relatively safe, nontoxic and biocompatible. | |
| Exhibit anti-cancer, anti-bacterial impacts because these induce apoptosis, formation of reactive oxygen species, anti-viral and as an adjuvant for vaccine. | |||
| In solution of citric acid and glucose | Enhances activity of platelets, do not result in thrombin formation, being photosensitive in nature it generates ROS, hence, not safe for use. | ||
| 6 | Copper, iron and cadmium sulfide | Result in ADP pro-aggregatory activity in platelets. | |
| 7 | Titanium oxide | Cytotoxic, exhibit photocatalytic nature and generate ROS activate platelets in dogs and in humans this activation reduces. | |
| 8 | Titanium dioxide rutile and Titanium dioxide anatase | Murine platelets aggregate, do not induce activation of platelets in mice. |
Table 1: Metal and metal oxide nanoparticles and their effects on platelets.
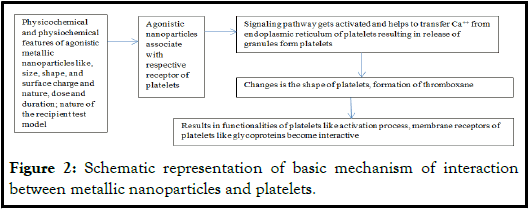
Figure 2: Schematic representation of basic mechanism of interaction between metallic nanoparticles and platelets.
Interaction between platelets and carbon nanomaterial
Carbon nanomaterials are one of the most sought-after nanomaterials in industrial, biomedical fields and investigatory studies. Carbon nanoparticles exist as tubular, spherical, sheet and other multidimensional shapes. These occur naturally and are fabricated as per the mode of application. The carbon nanoparticles have higher degree of biocompatibility and biodistribution in a given bio-system. The specific properties such as noncovalent functionalization ensure the van der Waals π-π bonding and hydrophobic and hydrophilic interactions. The nanoparticles act as favourable vehicles for drugs, biomolecules to be delivered to specific target. The common carbon nanoparticles used are carbon nanotubes (hollow, single walled and multiwalled), spherical, elliptical, and one can readily modify them structurally and functionally to a suitable specific applications for biomedical field. Their specific features like higher degree of biocompatibility and bio-distribution play major roles during their applications. The physicochemical properties like van der Waal, π-π bonding, hydrophobic and hydrophilic nature facilitate their functionalization involving carboxyl, ammonium group, and non-covalent bonds. Such functionalizations render these carbon nanoparticles suitable agents to load drug, bio-molecular cargo for appropriate delivery to a specific site in bio-system. Carbon nanomaterials such as carbon nanotubes, carbon quantum dots, carbon onions, fullerenes, nanodiamonds are among the most commonly used carbon nanomaterials in most of the biomedical fields [17].
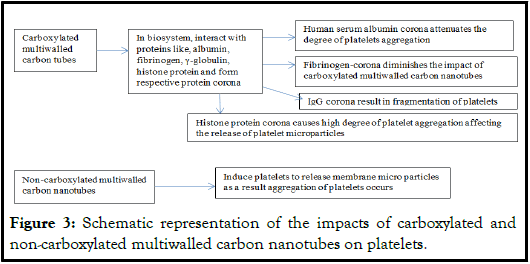
Carbon nanotubes, either functionalized or non-functionalized need caution during their applications in biomedical and other fields. These nanomaterials generally form protein corona when come in contact within proteins of biosystem or otherwise. These interactions involve non-covalent interactions which influence the physicochemical properties of carbon nanotubes. The development and functionalization of nanoparticles are likely to influence the biological processes in a bio-system and may affect their efficacies. In spite of the improved bottom-up nanoengineering techniques, chemistry of their surfaces and reductional approach affect the behavior of their complex interfaces specifically with components of biosystem. These products can be enclosed in the membrane of specific cell such as human platelets. These platelets participate in different pathological conditions. The carboxylated multiwalled carbon nanotubes form protein corona with proteins like albumin, fibrinogen, gamma-globulins, and histone proteins. There is a pronounced effect of these respective protein multiwalled carbon nanotubes coronas on the blood platelets. Noncarboxylated multiwalled carbon nanotubes induce platelets to release membrane microparticles, and aggregation among platelets. The fibrinogencorona on carbon multiwalled nanotubes agglomerates diminishes the impact of carboxylated carbon multiwalled nanotubes. The serum human albumin corona on carboxylated carbon nanotubes attenuates the degree of platelets aggregation while IgG corona on carboxylated nanotubes fragments the platelets. The histone protein corona brings about high degree of platelet aggregation affecting the release of platelet microparticles (Figure 3).
Figure 3: Schematic representation of the impacts of carboxylated and non-carboxylated multiwalled carbon nanotubes on platelets.
The activation of platelets is of great therapeutic significance and their inactive state is significant during the treatment of arterial thrombotic disorders. Any administered materials in human body or any vertebrate that may affect the inactive state of platelets requires lot of caution and attention of researchers. The carbon dots nanoparticles appear to be one of the potential antiplatelet agents [18]. These nanoparticles exert inhibitory impacts on collagen induced human platelet aggregation. These nanoparticles do not cause cytotoxic influence and this factor can be evaluated using lactate dehydrogenase activity. Further, these nanoparticles prevent the activation of collagen activated Protein Kinase (PKC), protein kinase B, C-Jun N-terminal Kinase (JNK) and phosphorylation of p38 mitogen activated protein kinase (MARK). In mice, the carbon nano-dots as an anti-platelet agent do not influence the bleeding time but these nanoparticles reduced the degree of mortality and enhanced ADP-induced thromboembolism. This observation indicates the possibility of carbon nano-dots as the possible therapeutic agent for treating thromboembolic conditions (Table 2).
Hallocyte nanotubes are having unique cylindrical configuration and high aspect ratio; with chemical formula Al2Si2O5(OH)4.nH2O and aluminosilicate present as layer. Generally, they have length ranging 0.5-1.5 μm, inner diameter within 10-40 nm and outer diameter 40-70 nm. Its outer surface exhibits chemical properties similar to those of SiO2 and inner surface shows similar properties to Al2O3. These functionalized nanotubes favour blood coagulation and promote activation of platelets. The degree of platelet activation is dose dependent. The cylindrical configuration of Hallocyte nanotubes enhances the degree of contact in comparison to spherical configuration and surface properties also affect the capability of platelets to aggregate. The Hallocyte nanotubes also affect the recalcification duration in a dose dependent manner and this behavior reflects on its procoagulant efficacy (Table 2). These features indicate that Hallocyte nanotubes have better hemocompatibility in comparison to other nanotubes and are suitable for biomedical applications.
| No. | Carbon nanomaterials | Nature and probable impact |
|---|---|---|
| 1 | Carbon dots | Mostly nontoxic, inhibit collagen induced human platelets aggregation; in mice, these do not affect bleeding time but reduce the degree of mortality and enhance ADP induced thromboembolism. |
| 2 | Carbon nanotubes | Mostly non-cytotoxic, antiplatelet agents, prevent collagen activated protein kinase, protein kinase-B, N-terminal kinase and phosphorylation of p38 Mitogen Activated Protein Kinase (MARK). |
| 3 | Hallocyte nanotubes | Promote activation of platelets, increases ability to contact and change in the nature of surface of platelets that alter their capability to aggregate, dose dependent recalcification and this nature affects their procoagulatory efficacy. |
| 4 | Pristine and functionalized graphene | Have good compatibility towards platelets and do not interrupt their functional aspects. |
| 5 | Graphene | The surface chemistry of graphene and physiological nature of platelet membrane influence the hemocompatibility of platelets. |
| 6 | Graphene functionalized with Dextran | Does not result in platelets activation. |
| 7 | Functionalized graphene GO nanosheets | Maintains the morphology of the treated platelets without triggering aggregation; Have higher degree of thrombo-inducing and thrombogenic abilities; possibly due to the distribution of surface charge |
| 8 | Fullerene | Show potential scavenging activity, generation of photoactive reactive oxygen species, and ability to confine with metal atoms and clusters. |
| Hydrated fullerene | Maintain thrombolytic enzymes and act favorable components for anticoagulants. | |
| 9 | Nanocomposites having fullerene C60 in polyvinyl pyrolidone solution | Do not induce any impact on the degree of aggregation of platelets. |
Table 2: Carbon nanomaterials and their impacts on platelets.
Interaction between platelets and quantum dots
Quantum dots are important entities in the field of material science exhibiting nanodimensions and semiconductor nature. These nanoparticles have specific optical and electronic properties that exhibit improved biocompatibility and hemocompatibility. These unique features of quantum dots make them one of the most suitable probes as imaging agents; normally, lower dose ranging between 300 pmol/g to 0.01 pmol/g is preferred at least for in vivo experiments. The UV light illumination excites the electrons present in quantum dots to a higher level/state of energy; this excitation process is in congruence with the transition of electron from valence band to the conductance band. The excited electron releases energy in the form of light and this process is photoluminescence. The difference of light emitted represents the difference in their respective conductance band and valance band. It also indicates the state of discrete energy and functionality of a quantum dot [19]. The quantum dots exhibit electronic wave functions and this behavior is similar to that of atoms or molecules and brings them in the category of artificial atoms. The optoelectronic properties of quantum dots vary with their size and shape.
A typical quantum dot consists of semiconductor material like cadmium selenide, indium arsenide or cadmium telluride and their quantum size correlates with the specific size i.e., their optoelectronic properties are size dependent. A quantum dot has a core-shell configuration. Core component is semiconductor material and the shell is protective and engulfs the core. The semiconductor material of core has high-energy band-gap. The higher energy band-gap of semiconductor reflects on the need of more energy to excite the electron. The affected electron is at its lower energy band (valance band) and attains the higher energy level (conduction band) i.e., need for more energy (absorption of higher-energy photons). The core-shell configuration is suitable to permit the manipulation of optoelectronic features. The composition of core and shell play prime roles in this process. The material of shell of quantum dots is also semiconductor in nature but has lower energy-band gap, like Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS). This shell in protective in nature and favours the tuning of optical properties. The quantum efficiency is the ratio between the number of photon emitted and the number of excitons formed, i.e., electron-hole pairs formed. Generally, smaller sized quantum dots show higher quantum efficiency because of their denser state which in turn provides more energy levels to accommodate more excitons. The optoelectronic properties of quantum dots vary with their size and shape. The quantum dots having larger size i.e., 5-6 nm effuse light of larger wave length like, orange or red while smaller ones with size 2-3 nm emit light of short wave length like blue and green light. The color of emitted light depends on the specific conformation of the quantum dot. Because of the specific physicochemical and optoelectronic properties quantum dots are among the most suitable for various applications, namely, display technology (TVs and monitors), light absorbing materials in solar cells, biomedical imaging, to produce energy efficient white light (it has longer life than other tradition sources), as sensors for detection of chemical toxins, temperature and pressure, as vehicles for drug targeted delivery, as catalyst in chemical field to improve efficiency and to enhance rate, as optoelectronics devices like laser, LEDs (Light Emitting Diodes), as agents to enhance the efficiency and speed of telecommunication, and as a component in data storage application to elevate the storage density, and data transfer devices like hard disc.
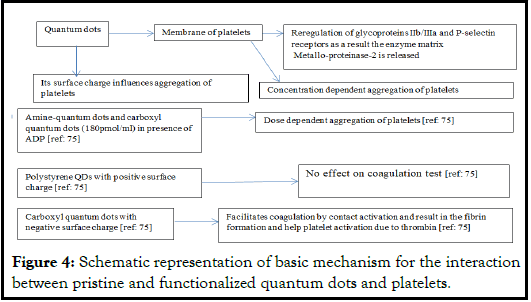
The transmission electron microscopy illustrates the interactions between quantum dots and platelets. Other techniques useful in such study are light aggregometry, quartz crystal microbalance with dissipation, flow cytometry, and gelatin zygometry. The changes in the morphology of platelets can be investigated using phase contrast microscopy, immunofluorescence, and atomic-force and transmission electron microscopy. Generally, when quantum dots get linked with the plasma membrane of platelets, there is a structural reregulation of the glycoproteins-IIb/IIIa and P-selectin receptors located at the site resulting in the release of matrix metalloproteinase-2 enzyme (Figure 4).
The quantum dots may exhibit lower degree of internalization in biological cells but fluorescent quantum dots find promising applications in biomedical field as sensors and imaging components. Some of the nanoparticles may also show lower degree of biointernalization. This problem can be handled by functionalizing them suitably. In HeLa cells, the fluorinated (functionalized) CdSe/ZnS quantum dots exhibit higher degree of surface activity i.e., elevated cellular internalization (in-vitro). The experimental and computational observations based on Langmuir monolayers of DPPC phospholipid membrane (1,2-dipalmitoyl-sn-glycero-3- phosphocholine, membrane used as model cell membrane) in vitro experiments reflected that there is an improved surface molecular internalization. The surface pressure-molecular area isotherm indicates that fluorinated quantum dots influence the physical state of the DPPC phospholipid membrane used. The significantly elevated concentration of quantum dots on the surface and any disruption to the membrane can be confirmed with UV-Vis reflections spectroscopy and Brewster Angle Microscopy. These observations and techniques illustrate the concerned mechanism involved during surface activity and molecular cellular internalizations.
Amine-QDs, and carboxyl-QDs (both having 180 pmol/ml) cause dose dependent ADP aggregation in murine platelets. Carboxyl-QDs result in higher degree of aggregation at 540 pmol/ml and minimal at 144 pmol/ml but no aggregation when ADP is absent. The shape of platelets changes at concentration 1620 pmol/ml of carboxyl QDs. The QDs induce varied degree of aggregation of platelets depending in the concentration, individual specificity, and the species used as experimental model. But higher concentrations result in elevated aggregation while lower concentrations exhibit lower degree [20]. The degree of P-selectin and the heparin can also affect aggregatory behavior of platelets. This aspect reflects the impact of surface charge on QDs during such aggregatory activity. The negative charge on the surface of carboxyl-QDs facilitates coagulation involving contact activation resulting in the fibrin generation and helping platelet activation due to thrombin. Thrombin is a potential agonist for activation of platelets using receptors like PART-1 PROTASE present on the platelets. The polystyrene QDs having positive surface chare does not affect the coagulation test (Figure 4). There is a need of more detailed investigation related to the impact of surface charge on the QDs and activation, aggregation and coagulation of platelets.
Discussion
Interaction between platelets and dendrimers
The dendrites as nanomaterials have tree like branching appearance with spherical morphology. These show repeated dichotomous systematic branches and the branching may vary from lower to intense. These nanomaterials are synthesized as per the need or set target and have predetermined functions. The dendrimers are spherical polymeric, three dimensional nanomolecules that can be conveniently formulated as carriers of drugs or any molecules that can be trapped in its structural branches. This is so because dendrimers have enormous surface area in relation to their respective volume between its branching and that space can be easily used for loading the molecules to be carried in a biological system for better biodistribution. The surface to volume ratio may extend up to 1000 m2/g. Dendrimers are specific perfect symmetrical polymeric molecules and have monodispersive nature along with higher surface available for the cargo. Dendrimers are polymeric molecules either having low molecular weight or high molecular weight. The low molecular species are referred as dendrones and dendrimers while higher molecular species are called polymer brush as they have hyper branched polymers. In most of the cases the properties of dendrimers are dependent on the functional group present in these polymeric molecules. Dendrimers have improved physicochemical properties which depend on the specific controlled mode of synthesis. Because of the unique architecture dendrimers can be functionalized at their core component as well as at their periphery zone. The incorporation of specific group within the interior of dendrimer is related to the parameters like design of monomer, mode of synthesis, properties of the type of back bone of dendrimer. Further, during such activities, an effort is made to adhere to the covalent modifications. Internalization of small guest molecules within the inner zone is done successfully and it is based on olefin metathesis (metathesis: a chemical reaction involving different kinds of molecules exchange parts forming other kinds of molecules) for example, ruthenium based Grubbs catalyst; in this case, the allylated pyrene and imidiazolidione get cross metathesis. Structurally, dendrimers have a core component to which branching are linked and is called GO or zero generation. The zone of branching constitutes the shell and is differentiated into peripheral and intermediate zones. Dendrimers are one of the most suitable carriers for drug and biomedical cargo because their internal units can be manipulated for the attachment or loading drugs. Their external surface can be readily liganded to a specific target and still retain their specific stability, thus avoiding the mononuclear phagocyte system of the receiver.
Platelets exhibit sensitivity to chilling temperatures and show normal structural and functionality between 22°C to 24°C at least for 3 to 5 days. When platelets are stored the positively charged dendrimers induce activation and aggregation in the platelets involving their amine residues. The dendrimers with high generations and molecular weight in higher concentration are suitable for such experimentation. The charge on the dendrimers also plays significant role during the preservation of platelets. The negatively charged dendrimers with lower generations, smaller size than 100 nm, and having suitable molecular weight are favorable in the studies of storage of platelets. Further, PEGylated dendrimers also facilitate the conditions related to storage of platelets and do not affect their structural and functional aspects.
The impact of amine-terminated PAMAM (polyamidoamine) dendrimer exposure to platelets results in conditions similar to disseminated intravascular coagulation which are ultimately lethal. The cationic G7 PAMAM dendrimers activate platelets and change the morphological and functional aspects of the platelets involved. The affected platelets show elevated degree of aggregation and adherence of platelets to the interacting surfaces. Even there is a declined in the degree of platelet-dependent formation of thrombin; this reflects on the disturbed functionality of the exposed platelets to amine-terminated PAMAM (polyamidoamine) dendrimers. This observation indicates that there can be adverse effects of amine-terminated PAMAM dendrimers on the mechanism involved during this interaction and the toxic nature of the dendrimers.
Triazine dendrimers having same size, numbers of cationic surface groups with molecular weights ranging between 8k Da to 130 k Da and in surface groups 16 to 156 exhibit different interactions with platelets. G3, G5, G7 and polyamidoamine dendrimers with G3, molecular weight 7 k Da, 32 surface groups and G6, 58 kDa and 256 surface groups interacted and exhibit varied degree of aggregation in platelets in human platelets rich plasma. Triazine dendrimers having size 0.01 to 1 μm do not cause any significant level of aggregation of platelets but the species with larger generation result in higher degree of aggregation. Thus, the interaction of these dendrimers relates to their size and charge. The activity of triazine dendrimer depends on the surface density of amines. Possibly, there is an addition of proton or hydron to other molecule (protonation) among the inner amine groups that cause change in the pH bringing it closer to neutral pH. Systemic administration of cationic polyamidoamine effectively causes a thrombogenic disorder, a consumptive coagulation; thus, rendering them defective carrier for drugs. This is a challenging aspect for further investigations (Table 3). Anionic dendrimers as polymers and functionalized with antibacterial drugs are of interest. Such dendrimers polymers (PPI-G3- DS-Mal) glycodendrimer and a G4 phosphorus anionic dendrimer, with 96 carboxyl surface groups) and conjugated with levofloxacin exhibit an effective antibacterial activity against Gram-negative bacteria like Escherichia coli and Proteus hauseri and gram-positive species Staphylococcus aurous. Two maltose units linked with PPI-G3-DS results in formation of a conjugated product PPI-G3-DS-Mal glycodendrimer. The PPI dendrimers G4 and G5 with terminal amine group result in significant hemolysis and their toxicity is relatively lesser than that of galactose PPI dendrimers. Triazine dendrimers having higher generations, exhibit milder intensity of interference of platelets activity and formation of blood clot in comparison to PAMAM (polyamidoamine) dendrimers.
In spite of the known physicochemical features of dendrimers it is rational to understand the level of toxicity caused in a biological system. This helps to have an idea about overall efficacy of the test compound. The chemical and molecular structures of dendrimer play a crucial role in testing its toxicity. This aspect also guides the process of synthesis and functionalization of dendrimer under consideration. The surface, nature and terminal functional groups play a significant role during the toxicity testing and also during interaction between platelets or any other cells of bio-systems. There exists correlation between the generation of dendrimer and of toxicity caused.
Dendrimer in biomedical applications exhibit cytotoxicity, hence, it is of routine activity to know the degree of cytotoxicity of dendrimers under consideration. Basically, degree of their cytotoxicity depends on parameters like numbers of generations, surface nature, concentration, and type of terminal groups. The cationic and amine dendrimers show higher degree of cytotoxicity. The grafted polyethylene carbosilane dendrimers, dendrimers with anionic terminal groups cause relatively milder degree of cytotoxicity (Table 3). The cationic dendrimers interact with cell membrane having negative charge resulting in the disruption of the structural and functional integrity of platelets.
| S. no | Dendrimers and the specific conditions | Nature and the impact on platelets |
|---|---|---|
| 1 | General dendrimers. | Monodispersive nature with greater degree of internalization may cause toxic impact. |
| 2 | Positively charged dendrimers with higher molecular weight and in higher concentration. | Induce activation and aggregation in the platelets involving their amine residues, platelets are well preserved. |
| Negatively charged dendrimers with lower generation and less than 100 nm. | Suitable for storage and investigating stored platelets. | |
| 3 | PEGylated dendrimers. | Facilitate the conditions related to storage of platelets and do not affect their structural and functional aspects. |
| 4 | Amine-terminated PAMAM (polyamidoamine) dendrimer Cationic G7 PAMAM dendrimers. |
Induce conditions similar to disseminated intravascular coagulation which are ultimately lethal. The cationic G7 PAMAM dendrimers activate platelets and change the morphological and functional aspects of the platelets, toxic to platelets. |
| 5 | Triazine dendrimers having higher generations | Exhibit milder intensity of interference of platelets activity and formation of blood clot in comparison to PAMAM (polyamidoamine) dendrimers. |
| 6 | The grafted polyethylene carbosilane dendrimers, dendrimers with anionic terminal groups. | Mild cytotoxic. |
| The grafted polyethylene cationic dendrimers. | Interact with cell membrane having negative charge and disrupt the structural and functional integrity of platelets. |
Table 3: Overall interactions between dendrimers and platelets.
Nanotoxicity, risk assesment and safety of bio-systems
Nanotoxicity is relatively a complex phenomenon and there is a possibility that simple methodology may lead to erroneous predictions regarding a particular nanoparticle or nano-formulation. These may reflect on the misleading preclinical, clinical observations regarding pharmacokinetics, cellular and organ toxicity, and physiological toxicity resulting in unexpected delivery of the concerned product. In vivo and in vitro investigations both form an integral part of studying toxicological aspects as these play important roles in developing and exploration of novel methods and evaluation of safety limits of a nano-formulation. This aspect gets a setback due to the restrictions put on the use of animal models as a result relevant toxicological out-come pathway becomes tedious. There exists greater degree of risk during applications of nanomaterials because still there is a scope for further research in the field of nanomaterials and nanotoxicity. There should be a fairly good balance between the development and applications of nanomaterials in the biomedical and environmental sciences. This aspect emphasizes on the distinctly clear and well defined development in the field of nanotoxicology and strategically ‘defensive database’ related to nanomaterials and their probable risk involved. This should also involve ecotoxicological profile of natural and man-made nanomaterials. In this pursuit local, the state governmental institutions and national and international agencies should participate more actively in providing unbiased and specific guidelines in the field of nanoscience to ensure maximum safety of mankind, ecosystem and biosphere. Since, there seems to be relatively deficient and unconfirmed data available, the researchers from governmental, academia from fields like material sciences, material engineering, chemistry, biophysics, biological sciences, and environmental sciences, and industrial agencies be actively involved to set-up a precise protocol and ‘safety data sheet’ for nanomaterials. There should be a free and open exchange of data to ascertain a common and most feasible guideline in this field. The international council of nanotechnology, international organization of standardization and other various national organizations have greater roles to play. The nanotoxicological investigations relate to the size, physicochemical features, and nature of the nanoparticles and such studies involve the probable toxicological threats to biosystem and environment/ecosystem.
Nanoparticles and nanoformulation have multiple applications in almost every field of life, therefore these nanoscaled particles have become part and parcel of life. These are fabricated, and functionalized as per the needs. Non-judicial and random applications are likely to result in their toxic impacts. This feature of nanoparticles can be implied as tools to investigate derogative effects of nanomaterials on biosystems. Nanoparticles can readily enter body fluids, cell, tissues, and systems of a biosystem. A good numbers of nanomaterials are useful as diagnostic and imaging agents, such as magnetic nanoparticles. During such investigations one must take precautions about the toxicity of the nanomaterials to the specific tissue and the system under consideration. The physicochemical features of carbon nanoparticles can result in adverse biological out-come due to unfolding of protein, fibrillation, thiol-cross linking and loss of enzyme energy during their interactions within biosystems. Such abnormal biological out-comes are likely to cause toxic and physiological dysfunctions.
Regarding platelets and carbon nanomaterials, there exist a correlation between the degree of aggregation of platelets and the ADP in relation to nanoparticles, specifically in case of metal nanoparticles. There is a threshold value of ADP at which the aggregation of platelets occurs. Nanoparticles influence this threshold value of ADP in a step-wise manner. Primarily, on contact nanoparticle trigger the formation of granules in platelets as a result of auto-catalysis occurs that leads to aggregation. In human there are variations in the degree of aggregation of platelets. This also reflects on the fluctuations of the threshold values of ADP involvement during aggregation of platelets in response to their interaction with nanoparticles. This parameter can be used as is reliable fingerprint of an individual to act as a ‘nano safety index’. This index is of great pharmaceutical value while considering nanoparticle carrier for loading.
Nanomaterials are one of the most suitable materials preferred over larger counter materials for the applications in biomedical, biomolecular, bioimaging, as delivery agents, and other fields. The TiO2 nanoparticles having 115 nm size induced proliferating, genotoxic and gentle oxidative stress conditions in mucosal epithelial cells during their absorption resulting in the impaired proliferation and interrupt renewal of the oral mucosal epithelium. This nanomaterial is mostly used as food additive, in tooth paste and many pharmaceutical preparations. Its nano-toxic effects are to be considered during the applications.
Future perspectives
Nanoscience and nanomaterials have centuries old history dating back 600 BC. In India, blacksmiths adopted this science and technology. Slowly it spread to the current Middle-East and European world and its wondrous products came in the form of ‘wootz steal’, ‘Lycurgus cup’, ceramic glazes, coloured glass windows of cathedrals during 6th to 15th centuries, etc. There have been tremendous changes in the modes of applications of the nanoscience and nanotechnology from time to time. During last few decades, nanoscience and nanomaterials have undergone metamorphosis and have come-out as most favorable materials to be applied in almost all spheres of life including ecosystems, and the biosphere. In this pursuit, researchers have devoted to explore and possible functionalize nanomaterials to gain their maximum utility as a result large numbers of both pristine and functionalized nanomaterials are available. Many such nanomaterials relate to biomedical field and have been presented to mankind that facilitate the biomedical investigation, distribution of the drugs in a bio-system, formulation of pharmaceutical products for mankind and different aspects of biosphere.
On-going discussion reveals that nanomaterials like metal and metal oxide, carbon nanoparticle, quantum dots, dendrimers etc., in relation to platelets are in forefront with respect to biomedical field, human and the test models. Since, platelets are one of the major blood components that interact with any material administered in vertebrate body need comparatively more consideration. Pristine and engineered metal and metal oxide nanoparticles with different shapes, physicochemical combinations may guide to evaluate toxic level and maintaining normal structural and physiological functionalities of platelets in healthy and pathological conditions. Carbon nanomaterials like multiwalled nanotubes, fullerene, and derivatives of graphene should be explored further to ensure safe, uniform and target oriented delivery of cargo of biomedical molecules as per the investigation and pathogenesis. The carbon dots can act as possible remedial agents in treating arterial thrombotic conditions. The role of carbon dots shows functional relationship with lactate dehydrogenase activity and this parameter can depict the degree of influence of carbon dots in a given test model. Quantum dots do not readily internalize the cell; such nanomaterials can be amalgamated with fluorescent agents and can be internalized in cell and used as effective sensors and imaging agents. Fluorinated quantum dots are suitable for investigating distribution of biomolecules within a test cell. Functionalized graphene is a favorable tool to investigate intraplatelet functionality in nonaggregated platelets. This aspect may be helpful to understand various physiological phenomena occurring within platelets (preserved or nonaggregated) and their ambient environment. This will be more suitable to investigate the role of nanomaterials on thrombo-inducing and thrombogenic abilities. Fullerene seem to be an effective agent to describe genetic impact of single gene on multiple traits and can be a useful to study phenotypic traits (pleiotropic); thus, it can be useful to investigate varied genetic conditions along with mechanism related to epigenetic studies. Fullerene as anticoagulants maintains the inactive state of platelets and it also inhibits coagulation. This feature can be the bases of investigating physiology of platelets in an inactive state under non-coagulated condition. Fullerene can be used to put a check on the production of reactive oxygen and nitrogen species utilizing their scavenging potential and also their efficacy to confine metal atoms and clusters. Functionalized fullerene can be explored as pharmaceutical material.
Further, investigations on activated platelets related to platelets growth factors and cytokines to enhance healing process within humans along with other vertebrates may elaborate more on the intricacies of healing process. Exploring effects of dendrimers can help to test the toxic levels of test compound in a biosystem. This aspect may help to evaluate the degree of efficacy of interaction between the test compound and activated platelets. This will be useful to frame a guide lines and toxic profile of a test compound. Most of the nanoparticles show correlation with ADP, collagen, restocetin, rate and degree of coagulation, and intraplatelet Ca++ distribution and concentration; these parameters may be explored to estimate the toxic levels of nanomaterials. Such observations are likely to guide formulation, engineering and suitable functionalization of the nanomaterials for various applications in different fields. These are only suggestions and need thorough consideration, experimentation with respect to their feasibility and universal validation.
Conclusion
Nanomaterials are suitably applied in many fields as a result these nanoscaled materials have become an integral part of life; more so because, these are readily fabricated, functionalized to suit a specific application. Non-judicial and random applications of these are likely to result in their toxic impacts not only on humans but also on ecosystem and biosphere. In relation to nanomaterials and biosystems, in spite of many advances in the field of surface chemistry, reductionist functionalization surface of the manmade nanomaterials is not as suitable as natural membrane and the membrane of platelets is relatively more easily available and suitable for its use as an envelope for the materials to be delivered within biosystem. Any materials, may it be nanosized or non-nanosized, should be biocompatible, with fairly good degree of biodistribution, biocompatibility, nontoxic or with least harmful impacts on the cell, tissue, systems or a test model organism. These materials should be readily detectable during in vivo and in vitro studies. The ongoing discussion on the interactions between various nanomaterials and platelets reflects on beneficial and as well as expected harmful impacts. The combined studies on nanomaterials and platelets appear to be useful in the field of biomedical sciences and clinical applications.
Funding
No funding was used for this presentation.
Conflict of Interest Statement
The author declares no conflict of interest.
References
- Gremmel T, Frelinger III AL, Michelson AD. Platelet physiology. InSeminars in thrombosis and hemostasis 2016;42(03):191-204. Semin Thromb Hemost. Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
[Crossref] [Google Scholar] [PubMed]
- Mehrizi TZ, Ardestani MS, Kafiabad SA. A review study of the influences of dendrimer nanoparticles on stored platelet in order to treat patients (2001-2020). Curr Nanosci. 2022(3):304-18.
- Lahir YK. Review: Structural and functional aspects of platelets. World J Pharm Pharmaceut Sci. 2023;12(4):886-902.
- van der Meijden PE, Heemskerk JW. Platelet biology and functions: New concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166-79.
[Crossref] [Google Scholar] [PubMed]
- Lahir YH, Avti P. Nanomaterials and their interactive behavior with biomolecules, cells and tissues. Bentham Sci Pub. 2020.
- Speranza G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials. 2021;11(4):967.
[Crossref] [Google Scholar] [PubMed]
- Kumar LY. Role and adverse effects of nanomaterials in food technology. J Toxicol Health. 2015;2(2).
- Lahir YK, Samant M, Dongre PM. Role of Nanomaterials in the development of biosensors. Global J Biosci Biotechnol. 2016;5(2):146-63.
- Lahir Y. Graphene and graphene-based nanomaterials are suitable vehicles for drug delivery. IApplications Targeted Nano Drugs Deliv Syst. Elsevier. 2019:157-189.
- Hante NK, Medina C, Santos-Martinez MJ. Effect on platelet function of metal-based nanoparticles developed for medical applications. Front Cardiovasc Med. 2019;6:139.
[Crossref] [Google Scholar] [PubMed]
- Mohapatra S, Ranjan S, Dasgupta N, et al. Applications of targeted nano drugs and delivery systems. Nanoscie Nanotech Drug Deliv. Elsevier. 2018.
- Mariam J, Dongre PM, Kothari DC. Study of interaction of silver nanoparticles with bovine serum albumin using fluorescence spectroscopy. J Fluoresc. 2011;21:2193-9.
[Crossref] [Google Scholar] [PubMed]
- Hu CM, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118-21.
[Crossref] [Google Scholar] [PubMed]
- Bandaru R, Sanket AS, Rekha S, et al. Biological interaction of dendrimers. InDendrimer-Based Nanotherapeutics 2021;63-74.
- Deb S, Dasgupta AK. Thrombotic inception at nano-scale. Acute Coronary Syndromes. 2012.
- De La Cruz GG, Rodriguez-Fragoso P, Reyes-Esparza J, et al. Interaction of nanoparticles with blood components and associated pathophysiological effects. 2017.
- Deb S, Patra HK, Lahiri P, et al. Multistability in platelets and their response to gold nanoparticles. Nanomedicine. 2011;7(4):376-84.
[Crossref] [Google Scholar] [PubMed]
- Corbalan JJ, Medina C, Jacoby A, et al. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: Inflammatory and cytotoxic effects. Int J Nanomedicine. 2011;2821-35.
[Crossref] [Google Scholar] [PubMed].
- Dunpall R, Nejo AA, Pullabhotla VS, et al. An in vitro assessment of the interaction of cadmium selenide quantum dots with DNA, iron, and blood platelets. IUBMB Life. 2012;64(12):995-1002.
[Crossref] [Google Scholar] [PubMed]
- Aseychev AV, Azizova OA, Beckman EM, et al. Effect of gold nanoparticles coated with plasma components on ADP-induced platelet aggregation. Bull Exp Biol Med. 2013;155:685-8.
[Crossref] [Google Scholar] [PubMed]