Studies on polyethylene glycol-monoclonal antibody conjugates for fabrication of nanoparticles for biomedical applications
Washington
, USA, Email: fisusi@howard.eduReceived: 30-Dec-2019 Accepted Date: Jan 27, 2020; Published: 03-Feb-2020
Citation: Fisusi F, Brandy N, Jingbo Wu and Akala EO. Studies on polyethylene glycol-monoclonal antibody conjugates for fabrication of nanoparticles for biomedical applications. J Nanosci Nanomed January 2020;4(2):1-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: The objective of this work is to synthesize and characterize PEGylated monoclonal antibody using the reactivity of oligosaccharide residues in the Fc region of trastuzumab and pertuzumab with a view to preserving their activities. METHODS: The hydrazide-functionalized PEG monomethacrylate was synthesized and reacted with NaIO4-generated aldehyde groups on glycans in the Fc-domain of trastuzumab and pertuzumab. The conjugates were purified by HPLC. SAMSA-fluorescein substitution method and MALDI MS spectroscopy were used to determine the number of PEG per antibody. Preliminary biological studies involved antiproliferative studies and binding (flow cytometry) following treatments with SKBR3 (HER2-overexpressing) cells and the control. RESULTS: 1H NMR and 13C NMR confirmed the formation of hydrazide- functionalized PEG monomethacrylate. MALDI mass-spectrometry showed that there are two PEGs per each antibody and it appears more reliable than the degree of SAMSA-fluorescein substitution method. HER-2 binding assay showed that PEGylated monoclonal antibody bound less efficiently to SKBR3 (high HER-2 expressing) cells than unmodified trastuzumab and pertuzumab. In vitro growth inhibitory effects of unmodified monoclonal antibodies increased with increase in concentration; while the in vitro growth inhibitory effects of PEGylated monoclonal antibodies also increased (but less than the pure antibody) with concentration and it appeared to be more active than unmodified mAbs at higher concentration. CONCLUSION: The results indicate that PEG can be site-specifically attached via the oxidized glycans in the Fc domain of monoclonal antibodies but the process needs further optimization in terms of PEG size and biological testing at each stage of development.
Keywords
Trastuzumab; Pertuzumab; PEGylated monoclonal antibodies; MALDI mass-spectrometry; Flow cytometry; Antipoliferation; SKBR3 cells
Introduction
The human epidermal growth factor receptor type 2 (HER-2) encoded by the HER-2 gene is overexpressed in about 20-30% of breast cancers in which the oncogene is amplified [1-3]. HER-2 overexpression had been previously associated with more aggressive disease and poor outcomes [4]. However, targeted therapy with the humanized monoclonal antibody, trastuzumab, has revolutionized treatment and outcomes of patients with HER-2 positive breast cancer, especially in combination with chemotherapy as adjuvant therapy [2,3]. Also, combination of trastuzumab with another humanized monoclonal antibody, pertuzumab, for targeting HER-2 was shown in a clinical (CLEOPATRA) study to be more beneficial than trastuzumab alone [5]. The result was the approval of a trastuzumab, pertuzumab and docetaxel combination regimen as the first-line of treatment in metastatic HER2- positive breast cancer [6]. Thus mAbs have a great potential as therapeutic agents. They can modify tumor cell signaling cascade or tumor-stroma interaction. Aside from trastuzumab and pertuzumab, other examples include rituximab (anti-CD20 mAb) and bevacizumab (anti-vascular endothelial growth factor (VEGF) mAb) [7]. In recent time, a class of mAb- based immunotherapy named immune-checkpoint inhibitors, which represent a breakthrough in the cancer treatment, was approved by FDA [8,9].
Monoclonal antibodies (mAbs) have found applications in target-specific delivery. They may be used alone, in combination, or may be conjugated to bioactive agents or other delivery systems such as micelles, polymeric nanoparticles, liposomes, etc. to allow specific delivery to target sites. For example, urokinase was conjugated to an antifibrin mAb to dissolve fibrin clots [10]. Trastuzumab-emtansine, a HER-2-targeted antibody-drug conjugate, has shown great effectiveness for treating HER-2+ breast cancer. Antibody-drug conjugates (ADCs) are developed to deliver cytotoxic drugs specifically to cancer cells [11].
Whether mAbs are used in diagnosis, drug delivery or targeting and other purposes, certain substances are often conjugated to them to form mAb- conjugates and to give them the desired characteristics. MAb-conjugates, which include antibody−drug, antibody−enzyme, antibody−hapten, antibody-nanoparticles, antibody-PEG, etc., have been used for a wide variety of applications in the biomedical sciences. The importance of PEG as a starting material in the fabrication of various conjugates cannot be overemphasized. Some of the PEG-modified materials such as proteins (e.g., mAbs), liposomes, nanoparticles, and blood-contact materials are available for commercial use. The advantages that PEG provides include reduced enzymatic degradation, extended plasma lifetime, diminished uptake by reticuloendothelial (RES) system, or from the reduction of other unwanted manifestations of biological recognition [12]. PEG-protein conjugates are also used in enzymatic catalysis in organic solvents as well as two-aqueous phase partitioning systems for purification and analysis of various biologically derived mixtures. The unique properties of PEG make it suitable for many biomedical applications [13-15]. Compared with other polymers, PEG has a relatively narrow polydispersity (Mw) in the range of 1.01 for low molecular weight PEGs (<5 kDa) to 1.1 for high molecular weight PEGs (>50 kDa). The unique ability of PEG to be soluble in both aqueous solutions and organic solvents makes it suitable for end group derivatization and conjugation to biological molecules under mild physiological conditions. Due to both high flexibility of the backbone chain and the binding of water molecules, the PEG molecule acts as if it were five to ten times as large as a soluble protein of comparable molecular weight. These factors have been suggested as the reason that PEG exhibits the ability to precipitate proteins, exclude proteins and cells from surfaces, reduce immunogenicity and antigenicity and prevent degradation by mammalian cells and enzymes. Anti-PEG antibodies are not generated under normal biomedical use of a PEG-modified protein.
Efforts are constantly being made to develop novel strategies for conjugation of PEG with proteins (especially mAbs). To avoid adverse effects on the binding activity of an antibody or its biological activity, consideration needs to be given to the chemistry of PEG attachment to antibody. Conventional methods for covalent protein modification typically involve reacting the appropriate amino acids (lysine, tyrosine, aspartic acid or glutamic acid) with reactive agents (activated PEGs) [16]. The modification methods are not site-specific and there is no control over which amino acids are modified. It is common for resulting conjugates to be modified in positions that weaken or even abrogate the binding to the antigen, which in turn decreases the efficacy of the targeting system [16-18]. Amines of lysine are commonly used for linking PEG or other bioactive agents to mAbs because lysines are usually exposed on the surface of the antibodies and therefore are easily accessible. Antibodies contain up to 80 lysines [19,20] and conjugation through lysine residues inevitably leads to two-fold heterogeneity: (a) different number of PEGs per antibody; and (b) antibodies with the same number of PEGs attached at different sites (such conjugations can result in a diverse population of mAb-PEG conjugates with a wide distribution of PEGS per antibody) [21,22].
Site-specific attachment of a suitably functionalized polymer is one way to produce PEG-mAb conjugates without introducing a steric barrier into an essential binding site on the protein molecule [23]. The ability of hydrazides to form stable hydrazones with aldehyde-containing compounds is particularly useful for site-specific modification of glycoproteins. The reactivity of oligosaccharide residues in glycoproteins is utilized for attachment of PEG chains without affecting the biding portion of the mAbs [23-25]. Rodwell et al. [26] modified a murine anti-phosphocholine IgM-mAb with DTPA via oxidized carbohydrates as well as via random conjugation to lysine residues. The immunoconjugates were radiolabeled with 111 In and directly compared. While the carbohydrate-coupled conjugate showed no loss of binding affinity in vitro and significant tissue accumulation in vivo, the randomly conjugated mAb lost binding affinity and preferentially localized in the liver.
Polymeric nanoparticles can be prepared mainly by two methods: (i) dispersion of preformed polymers and (ii) polymerization of monomers (i.e., in situ polymerization) [27-31]. The first method has been used successfully in the fabrication of polymeric nanoparticles. The main challenge with this method is that it is very difficult to tether targeting ligands, like mAbs, to the corona/surface of the nanoparticles for biorecognition events. Any attempt to modify the surface of nanoparticles fabricated by dispersion of preformed polymers often results in a substantial loss of encapsulated bioactive agents, contrast agents for imaging or other materials.
In situ co-polymerization of monomers/macromonomers, including crosslinkers, is another method for the fabrication of polymeric nanoparticles. The method allows one-pot synthesis of nanoparticles. It offers many advantages such as functionalization of the polymeric nanoparticles ’ surface, incorporation of pH-sensitive monomers and crosslinking agents for controlled/sustained drug release. Theranostics nanoparticles (multifunctional nanoscale devices which allow for a combination of diagnostic agent with a therapeutic agent and even a reporter of therapeutic efficacy in the same nanodevice package) can be easily made by in situ polymerization method [32]. Our laboratory has developed an in situ polymerization technique (dispersion polymerization) for the fabrication of nanoparticles; it can be carried out at an ambient temperature. The technique involves the use of biodegradable macromonomer (poly lactide or poly-ε-caprolactone) or monomer that forms the core of the nanoparticles, a pH-responsive crosslinker that crosslinks the core, a redox initiator system for polymerization at ambient temperature, and a hydrophilic macromonomer (PEG) which confers the stealth property to the nanoparticles and which also serves as a stabilizer [27-31,33-37]. The core-shell/corona stealth polymeric nanoparticles developed in our laboratory by dispersion polymerization at ambient temperature are suitable for varied and diverse biomedical applications: targeted delivery of bioactive active agents such as, drugs, nucleic acids and contrast agents for imaging. These nanoparticles can be made multifunctional by tethering monoclonal antibody (targeting moiety) to the PEG component of the materials of fabrication. These multi-functional polymeric nanoparticles will incorporate the bioactive agents needed for different therapeutic purposes within the core but will also be “decorated” on the surface with a targeting moiety to specifically target cellular receptors thereby serving as molecular targeted delivery system. The multifunctional polymeric nanoparticles will be capable of specific delivery of large amounts of bioactive agents per targeting biorecognition event compared to simple immunotargeted bioactive agents such as antibody-drug conjugates [38]. The thrust of the work reported in this paper is to make the PEG macromonomer component of the materials of fabrication of the polymeric nanoparticles heterobifunctional derivative of PEG: polymerizable group on one end and mAb tethered to the other end. We want to test the hypothesis that site specific conjugation of PEG-macromonomer to mAbs using the reactivity of oligosaccharide residues in the Fc region of trastuzumab and pertuzumab will be successful and will preserve the activities of the ligands (mAbs) (will not affect the biding portion of the mAbs) after conjugation and attachment to the surface of the polymeric nanoparticles. Our approach to antibody PEGylation is to site-specifically attach the PEG to targeted molecules in the antibody. Human IgG is a glycoprotein that displays glycans within the Fc region [39]. This glycan moiety can be selectively oxidized by sodium periodate to yield aldehyde groups, followed by reaction with PEG-hydrazide to form a hydrazone. Using this sciff-base chemistry, a site-specific chemical modification, with no significant effect on the binding affinity between transtuzumab and pertuzumab and HER2 receptor [40] is expected.. We will incorporate hydrazide-functionalized monomethacryate separately into trastuzumab and pertuzumab (mAbs), which can be used as a macromonomer to prepare polymeric nanoparticles.
Materials and Methods
Materials
1,1-Carbodiimidazole (CDI), sodium periodate (NaIO4), sodium cyanoborohydride (NaBH3CN), sodium acetate, sinapinic acid and anhydrous hydrazine, solvents for reactions and HPLC analysis were purchased from Sigma-Aldrich. Sodium hydroxide, Trifloroacetic acid (TFA), Millipore Amicon Spin filter (0.5 mL, MWCO=30 kDA, SAMSA Fluorescein were purchased from Fisher Scientific. PEG Monomethacrylate (Mav 2000) was purchased from Polysciences. Pertuzumab and Trastuzumab were purchased from Genentech (South San Francisco). SKBR3 cell line (American Type Culture Collection (ATCC) (Manassas VA, USA).
Instrumentation
The 1H and 13C- NMR spectra were obtained on a Bruker Avance 400 and 500 MHz in deuterated chloroform (CDCl3). Chemical shifts are in δ units (ppm) with TMS (0.0 ppm). PEG conjugates were purified using HPLC on the Hewlett- Packard liquid chromatography system Series 1100. Ultraviolet-Visible detection was performed using HP photodiode array detector. For purification and analysis, Waters X-Bridge BEHC4 column (4.6 mm × 100 mm, 10K-500K) (analytical column) was used with a flow rate at 1.0 ml/min, at 80℃ and was monitored at 280 nm as well as Agilent Zorbax 300SB-C18 4.6 × 250 mm (Semi-Prep column) at 3.0 ml / min at 45 ℃ and was monitored at 280 nm. MALDI-ToF measurements were performed on Bruker Autoflex MALDI-ToF. BD FACSVerse flow cytometer was used for flow cytometry work.
Methods
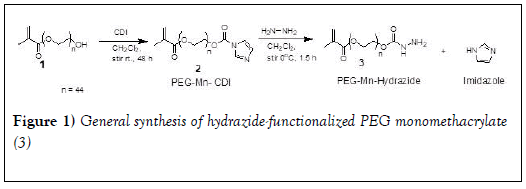
Preparation of PEG monomethacrylate-CDI: We followed the method used previously in our laboratory [41]. CDI (0.35 mmol) was added to a three-neck round bottom flask and purged with nitrogen for 10 minutes. PEG Monomethacrylate 2000 (1 in Figure 1) (0.25 mmol) was dissolved in dichloromethane (3 mL) and was added to the flask using a syringe. The reaction was stirred for 48 h at room temperature. Upon completion, an additional (30 mL) of dichloromethane was added to the round bottom flask before transferring to a separatory funnel. The reaction mixture was washed with saturated sodium carbonate (5 ml) three times and dried over anhydrous sodium sulfate. Dichloromethane was evaporated to produce a white solid residue.
PEG-2000-CDI (2 in Figure 1): 1H NMR (400 MHz, CDCl3) δ 8.0 (s, 1H, C-H), 7.30 (s, 1H, C-H), 6.91 (s, 1H, C-H), 5.97 (m, 1H, vinyl C-H), 5.42 (m, 1H, vinyl C-H,), 4.42- 4.39 (m, 2H, CH2-O), 4.14-4.12 (m, 2H, CH2-O), 3.3-3.7 (s, 222 H, O-CH2 CH2-O), 1.79 (s, 3H, CH3).
13C NMR (400 MHz, CDCl3) δ 167.09, 148.54, 137.02, 136.02, 130.51, 125.56, 117.08, 70.83, 70.64, 70.50, 68.46, 67.05, 63.75, 18.20.
Preparation of hydrazide-functionalized PEG monomethacrylate: PEG CDI Monomethacrylate 2000 (2 in Figure 1) (0.25 mmol) and anhydrous hydrazine (1 mmol) were dissolved in dichloromethane (2 mL). Then the reaction was left to stir on an ice-bath for 1.5 hours, followed by an additional one hour at room temperature. 30 mL of dichloromethane was added to the reaction flask. The organic layer was washed with saturated sodium chloride (1 mL) three times and dried using anhydrous sodium sulfate. Dichloromethane was removed in-vacuo and the residue was chilled to produce white residue [42].
1H NMR (400 MHz, CDCl3) δ 6.03 (s, 1H, vinyl C-H), 5.48 (s, 1H, vinyl C-H,), 4.14-4.21 (m, 4H, CH2-O), 3.3-3.7 (s, 228 H, O-CH2 CH2-O), 1.85 (s, 3H, CH3).
13C NMR (400 MHz, CDCl3) δ 167.11, 158.51, 136.03, 135.41, 125.55, 72.51, 71.62, 70.83, 70.64, 69.2, 68.98, 64.19, 63.74, 18.18
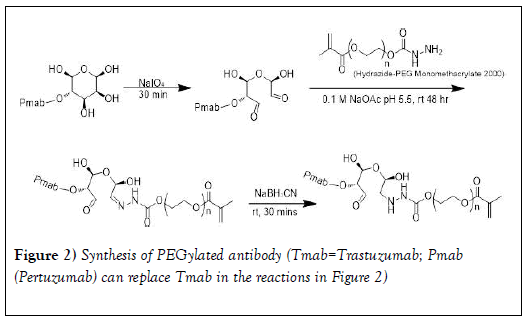
Site-specific conjugation of hydrazide-PEG monomethacrylate 2000 to antibody (PmAb or TmAb): As shown in Figure 2, the site-specific conjugation of PEG to trastuzumab or pertuzumab was performed according to previous studies via the reaction of the terminal hydrazide groups of PEG with NaIO4 -generated aldehyde groups on glycans in the Fc- domain of the mAb [42-45]. Pertuzumab (Pmab) (3 mL) was transferred to Amicon 30kDa MWCO spin filter and centrifuged at 14000 rpm (4℃ for 10 minutes). The antibody was purified by washing 6 times to remove preservatives with sodium acetate buffer (0.1 M, pH=5.5) [45]. The antibody concentration was measured at 280 nm using UV-VIS spectrometer. Purified Pmab was then oxidized by incubating in the dark at room temperature using NaIO4 for 30 minutes with slight shaking at molar ratio of 200:1 (NaIO4: Pmab) [43]. Oxidized Pmab containing reactive aldehydes was purified with sodium acetate buffer (0.1 M, pH 5.5) using the Amicon 30kDa MWCO spin filter to remove excess NaIO4. Oxidized Pmab concentration was then measured at 280 nm using the UV-Vis spectrometer [43].
The procedure used for the oxidation of trastuzumab (Tmab) was similar to that of Pmab except that Tmab (102mg) was dissolved in sodium acetate buffer (4 ml, 0.1 M, pH=5.5). The mixture was transferred to the Amicon 30kDa MWCO spin filter and centrifuged at 14000 rpm (4 ℃ for 10 minutes). The antibody was purified by washing 6 times to remove preservatives with sodium acetate buffer (0.1 M at pH 5.5). Purified Tmab was then oxidized by incubating in the dark at room temperature using NaIO4 for 30 minutes with slight shaking at molar ratio of 200:1 (NaIO4: Tmab). The oxidized antibody containing reactive aldehydes was then purified with sodium acetate buffer (0.1 M, pH 5.5) using the Amicon 30kDa MWCO spin filter to remove excess NaIO4.
Conjugation was done by reacting the purified oxidized antibody with a 50- fold excess of the hydrazide PEG at room temperature for 48 hours. The hydrazone linkage was then reduced to a stable hydrazine linkage (Figure 2) using 5M cyanoborohydride solution prepared in 1M NaOH (0.02 ml) and stirred for an additional 30 minutes. Unconjugated PEG was removed by ultrafiltration using the Amicon spin filter (MWCO=30 kDa) and washed with sodium acetate buffer (0.1 M at pH 5.5). The conjugate was analyzed and purified using (RP-HPLC).
HPLC Purification and analysis of PEG-antibody conjugates: Fractions were collected in microcentrifuge tubes and analyzed to determine the fractions that contained the desired product and the percentage purity. Purification (semi-preparative scale) and analysis for purity (analytic scale) were done using XBridge Protein BEH C4, 300Å columns (Waters Corporation) at 80℃, followed by ZORBAX 300StableBond C18 column (Agilent Technologies) at 45℃. Purity was up to 92% and higher.
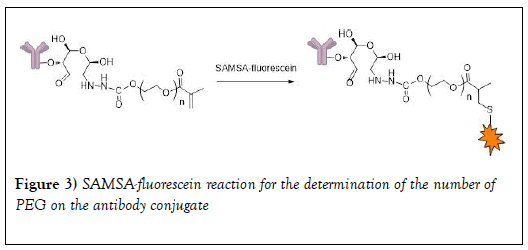
Determination of the number of PEG conjugated to the antibody using the dye method: 1.5mg of SAMSA-fluorescein was dissolved in 0.15mL of 0.1M NaOH. The solution was incubated for 30 minutes at RT in the dark and then neutralized with 1.5 μL of 6M HCl and 30 μL of phosphate buffer (pH 7.0). The activated probe was added to PEG-Tmab conjugate in sodium acetate buffer (0.1M, pH 5.5) at a 20-fold molar excess. After incubation for 5 h at RT, the PEG-Mab was purified with sodium acetate buffer (0.1 M, pH 5.5) using an Amicon spin filter (MWCO=30 kDa) to remove the excess of SAMSA-fluorescein (Figure 3). The degree of SAMSA-fluorescein substitution was determined by using the molar extinction coefficients of the trastuzumab (Ɛ280=2.25 × 105 M-1 cm-1) [45].
Determination of MALDI spectra of PEG and PEG-antibody conjugates: The matrix solution (sinapinic acid) was placed on the steel sampling grid. Then the sample solution (TmAb or PmAb) was added, followed by a further addition of the matrix solution. The sample was then left to air dry on the grid until completely dried. The sample (on the grid) was then loaded onto the instrument (Bruker Autoflex MALDI-TOF) for analysis. The analysis was carried out at a linear positive mode at laser power 60 to 80%.
A preliminary study on the biological activities of PEG-antibody conjugates: In order to determine the effect of PEG conjugation on the biological activities of the antibodies, the ability of conjugates to bind to HER2 receptors and their cytotoxicity [45-47] were assessed in SKBR3 cells. The ability of the conjugates to bind to HER2 receptors was assessed by flow cytometry while cytotoxicity was assessed by cell proliferation studies using CellTiter-Glo cell viability assay kit (CellTiter-Glo Luminescent Viability Assay is based on the measurement of ATP, which signals the presence of metabolically active cells).
Cell binding studies: The cells were harvested by trypsinization and a cell count was done to determine the concentration of the cell suspension. The cells were then centrifuged (150 g for 5 minutes at 4℃) and washed twice with phosphate buffered saline (PBS) supplemented with 2% FBS (PBS-2% FBS). The cells were then transferred into microcentrifuge tubes, each containing 2.5 × 105 cells/mL (1mL per tube). The cells were incubated with unconjugated (pure) antibody or PEG-conjugated antibody (20 μg/mL) in PBS-2% FBS for 1 h at 4°C under gentle agitation. A further tube was incubated with human IgG1 isotype control (20 μg/mL) as the non-binding antibody control. The cells were subsequently washed twice with PBS-2% FBS and incubated with FITC-conjugated anti-human IgG1- Fc antibody (20 μg/mL) diluted with PBS-2% FBS in the dark, for 30 minutes at 4°C under gentle agitation. Cells were then washed twice with PBS-2% FBS and resuspended in 0.4 mL of PBS- 2% FBS containing propidium iodide (0.1 μg/mL). The cells were then subjected to flow cytometry analysis using the BD FACSVerse flow cytometer and the data obtained were analyzed using FlowJo V10 software. Data are presented as overlay plots of histograms for cells treated with either of the pure antibodies [trastuzumab (TMAB) or pertuzumab (PMAB)], pertuzumab- PEG 2k conjugate (P2K), trastuzumab-PEG 2k conjugate (T2K) and control cells.
Cell proliferation studies: The cells were seeded in 96-well microplates (5,000 cells in 100 μL of culture medium per well) and incubated for 24 hours to allow the cells to attach. The culture medium was then removed from the wells and replaced with fresh medium containing different concentrations of pure antibody or PEG-conjugated antibody (1-30μM). The plates were then incubated with the treatments for 5 days. Cell viability was assessed using the using CellTiter-Glo cell viability assay according to the manufacturer’s protocol.
Results and Discussion
The HER-2 (ErbB2) receptor tyrosine kinase is a member of the epidermal growth factor receptor family of transmembrane receptors. These receptors, which also include the epidermal growth factor receptor (EGFR, ErbB1), HER-3 (ErbB3) and HER-4 (ErbB4), are known to play critical roles in both normal development and cancer. A subset of breast cancers is associated with the HER-2 gene, which is amplified and/or overexpressed in 20–25% of invasive breast cancers and is correlated with tumor resistance to chemotherapy and poor patient survival. HER-2 drives the cancer cells to develop a more aggressive phenotype, to metastasize to viscera and the central nervous system, and to be less sensitive to chemotherapeutic agents [48]. HER-2 has been validated as a target for directed therapies. Trastuzumab (Herceptin) is a recombinant humanized monoclonal antibody (mAb) directed against the extracellular domain of HER-2 and it binds with high affinity and avidity. Current treatment in the adjuvant setting for HER-2-positive tumors includes the use of trastuzumab with either concurrent or sequential systemic chemotherapies, with taxanes being incorporated along with trastuzumab as one of the most commonly used regimens. In addition, trastuzumab in combination with chemotherapy is often used as first line therapy for metastatic HER-2 positive breast cancers [49,50].
In 2012, FDA granted regular approval to pertuzumab for use in combination with trastuzumab and docetaxel for treatment of patients with HER-2 positive metastatic breast cancer who have not received prior anti- HER-2 therapy or chemotherapy for metastatic disease. However, in the metastatic setting, the vast majority of patients who achieved an initial response to trastuzumab based regimens developed resistance within months to years (acquired resistance); while other patients demonstrate intrinsic resistance (de novo resistance).
As a potential path to overcome trastuzumab resistance, the small molecules tyrosine kinase inhibitors, as exemplified by lapatinib, are also FDA approved or undergoing clinical trials for the treatment of HER-2- overexpressing breast cancers as a second line therapy [51,52]. However, lapatinib is relatively toxic and therapeutic resistance to lapatinib had occurred [53]. FDA granted accelerated approval to pertuzumab in 2013 as neoadjuvant treatment to be used in combination with trastuzumab and chemotherapy for patients with HER2-positive, locally advanced, inflammatory, or early stage breast as part of a complete treatment regimen for early breast cancer.
To overcome these problems of resistance, we have embarked on the development of multifunctional polymeric nanoparticles to test the hypothesis that tri-modal combination nanoparticles will prove more effective with less toxicity than current standard of care therapies for HER-2 positive breast cancers. These multi-functional polymeric nanoparticles will incorporate paclitaxel or docetaxel and low molecular weight tyrosine kinase inhibitor within the core, but will also be “decorated” on the surface with trastuzumab or pertuzumab as a targeting moiety to specifically target HER-2 receptors and also to serve as molecular targeted therapy. The core- shell nanoparticles (with biodegradable polyesters in the core and PEG on the shell/corona) developed in our laboratory using dispersion polymerization technique [27-37] are suitable for the development of multifunctional nanoparticles. Fabrication of mAb-PEG conjugates based on random site PEGylation is known to abrogate the binding property and the biological activity of monoclonal antibodies as indicated under introduction. Our approach is to carry out site specific conjugation of PEG- macromonomer to mAb using the reactivity of oligosaccharide residues in the Fc region of trastuzumab and pertuzumab. Reports have shown that this site-specific modification method has little effect on the affinity of antibodies to receptors and does not compromise their in vivo or in vitro biological functions. [45,54,55]. To incorporate monoclonal antibody (trazutuzumab or pertuzumab) separately onto the surface of our polymeric nanoparticles, we need to introduce poly(ethylene glycol) (PEG) monomethacrylate into antibody to form antibody-functionalized PEG macromonomer for the preparation of antibody-functionalized polymeric nanoparticles by dispersion polymerization. It is important to characterize the antibody-functionalized PEG macromonomer very well to be able to estimate the density of trastuzumab or pertuzumab per immuno- nanoparticle corona.
The synthesis of the hydrazide-functionalized PEG monomethacrylate reported in this work involves two steps as shown in Figure 1. First, the terminal hydroxyl group of PEG-derivative 1(Figure 1) was activated by CDI yielding its PEG-derivative 2. The reaction condition followed was as previously reported from our laboratory [41]. Slight excess of CDI was used to assure the complete consumption of PEG derivative 1. White powder was obtained, and the yield was 83%. The 1H NMR spectrum of PEG derivative 2 is similar to previous report [41]. Further characterization was carried out with 13C NMR Figure 4 and the result was as expected. Carbamate 2 (Figure 1) is stable at room temperature. It can be isolated in pure form and stored for a prolonged time in a freezer without degradation.
Figure 4: 13Carbon NMR of PEG-CDI (2 in Figure 1)
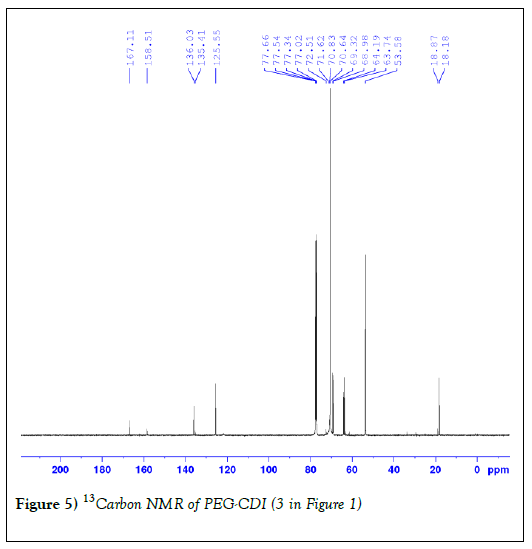
The second step involves nucleophilic substitution of imidazole with hydrazine in dichloromethane (DCM) without any catalyst at low temperature. Due to the potential reaction between hydrazine and the double bond of methacraylate group, the reaction was performed in an ice bath to minimize this side reaction. Slight excess of hydrazine was also found to be important. We chose the molar ratio of hydrazine and PEG derivative 2 (Figure 1) as 4:1. White powder was obtained and the yield was 90%. 1H NMR data are as reported previously [42]. Figure 5 shows the 13C NMR spectrum and the result is as expected.
Figure 5: 13Carbon NMR of PEG-CDI (3 in Figure 1)
Previous reports on conjugation of hydrazide to the glycan of monoclonal antibodies are as follows. A glycan (fucose bearing glycan)-specific conjugation of hydrazide derivatives to a vascular-targeting monoclonal antibody in IgG was reported by Zuberbu¨hler et. al. [56]. The method is similar to our approach in this paper (tagging PEG hydrazide to the glycan attached to the Fc domain of pertuzumab and trastuzumab so that PEG attachment would have a minimal influence on the antigen recognition and binding). Periodate oxidation was used to site-specifically modify the oligosaccharide structure of an IgG at the fucose residue. Specific and efficient conjugation of hydrazide-moieties to an IgG targeting the tumor neo vasculature was reported: the resulting chemically defined, homogeneous hydrazone-linked IgG conjugates remain immunoreactive. This method enabled specific modification of the aldehyde generated from fucose with a hydrazide, resulting in a homogeneous construct displaying two molecules per IgG at defined sites. Further, Lu et al. [44] employed a spectrophotometric assay to estimate the mean number of metal containing polymers per trastuzumab using a pyrene-labeled-metal containing polymers. The metal containing polymer content in the immunoconjugate was determined by comparing the absorbance of the solution at 345 nm (characteristic of pyrene) with that at 280 nm (trastuzumab) and the average the number of metal containing polymer per trastuzumab was 1.4 ± 0.5. HER-2 binding assays demonstrated that the metal containing polymers did not interfere with specific antigen recognition on SKBr3 cells. In another study, maleimide-modified trastuzumab was prepared by oxidizing polysaccharide residues on the Fc region with sodium periodate followed by conjugation with the maleimide-containing molecule 4-(4-N- maleimidophenyl) butyric acid hydrazide (MPBH). The number of maleimide residues per antibody molecule was determined indirectly by assaying the binding to the maleimide-modified antibody (Mal-Ab) of a thiol-containing fluorescent probe, SAMSA-fluorescein. The average number of maleimide groups on each antibody molecule was approximately 2. In our experiments, following the reaction of the terminal hydrazide groups of PEG (Figure 1) with NaIO4-generated aldehyde groups on glycans on the Fc-domain of trastuzumab and pertuzumab (Figure 2) and the use sodium cyanoborohydride solution to reduce the hydrazone linkage into a stable hydrazine linkage, the PEG-trastuzumab and PEG-pertuzumab conjugates were purified by HPLC. Purity ranged from 92-95%. In order to determine the number of PEG molecules per each of the monoclonal antibodies, the degree of SAMSA-fluorescein substitution [46] was determined using the molar extinction coefficient of the trastuzumab (the concentration of the dye (SAMSA-fluorescein) labeled monoclonal antibody was compared with the concentration of unlabeled PEGylated monoclonal antibody. A value of 1.48 ± 0.12 for trastuzumab was obtained. The value was less than two as expected for the two aldehyde groups (Figure 1). The result may be due to the dye substitution method (which is a chemical reaction) which may need optimization. We observed a slight difference in the value obtained depending on reaction time (16 hours compared to 24 hours). Lu et al. [44] also reported a value of 1.4 ± 0.5 for the average the number of metal containing polymer per trastuzumab. Thus a spectrophotometric assay method may not be as accurate as expected.
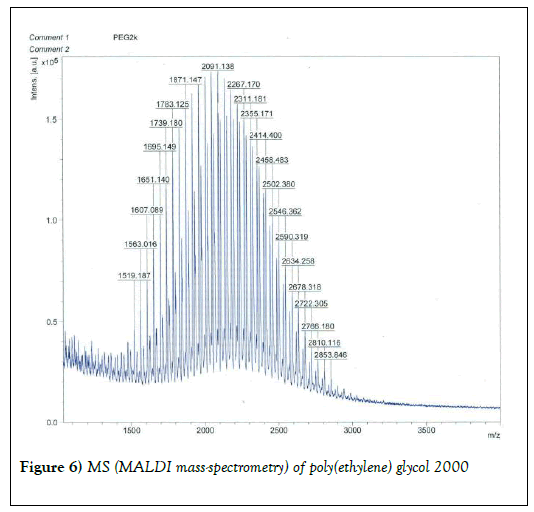
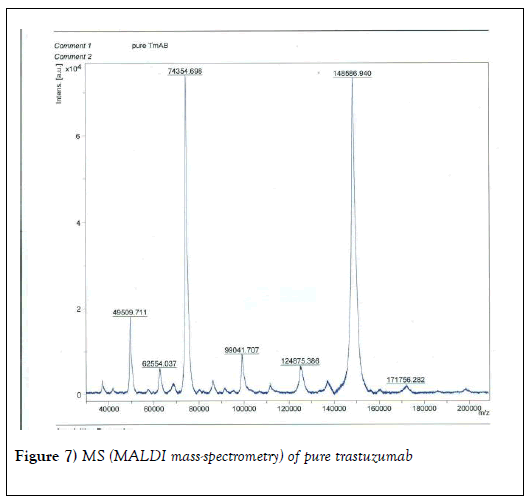
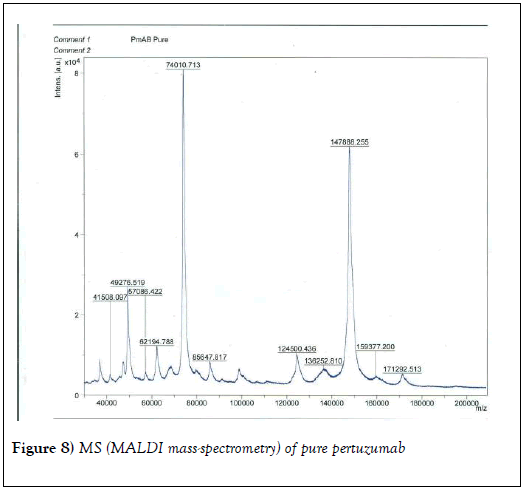
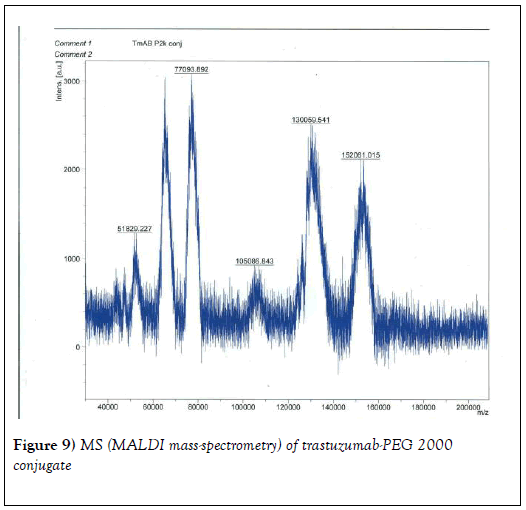
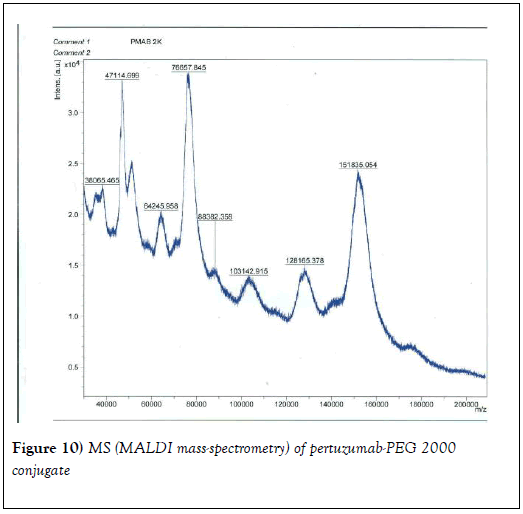
We resorted to the use of MALDI MS spectroscopy for PEG-antibody conjugates. Informed by our experience over the years on the use of PEG derivatives in the fabrication of core-shell nanoparticles by dispersion polymerization, we first carried out the MALDI spectrum of PEG monomethacrylate (Mav 2000). Figure 6 shows the spectrum. The weight average molecular weight of PEG monomethacrylate is 2091.138 g•mol-1. Then we determined the molecular weight of unmodified (pure) trastuzumab and pertuzumab using MALDI. The results are shown in Figures 7 and 8. The molecular weights of trastuzumab and pertuzumab are 148586.940 g•mol−1 and 147888.255 g•mol−1 respectively. The results are in agreement with varied and diverse literature reports (including National Cancer Institute Formulary) that trastuzumab and pertuzumab have molecular weights of about 148 kDa. The purified PEGylated trastuzumab and pertuzumab MALDI spectra are shown in Figures 9 and 10 and the molecular weights of trastuzumab-PEG 2000 and pertuzumab-PEG 2000 conjugates are 152061.015 and 151836.054 respectively. Thus the two PEGylated monoclonal antibodies have two PEGs per antibody. It appears that MALDI spectra determination is a better approach for the determination of the number of PEGs per antibody.
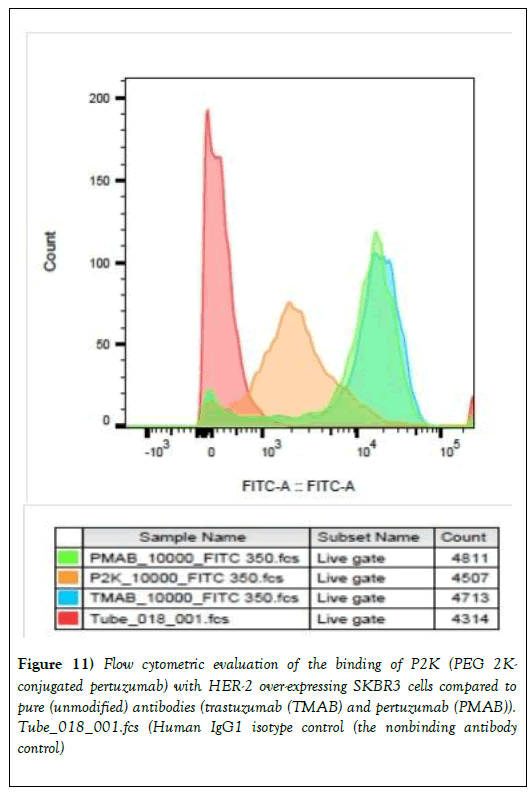
We proceeded to carry out preliminary evaluation of the biological properties of trastuzumab-PEG 2000 and pertuzumab-PEG 2000 conjugates. Figure 11 shows the comparison of the binding of the unmodified trastuzumab and pertuzumab and pertuzumab-PEG 2000 conjugate with SKBR3 (HER-2-overexpressing) cells by flow cytometry. The cells were subjected to flow cytometry analysis using the BD FACSVerse flow cytometer and the data obtained were analyzed using FlowJo V10 software. Data are presented as overlay plots of histograms for cells treated with either of the pure antibodies [trastuzumab (TMAB) or pertuzumab (PMAB)], pertuzumab-PEG 2k conjugate (P2K) and control cells. There is little difference, in terms of binding as judged by the cell-associated fluorescence intensity in the flow cytometry analysis, between trastuzumab and pertuzumab. They are known to bind to HER-2-receptors strongly.
Figure 11: Flow cytometric evaluation of the binding of P2K (PEG 2K- conjugated pertuzumab) with HER-2 over-expressing SKBR3 cells compared to pure (unmodified) antibodies (trastuzumab (TMAB) and pertuzumab (PMAB)). Tube_018_001.fcs (Human IgG1 isotype control (the nonbinding antibody control)
However, the pertuzumab-PEG 2000 conjugate treated SKBR3 cells showed a different pattern from the unmodified antibodies with pertuzumab and trastuzumab treated cells being more positive for FITC, suggesting that the modified antibody (pertuzumab-PEG 2000 conjugate) had affinity for HER-2 receptors expressed by SKBR3 but the binding was less than those of the pure antibodies (trastuzumab and pertuzumab). The human IgG1 isotype control (the nonbinding antibody control) did not bind to the cells. A similar pattern was observed for trastuzumab-PEG 2000 conjugate (data not shown). Experiments are being planned to improve upon the result by determining the bioactivity of the monoclonal antibodies immediately after oxidation and to use PEGs of shorter chain lengths.
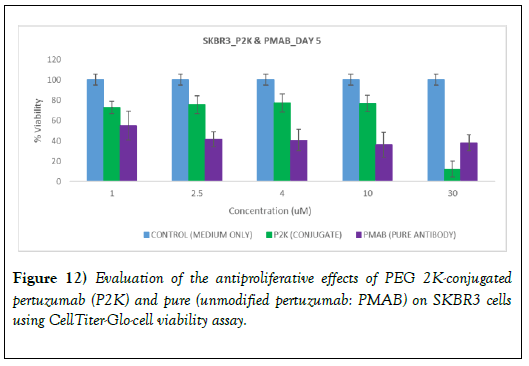
The results of the antiproliferative studies are shown in Figure 12 for pure (unmodified pertuzumab and pertuzumab-PEG 2000 conjugate following treatment with SKBR3 (HER-2-overexpressing) cells. Cell viability was assessed using the CellTiter-Glo luminescent cell viability assay. To allow direct comparison, the amount of pertuzumab in pertuzumab-PEG 2000 conjugate as the unmodified pertuzumab in solution was used. The viability of the cells was reduced as unmodified pertuzumab concentration increased. The viability of the cells was also reduced with increase in the concentration of pertuzumab-PEG 2000 conjugate but less than the pure (unmodified) monoclonal antibody). At a high concentration (30 μM) of pertuzumab in pertuzumab-PEG 2000 conjugate, the cell viability decreased sharply and it appeared to be more active than the unmodified pertuzumab. Chan et al. [57] reported that PEG (40 kDa)−trastuzumab conjugate showed a 5-fold reduction in HER2 binding affinity; however the in vitro growth inhibitory effects were preserved as a result of changes in cellular trafficking when compared to native trastuzumab. As indicated earlier, further work is being planned. Studies involving different cells (high HER-2 expression (SKBR3 cells), low HER-2 expression (MCF7 cells) and no HER-2 expression (MDAMB-468 cells) will be used. PEGs with lower molecular weights and level of biological activity after oxidation but before conjugation with PEGs will be carried out. We hope we will be able to account for the differences in binding and anti-proliferation effects of unmodified trastuzumab and pertuzumab and the corresponding PEG conjugates.
Figure 12: Evaluation of the antiproliferative effects of PEG 2K-conjugated pertuzumab (P2K) and pure (unmodified pertuzumab: PMAB) on SKBR3 cells using CellTiter-Glo-cell viability assay.
Conclusion
The objective of this study was to prepare and characterize PEGylated trastuzumab macromonomer and PEGylated pertuzumab macromonomer suitable for the development of multifunctional nanoparticles (using dispersion polymerization technique) for active targeting in the treatment of HER-2 positive cancers, especially HER2 positive breast cancer. We synthesized and characterized PEG-2000 derivative with a hydrazide end. The hydrazide-functionalized PEG monomethacrylate was conjugated/ tethered to aldehyde groups generated by NaIO4 oxidation of the pendant glycan in the Fc domain of trastuzumab and pertuzumab. MALDI mass- spectrometry showed that there are two PEGs per each antibody. We found that MALDI mass-spectrometry is more reliable than the dye substitution method (the degree of SAMSA-fluorescein substitution) in determining the number of PEG per antibody. HER-2 binding assays showed that PEGylated pertuzumab macromonomer bound less efficiently to SKBR3 (HER-2- overexpressing) cells than unmodified trastuzumab and pertuzumab. In vitro growth inhibitory effects to pure (unmodified) pertuzumab increased with increase in concentration of pertuzumab; while the in vitro growth inhibitory effects of PEGylated pertuzumab macromonomer also increased with concentration and at high concentration of PEGylated pertuzumab macromonomer, it appeared more active than the unmodified pertuzumab. The results indicate that PEG can be site-specifically attached to monoclonal antibodies via oxidized glycans in the Fc domain of monoclonal antibodies. It needs optimization using different PEG size and cancer cell lines with different expression of HER-2 receptor.
Conflicts of Interest
The authors confirm that the content of this research article has no conflict of interest.
Acknowledgements
We thank Kenneth Blakeslee (Principal Technical Support Specialist, Waters Mid-Atlantic Office Columbia, MD) for his efforts in the selection of the column and HPLC separation conditions for the monoclonal antibodies and the PEG conjugates. We thank Professor Sergei Nekhai (Howard University) for access to BD FACSVerse Flow Cytometer. Research reported in this publication was supported by NCI/NIH Grant #: 1SC1CA199810-01. This work was carried out in facilities supported by NCRR/NIH Grants #1 C06 RR 020608-01 and #1C06 RR 14469-01.
REFERENCES
- Prat A, Carey LA, Adamo B, et al. Molecular Features and Survival Outcomes of the Intrinsic Subtypes within Her2-Positive Breast Cancer. J Natl Cancer Inst. 2014;106(8):1-8.
- Nuciforo P, Thyparambil S, Aura C, et al. High Her2 Protein Levels Correlate with Increased Survival in Breast Cancer Patients Treated with Anti-Her2 Therapy. Mol Oncol. 2016;10(1):138-47.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of Chemotherapy Plus a Monoclonal Antibody against Her2 for Metastatic Breast Cancer That Overexpresses Her2. N Engl J Med. 2001;344(11):783-92.
- Ross JS, Slodkowska EA, Symmans WF, et al. The Her-2 Receptor and Breast Cancer: Ten Years of Targeted Anti-Her-2 Therapy and Personalized Medicine. Oncologist. 2009;14(4):320-68.
- Baselga J, Cortés J, Kim S-B, et al. Pertuzumab Plus Trastuzumab Plus Docetaxel for Metastatic Breast Cancer. New England Journal of Medicine. 2012;366(2):109-19.
- Swain SM, Kim SB, Cortes J, et al. Pertuzumab, Trastuzumab, and Docetaxel for Her2-Positive Metastatic Breast Cancer (Cleopatra Study): Overall Survival Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2013;14(6):461-71.
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(1):278-87.
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Brit J Cancer 2014;111(12):2214-9.
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56-61.
- Hagemeyer CE, Tomic I, Weirich U, et al. Construction and characterization of a recombinant plasminogen activator composed of an anti-fibrin single-chain antibody and low-molecular-weight urokinase. J Thromb Haemost. 2004;2(5):797–803.
- Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90.
- Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and Protein PEGylation. Adv Drug Deliv Rev. 2002;54(4):459-76.
- Polson A. A theory for the displacement of proteins and viruses with polyethylene glycol, Prep. Biochem. 1977;7(2):129–54.
- Gombotz WR, Guanghui W, Horbett TA, et al. Protein adsorption to and elution from polyether surfaces. Poly(Ethylene Glycol) Chemistry. 1992;25(12):247-261.
- Abuchowski A, van TE, Palczuk NC, et al. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol, J Biol Chem. 1977;252(11):3578–81.
- Rodwell JD, Kearn TJ, Linker technology - Antibody-mediated delivery systems. Nat Biotechnol. 1985;3:889-94.
- Garnett MC, Targeted drug conjugates: principles and progress. Adv Drug Delivery Rev. 2001;53(2):171-216.
- Firestone RA, et al. Synthesis and antitumor activity of the immunoconjugate BR96-Dox. J Control Release. 1996;39(2-3): 251-59.
- Karr LJ, Donnelly DL, Kozlowski A, et al. Use of poly(ethylene glycol)-modified antibody in cell extraction, Methods Enzymol.1994;228:377–390.
- Mueller BM, Wrasidlo WA, Reisfeld RA. Determination of the number of e-amino groups available for conjugation to monoclonal antibodies. Hybridoma 1988;7(5):453–56.
- Wakankar AA, Feeney MB, Rivera J, et al. Physicochemical stability of the antibody-drug conjugate Trastuzumab-DM1: Changes due to modification and conjugation processes. Bioconjug Chem. 2010;21(9):1588–95.
- Widdison WC, Wilhelm SD, Cavanagh EE, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49(14):4392–4408.
- Zalipsky S. Chemistry of Poly(ethylene glycol) conjugates with biologically active molecules. Adv Drug Deliv Rev. 1995;16(2-3):157-82.
- O'Shannessy DJ. A novel procedure for labeling immunoglobulins by conjugation to oligosaccharide moieties. Immunol Lett. 1984;8(5):273-77
- O'Shannessy DJ, Quarles RH. Labeling of the oligosaccharide moieties of immunoglobulins. J Immunol Methods 1987;99(2):153-61.
- Rodwell JD, Alvarez VL, Lee C, et al. Site-specific covalent modification of monoclonal antibodies- In vitro and in vivo evaluations. Proc Natl Acad Sci. 1986;83(8):2632-36.
- Oluwaseun O, Kumari N, Kahli A, et al. “Antiretroviral Drugs-Loaded Nanoparticles Fabricated by Dispersion Polymerization with Potential for HIV/AIDS Treatment” Infect Dis. 2016;9:21-32.
- Akala EO, Adesina S, Ogunwuyi O. “Computer Optimization of Biodegradable Nanoparticles Fabricated by Dispersion Polymerization”. Int J Environ Res. 2016;13(1): 47- 63.
- Ogunwuyi O, Adesina S, Akala EO. D-optimal mixture experimental design for stealth biodegradable crosslinked docetaxel-loaded poly-ε-caprolactone nanoparticles fabricated by dispersion polymerization. Pharmazie. 2015;70(3): 165–76.
- Adesina SK, Holly A, Marek GK, et al. Polylactide- based Paclitaxel-loaded Nanoparticles Fabricated by Dispersion Polymerization: Characterization, Evaluation in Cancer Cell Lines, and Preliminary Biodistribution Studies. J Phar Sci. 2014;103(8):2546 –55.
- Simeon K, Adesina SA, Akala EO, et al. Optimization of the fabrication of novel stealth PLA- based nanoparticles by dispersion polymerization using D-optimal mixture design. Drug Dev Ind Pharm. 2014;40(11):1547-56.
- Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem. 2011;22(10):1879–1903.
- Puri R, Solomon A, Berhe and Akala EO. "pH-Sensitive Polymeric Nanoparticles Fabricated By Dispersion Polymerization for the Delivery of Bioactive Agents”. Pharm Nanotechnol. 2017;5(1):44-46.
- Akala EO, Okunola O. Novel stealth degradable nanoparticles prepared by dispersion polymerization for the delivery of bioactive agents Part I. Pharm Ind. 2013;75(7):1191-96.
- Akala EO, Okunola O. Novel stealth degradable nanoparticles prepared by dispersion polymerization for the delivery of bioactive agents Part II. Pharm Ind. 2013;75(8):1346-52.
- Simeon K, Adesina, Akala EO. “Nanotechnology Approaches for the Delivery of Exogenous siRNA for HIV Therapy”. Mol Pharm. 2015;12(12):4175–87.
- Akala EO, Simeon K, Adesina. “Fabrication of polymeric core-shell nanostructures” in Nanoscale Fabrication, Optimization, Scale-up and Biological Aspects of Pharmaceutical Nanotechnology. Alexandru Mihai Grumezescu. (Elsevier, William and Andrew, Applied Science Publishers, United Kingdom). 2017:1-49.
- Puri R, Adesina S, Akala E. Cellular uptake and cytotoxicity studies of pH-responsive polymeric nanoparticles fabricated by dispersion polymerization. J Nanosci Nanomed. 2018;2(1):1-18.
- Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(-caprolactone) Nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005;293(1-2):261-70.
- Sung MC, Scheinberg DA, Avdalovic NM, et al. Genetically engineered deglycosylation of the variable domain increases the affinity of an anti-CD33 monoclonal antibody. Mol Immunol. 1993;30(15):1361-67.
- Lu Y, Mbong GNN, Liu P, et al. Synthesis of Polyglutamide-Based Metal-Chelating Polymers and Their Site-Specific Conjugation to Trastuzumab for Auger Electron Radioimmunotherapy. Biomacromolecul. 2014;15(6):2027-37.
- Bol’shakov OI, Akala EO. MS-Monitored Conjugation of Poly(Ethylene Glycol) Monomethacrylate to RGD Peptides. J Appl Polym Sci Symp. 2014;131(14):40385.
- Huang L, Song J. Chen B. A Novel Targeting Drug Carrier to Deliver Chemical Bonded and Physical Entrapped Anti-Tumor Drugs. Int J Pharm. 2014;466(1–2):52–57.
- Lu Y, Mbong GNN, Liu P, et al. Synthesis of Polyglutamide-Based Metal-Chelating Polymers and Their Site-Specific Conjugation to Trastuzumab for Auger Electron Radioimmunotherapy. Biomacromol. 2014;15(6):2027-37.
- Mbong GNN, Lu Y, Chan C, et al. Trastuzumab Labeled to High Specific Activity with (111) In by Site-Specific Conjugation to a Metal-Chelating Polymer Exhibits Amplified Auger Electron-Mediated Cytotoxicity on HER2-Positive Breast Cancer Cells. Mol Pharm. 2015;12(6):1951-60.
- Shi M, Wosnick JH, Ho K, et al. Immuno-Polymeric Nanoparticles by Diels–Alder Chemistry. Angewandte Chemie. 2007;46(32):6238-6243.
- Escoffre JM, Deckers R, Sasaki N, et al. Mild Hyperthermia Influence on Herceptin (®) Properties. Radiol oncol. 2015;49(1):41-9.
- Davoli A, Hocevar BA, Brown TL. Progression and treatment of HER2-positive breast cancer. Cancer Chemother Pharmacol. 2010;65(4):611-23.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344(11):783-92.
- Vogel CL, Cobleigh MA, Tripathy D. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719-26.
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124-30.
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-43.
- Nahta R, Shabaya S, Ozbay T, et al. Personalizing HER2-targeted therapy in metastatic breast cancer beyond HER2 status: what we have learned from clinical specimens. Curr Pharmacogenomics Person Med. 2009;7(4):263-74.
- Awwad M, Strome PG, Gilman SC, et al. Modification of monoclonal antibody carbohydrates by oxidation, conjugation, or deoxymannojirimycin does not interfere with antibody effector functions. Cancer Immunol Immunother. 1994; 38(1):23-30.
- Chamow SM, Kogan TP, Peers DH, et al. Conjugation of Soluble CD4 without Loss of Biological Activity via a Novel Carbohydrate-directed Cross-linking Reagent. J. Biol. Chem. 1992;267(22):15916-922.
- Zuberbuhler K, Casi G, Bernardes GJL, et al. Fucose-specific conjugation of hydrazide derivatives to a vascular-targeting monoclonal antibody in IgG format. Chem Comm. 2012;48(56):7100-7102.
- Chan LJ, Bulitta JB, Ascher DB, et. al. PEGylation Does Not Significantly Change the Initial Intravenous or Subcutaneous Pharmacokinetics or Lymphatic Exposure of Trastuzumab in Rats but Increases Plasma Clearance after Subcutaneous Administration. Mol Pharm. 2015;12(3):794−809.