Molecular study of the ?-casein gene in water buffalo population of North-West of Iran
Received: 23-Jan-2020, Manuscript No. PULJCGG-22-2806; Editor assigned: 28-Jan-2020, Pre QC No. PULJCGG-22-2806(PQ); Reviewed: 11-Feb-2020 QC No. PULJCGG-22-2806; Revised: 01-Dec-2022, Manuscript No. PULJCGG-22-2806(R); Published: 29-Dec-2022
Citation: Firouzamandi M. Molecular study of the β-casein gene in water buffalo buffalo population of North-West of Iran. J Clin Gen Genomics 2022;5(1):1-3.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Milk protein comprises 80% Caseins (CN) and 20% whey proteins. Caseins genes in buffalo include the major protein component and encoded by CSN1S1, CSN1S2, CSN2, and CSN3 closely linked genes. In the current study, thirty meat samples were collected from water buffaloes of North-west of Iran (Azeri breed), slaughtered in Tabriz slaughterhouse. Amplification of exon seven of the β-casein gene (CNS27) and proximal promoter region of the β-casein gene (CNS2PP) was ran using specific primers and a 510 bp and a 382 bp obtained respectively for CNS27, and CNS2PP. PCR-SSCP analysis of buffalo β-casein exon seven showed polymorphism and revealed two types of SSCP patterns. Furthermore, PCR-SSCP analysis of the proximal promoter region showed the monomorphic in all of the studied samples. Genetic variation in the casein loci has studied extensively in bovine due to their inherent effect on milk composition and cheese-making properties and milk yield. However, buffalo milk has many benefit to bovine, its β-casein gene has remained mostly ignored. This is the first report of genetic variability of the exon seven and the proximal promoter region of β-casein gene in Iranian water buffalo (Azeri breed).
Keywords
Iranian water buffalo; Azeri breed; Exon VII of β-casein gene; Proximal promoter region of the β-casein gene
Introduction
Water buffalo is a local animal of Iran, with more than 80 percent of its populace moved in the North and North-west (Azerbaijan province) and 18 percent in the south of the Iran. Water buffalo or Asian buffalo classified under the genus Bubalus, species bubalis. Bubalus bubalis are major domesticated animals' species for milk and meat generation around the world, and are second in milk creation after dairy cows with 13% of all out milk creation. There are three breeds of water buffalo in the different regions of Iran: Azeri (70%); Kuhzestani (22%) and Mazandarani (8%).
Average of milk production of the Iranian breeds is as per the following: Kuhzestani: 1950 kg milk; Azeri: 1500 kg milk; Mazandarani: 1300 kg milk.
Caseins represent around 80% of the cow's milk protein and contents 36% alpha-casein, 27% beta-casein, 9% kappa-casein, 27% peptides and amino acids. The genes encoding four types of casein genes as CSN1S1, CSN2, CSN1S2, CSN3, and found as a cluster on the chromosome six in sheep, cattle, and goats. In buffalo milk, caseins include the main protein component and secretion of four closely related genes that structured in a cluster of around 250 kb on the chromosome 7. Within β-casein, many variants genetically determined to relate to the biological properties and structure of milk [1]. There are 12 genetic variants of β-casein as A1, A2, A3, B, C, D, E, F, H1, H2, I, G in cattle and at least 8 alleles as A, A1, B, C, C1, E, and 0, and 01 are existing in goats. Active peptides-opioids are a source from some milk proteins. Term ‘opioid’ mentions to chemical ingredients that have activity as morphine-like in the body. Some the opioid has played an important role in the reaction to pain and stress, and food intake control. The β-casein as active peptides are the most hydrophobic and has a main role in response for the surface properties micelles and curd formation of casein [2]. In buffalo, compared to goats and cattle, the β-casein protein and nucleotide sequence variations of this important gene had less extensively considered. Single-Strand Conformation Polymorphism (SSCP) technique had been useful for identified of many point mutations of β- casein in caprine, ovine and bovine. This technique was effective to the determination of new alleles in all of the studied species. In the present study, PCR-SSCP technique for the first time employed for characterization of the β-casein gene and its variants in North-west buffalo breed (Azeri) of Iran. Already, there is any molecular study on the β-casein gene in Iranian North-west buffalo [3-6].
Materials and Methods
Sampling and DNA extraction
Thirty meat samples were collect from water buffalo (Azeri breed) of Northwest of Iran. The samples were carrying out to DNA extraction by modified of the phenol-chloroform method [7].
Polymerase Chain Reaction (PCR)
PCR reactions for this study were used by the thermo-cycler instrument (MWG-BIOTECH, Germany) with the temperature cycles. Amplification of exon seven of the β-casein gene (CNS27) was conducted by the forward primer of 5'-CCT TCT TTC CAG GAT GAA CTC CAG-3', and the reverse primer 1 of 5′-AAT AAT AGG GAA GGG TCC CCG G-3′ which was used previously [8]. PCR carries out for exon seven of the β-casein gene by touchdown PCR as after an initial incubation at 95°C for 2 min, followed by five cycles at 95°C for 45 sec, 60°C and each cycle 1°C decreased until reached to 56°C for 30 sec and 72°C for 30 sec [9]. Then followed by 25 cycles as 94°C for 45 sec, 55°C for 30 sec, and 72°C for 30 sec and terminated with a final extension at 72°C for 5 min. Amplification of the proximal promoter region of the β-casein gene (CNS2PP) was run using specific primers that were used previously. Sequences of the forward primer were as 5’-CGT GTG GCC TGT GTA TTT CTG GTG TGT ATT-3’ and reverse primer was as 5’-AGG ACA AGA AGT GAA GGA GGA GCT GAA TGG-3’. The PCR program was: 1 cycle at 94°C for 2 min, 35 cycles by 94°C for 45 sec, 70°C for 30 s and 72°C for 30 s followed with final extension for 5 min at 72°C. Each PCR reagents consisted of 12.5 Master Mix (Ampliqon, Denmark), ten-pmol of each inner primer, 50 ng-100 ng of extracted DNA, and reached to a final volume of 25 μl. Electrophoresis of amplified fragments was run on 1.5% agarose gels containing DNA safestain (CinnaGen, Iran) and the size of products was estimated by DNA Ladder, 50 bp-1500 bp [10].
SSCP (Single-Strand Conformation Polymorphism)
SSCP protocol were carried out on 7% acrylamide gel comprise 30% polyacrylamide according to previously described study. For the preparation of 20 ml of 7% polyacrylamide gel; 5 ml of 30% ployacrylamid solution (Acrylamide: Bisacrylamide in 29:1) with 13 ml of distilled water was mixed, then 2 ml of TBE10X solution, 200 μl of APS10% and, 50 μl of TEMED(N, N, N', N'-tetramethylendiamine) were added. Then SSCP technique was carried out as follows: 5 μL of PCR products mixed with 7 μL of SSCP loading dye (95% deionized formamide with 5% DNA loading dye 6X), then this final volume denatured for 10 min at 95°C and snap cooled. The chilled solution directly loaded on the gel. Polyacrylamide gel electrophoresis was carrying out at continuous voltage of 150 V for 2 h-3 h. The gel stained by the silver staining protocol as described previously [11].
Briefly, silver staining of the gel was done by five solution buffer as bellows; 200 ml of a stabilization solution (ethanol 10% + acetic acid 1%) was used for 15 min, after decant the buffer a stain solution (AgNO3 0.1%) for 10 min-15 min was applied, after the, immediately the gel rinsed for 20 second with water, then applied an apparition solution (NaOH 1.5%+formalin 0.1%) for 20 min-30 min then decant the solution and applied a stopped solution (NaHCO3 0.75%) for 10 min, finally the gel keep in a preservative solution (1% acetic acid) [12].
Results and Discussion
PCR products
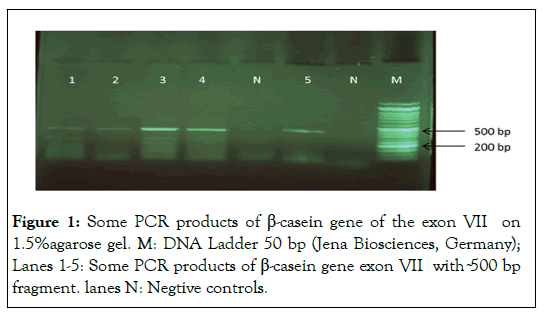
A fragment size with 510 base pairs of the buffalo β-casein exon VII including a part of exon seven and a part of intron 6 amplified (Figure 1).
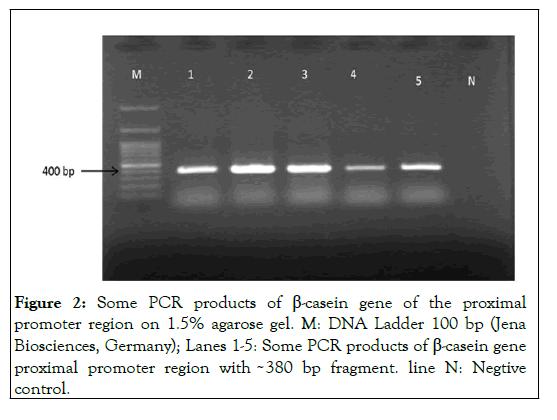
Besides, a fragment size with 382 bp base pairs of the buffalo β-casein proximal promoter region amplified by PCR (Figure 2) [13].
SSCP-PCR analysis
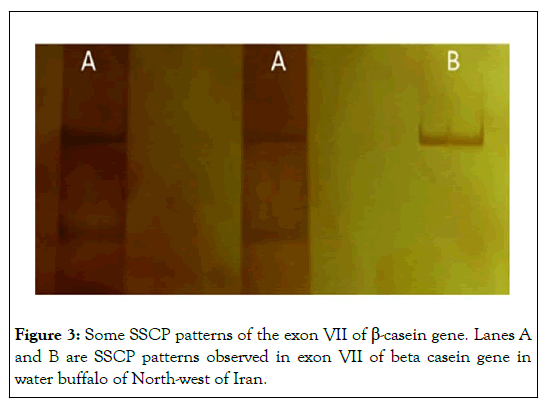
A ~510 bp amplification fragment represented whole exon seven of β- casein, which that was applied in SSCP technique to identify the point mutations of β-casein gene in this region. Two different patterns were exposed for the exon seven (named A and B) as shown in the Figure 3.
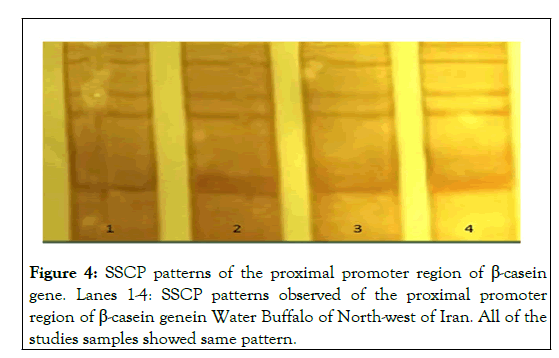
Twenty samples from thirty studied samples were showed A pattern (67%) and 10 samples were showed B pattern (33%). The pattern A is predominant than the pattern B. Whereas, PCR-SSCP analysis of the proximal promoter region of the β-casein gene in the all of the studied samples was showed mono-morph patterns as shown in Figure 4. A study by Vinesh et al on the exon seven of β-casein gene by SSCP was showed polymorphism in Indian riverine buffalo, and our results paralleled with their results [14]. However, the SSCP patterns in our study were different from them. This finding indicated that a sequence of seven exon of the β- casein gene in Iranian and Indian buffalo is diverse of each other. Besides, our finding showed that the proximal promoter region of the β-casein gene was mono-morphism. Whereas, according to study of Vinesh, this region had shown polymorphism also. Overall, results of our study revealed that genome of Iranian water buffalo (Azeri breed) in the exon seven and proximal promoter of β-casein gene in the studied samples was different between Indian riverine buffaloes and Iranian water buffaloes. The primary sequence of all food proteins as well as milk protein is subject to genetic variation [15]. Furthermore, genetic polymorphism within β-casein gene may dictate the type of bioactive peptides released from milk proteins. β- casein (variant A1, A1+B) in bovine is demanded to be associated with diabetes Type-I (Elliott), while, Bell et al. reported that individual with consuming of milk with β-casein A2 variant have lower incidence to cardiovascular diseases and Type-I diabetes. A similar amino acid sequence of these variant of β-casein gene has been seen in water buffalo milk.
Buffalo milk is rich source of essential amino acids and the predominant amino acids. Buffalo milk is healthy as it is richer in saturated fatty acids. Its greatly higher total solids are valuable for butterfat and cheese production.
In spite of its huge potential and advantage to cattle in many features, the buffalo has remained generally ignored. Currently, we have little direct information about genetic variants of buffalo milk protein in all buffalo breed, especially in Iranian breeds. The present study proposed to study of casein gene in buffalo to determine revealing polymorphism in β-casein exon seven and promoter regions.
Conclusion
A study on the incidence of polymorphic sites in the β-casein gene promoter showed eight polymorphic sites in this region. Cosenza et al. found that most of the β-casein promoter of buffalo transcription factor binding sites conserved. The present study at the proximal promoter region of buffalo suggests which region is mono-morph. Therefore, our results are in line with Cosenza et al. Information on the polymorphism status of buffalo casein gene, it is poorly explored. In the present study, for the first time, we analyzed the genetic variability of exon seven and the proximal promoter region of the β-casein gene in Iranian buffalo breed (Azeri breed). More studies are required to identification Iranian buffalo breed characterization in beta-casein gene regions.
Acknowledgement
The authors are thankful to Research affairs of Faculty of Veterinary Medicine, University of Tabriz.
References
- Borghese A, Mazzi M. Buffalo population and strategies in the world. Buffalo Prod Res. 2005;67:1-39.
- Bassam BJ, Caetano-Anollés G, Gresshoff PM. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991;196(1):80-3.
- Bell SJ, Grochoski GT, Clarke AJ. Health implications of milk containing β-casein with the A2 genetic variant. Crit Rev Food Sci Nutr. 2006;46(1):93-100.
- Silva SV, Malcata FX. Caseins as source of bioactive peptides. International Dairy J. 2005;15(1):1-5.
- Campos PF, Gilbert TM. DNA extraction from formalin-fixed material. Ancient DNA. 2012;840:81-5.
- Ceriotti G, Chessa S, Bolla P, et al. Single nucleotide polymorphisms in the ovine casein genes detected by polymerase chain reaction-single strand conformation polymorphism. J Dairy Sci. 2004;87(8):2606-13.
- Chessa S, Rignanese D, Chiatti F. Simultaneous identification of CSN1S2 A, B, C, and E alleles in goats by polymerase chain reaction-single strand conformation polymorphism. J Dairy Sci. 2008;91(3):1214-7.
- Chessa S, Rignanese D, Berbenni M. New genetic polymorphisms within ovine β-and αS2-caseins. Small Ruminant Res. 2010;88(2-3):84-8.
- Cosenza G, Feligini M, Mancusi A, et al. Italian Mediterranean river buffalo CSN2 gene structure and promoter analysis. Ital J Anim Sci. 2009;8(sup2):57-9.
- Dalgleish DG. Bovine milk protein properties and the manufacturing quality of milk. Livestock Produc Sci. 1993;35(1-2):75-93.
- Das P, Jain S, Tiwari G, et al. Rapid communication: Nucleotide sequence of the river buffalo kappa-casein cDNA. J Anim Sci. 2000;78(5):1389.
- Davies DT, Law AJ. The content and composition of protein in creamery milks in South-West Scotland. Res J Dairy Sci. 1980:47(1):83-90.
- Elliott RB, Harris DP, Hill JP, et al. Type I (insulin-dependent) diabetes mellitus and cow milk: Casein variant consumption. Diabetologia. 1999;42(3):292-6.
- Ferretti L, Leone P, Sgaramella V. Long range restriction analysis of the bovine casein genes. Nucleic Acids Res. 1990;18(23):6829-33.
- Jann O, Ceriotti G, Caroli A, et al. A new variant in exon VII of bovine β‐casein gene (CSN2) and its distribution among European cattle breeds. J Anim Breed Gene. 2002;119(1):65-8.