Study of the Ultrastructure of the placenta in gestational Diabetes Mellitus
2 Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Umm Alqura University, Saudi Arabia, Email: abdelghanyha@yahoo.com
Received: 14-Dec-2017 Accepted Date: Dec 26, 2017; Published: 04-Jan-2018, DOI: 10.37532/1308-4038.18.11.004
Citation: Abdelghany AH, Eissa TM, Idris S. Study of the ultrastructure of the placenta in gestational diabetes mellitus. Int J Anat Var. 2018;11(1):004-010.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The placenta is the organ situated between the mother and fetus. It plays critical roles during pregnancy and is essential for fetal growth and development. The placental functions are determined by the ultrastructure of the placental barrier which is an exchange surface area between the fetus and mother. Gestational diabetes mellitus (GDM) comprises unfit conditions for embryonic and feto-placental development and may result in placental abnormalities. This study was made to detect the ultrastructural changes of the placenta in women with gestational diabetes. The placentas of 10 control and 10 women with GDM were studied. Whole thickness placental samples were taken and prepared for light and transmission electron microscopy study. Light microscopic study of the control placenta showed numerous densely packed microvilli, thin walled blood vessels and narrow intervillous spaces. The placentas of GDM showed reduced number of microvilli, wide intervillous spaces, thick walled vessels, edematous spaces and areas of fibrosis and perivillous fibrinoid degeneration. Electron microscopic study of the placentas of control women showed terminal villi with a thick layer of syncytiotrophoblast (Sy) with a lot of cylindrical microvilli and a thin layer of cytotrophoblast (Cy). There were some endoplasmic reticulum cisternae beside few mitochondria. The underling villus core was harboring fetal capillaries lined with flat endothelial cells and thin basement membrane. In GDM placenta, there was hypertrophy of Cy with atrophy of the Sy with multiple vacuoles and areas for glycogen storage. The microvilli were scarce and the villous core showed congested capillaries, edematous spaces, glycogen storage areas and fibrosis. All the changes in placentas of GDM are suggested to be attributed to the associated hypoxia and oxidative stress due to the associated decreased uteroplacental flow that was aggravated by the thick placental barrier.
Keywords
Gestational diabetes mellitus; Fetal; Syncytiotrophoblast; Cytotrophoblast; Placenta; Microvilli
Abbreviations
GDM: Gestational diabetes mellitus; Sy: Syncytiotrophoblast; Cy: Cytotrophoblast
Introduction
The placenta is the organ situated between the mother and fetus. It is essential for fetal growth and development [1]. Placenta performs essential functions during pregnancy, including the exchange of nutrients, water, respiratory gases, and waste products together with the synthesis of various hormones which regulate the transport of maternal nutrients to the fetus and help maternal adaptation to different pregnancy stages. These functions are governed by the ultrastructure of the placental exchange barrier [2,3].
The fetal and maternal blood is separated by the placental barrier that allows the exchange between the fetus and mother with no mixing between both bloods. With the progress of pregnancy, it decreases in thickness to compensate for the increased fetal demands. The placental barrier is composed of different structures including a maternal-facing layer of syncytiotrophoblast (Sy) with multiple microvilli, a thin layer of cytotrophoblast (Cy) which gets thinner as pregnancy proceeds but persists until term, the endothelial lining and underlying basal membrane (BM) of the fetal capillaries, and the connective tissue of the villous core between them [3-5]. A proper coordination of trophoblast proliferation, differentiation and invasion is required for placental development [5,6].
The physiological changes that occur during pregnancy necessitate proper nutrient supply to ensure placental and fetal development. During gestation, the placenta continuously undergoes changes in weight, shape and structure to make sure that the supply of nutrients is appropriate for fetal survival. Some forms of intrauterine fetal growth abnormalities have been attributed to abnormalities of placental blood flow and transfer of nutrients from the mother to the fetus [1].
Gestational diabetes mellitus (GDM) is defined as the glucose intolerance with onset or first recognition during pregnancy. It is associated with morbidity in both offspring and mother. The fetal adverse outcomes include macrosomia, neonatal hypoglycemia, neonatal jaundice and diabetes in adolescence or early adulthood while maternal complications include preeclampsia, preterm delivery, cesarean delivery, obesity and abnormal glucose tolerance [7-9].
The diabetic environment can be regarded as a complex network of substances (hormones, nutrients, and cytokines) with varying concentrations [10]. In diabetes, the placenta undergoes a variety of structural and functional changes and the extent of these changes depend on a range of variables including the modality of treatment and the extent of glycemic control achieved during the critical periods in placental development [10,11].
Despite the availability of treatment, GDM provides unfit conditions for feto-placental development and placental structure. However, reports on the placenta of GDM are usually controversial regarding the pathological features. Some researchers found no unusual features while others claimed that there were pathological changes. This inconsistency was partly attributed to the superadded preeclampsia and other associated complications that may change the metabolic environment and partly to the fact that the category of GDM women is not always homogeneous [1].
Moreover, few studies have investigated the ultrastructural changes in human term placentas [2]. Thus, this study was made to detect the ultrastructural changes of the placenta in women with GDM.
Materials and Methods
The placentas of 20 women; 10 control and 10 with GDM, were studied with a free consent approved by the committee of ethics, Faculty of Medicine, Alexandria University. Women with a history of pregestational diabetes or hypertension or other chronic diseases were excluded. The age ranged from 28 to 30 years for the control and 27 to 31 years for the GDM. Fasting blood glucose level was performed after an overnight fast of at least 10 hours at 24–28 weeks of gestation. The diagnosis of GDM was made based on the American Diabetes Association (2011) [7]. The control women were within the accepted normal ranges of blood glucose level from 90 to 115 mg/dL throughout gestation while the GDM women initially showed levels ranging from 120 to 160 mg/dL. GDM women were asked to control diet beside insulin therapy in order that their fasting blood glucose levels were kept in the satisfying range from 100 to 135 mg/dL until delivery. Two GDM women had higher fasting blood glucose ranging from 140 to 165 mg/dL because of their poor diet control and interrupted therapy. The maternal weight at gestation ranged from 62 to 65.5 Kg for the control and from 65 to 68.5 kg for GDM. All GDM women were delivered by caesarian section and the gestational weeks at delivery ranged from 36 to 38 weeks for the diabetics. 6 control women were delivered vaginally while 4 were delivered by caesarian section at a gestational age ranging from 36 to 39 weeks. All deliveries were done in Gynecology and Obstetrics department, Faculty of Medicine, Alexandria University. The fetal birth weight ranged from 2.80 to 3.15 Kg in the control group while in the GDM group, it ranged from 3.210 to 3.40 Kg. The embryonic membranes and the umbilical cords were trimmed from the placentas, In the control group, the placenta weight ranged from 450 to 480 gm while in the diabetic group, it ranged from 470 to 500 gm. Placental samples including the whole thickness of the placenta were taken from the center of the placenta beside the site of attachment of the umbilical cord. Paraffin-embedded blocks from for formaldehydefixed tissues were prepared and sections (thickness 0.5 μm) were cut from each block and stained with hematoxylin and eosin (HE) to be examined by light microscopy (Optica-B-150). Other blocks were prepared for electron microscopy study. They were fixed in the fixative (3% glutaraldehyde and 2% paraformaldehyde in 0.1 mol/lcacodylate buffer (pH 7.3) for 24 h at 4°C. After fixation in 1.0% OsO4 in 0.1 mol/l cacodylate buffer (pH 7.3) for 2 h at room temperature, the tissue specimens were subjected to dehydration in graded ethanol After immersion in propylene oxide (three times for 10 min each), the samples were immersed overnight in a mixture (1:1) of propylene oxide and Epon-812 resin to be finally embedded in Epon-812 resin (Spa, USA). Semithin sections (0.5 μm-thick) were cut using ultramicrotome (Leica Ultracut; Leica, Berlin, Germany) that were picked on copper grids and counter stained with 2% uranyl acetate and lead citrate [12]. The specimens were examined by transmission electron microscopy (JEOL- JEM- 100CX-JAPAN) and analyzed in the electron microscope unit, Faculty of Science, Alexandria University.
Statistical analysis: The results were analyzed statistically using SPSS software version 17 as mean± standard deviation and the 2 groups are compared using Paired-Samples T test and the level of significance was accepted as p<0.05.
Results
1. Parameters of the studied cases: The gestational age was increased in the control than in GDM women with no significant difference. On the other hand, the neonatal weight was significantly increased in GDM than in control women. Regarding the maternal age, GDM women were older than the control with no significant difference. Comparing the maternal weight, it was significantly increased in GDM than in control women. Finally, the placental weight was significantly increased in GDM than in control women (Table 1).
| Variables | Control | GDM | t-test | P value | |
|---|---|---|---|---|---|
| Gestational age | 36.90 ± 0.91 | 36.40 ± 0.60 | 1.88 | 0.076 | |
| Neonatal weight | 2.93 ± 0.127 | 3.15 ± 1.91 | 5.955 | 0.001* | |
| Maternal age | 28.85 ± 0.88 | 29.20 ± 1.36 | 0.941 | 0.358 | |
| Maternal weight | 63.97 ± 1.14 | 65.64 ± 0.53 | 5.807 | 0.001* | |
| Placental weight | 460.25 ± 8.96 | 485.25 ± 11.47 | 6.216 | 0.001* | |
Table 1: Parameters of the studies control and GDM cases
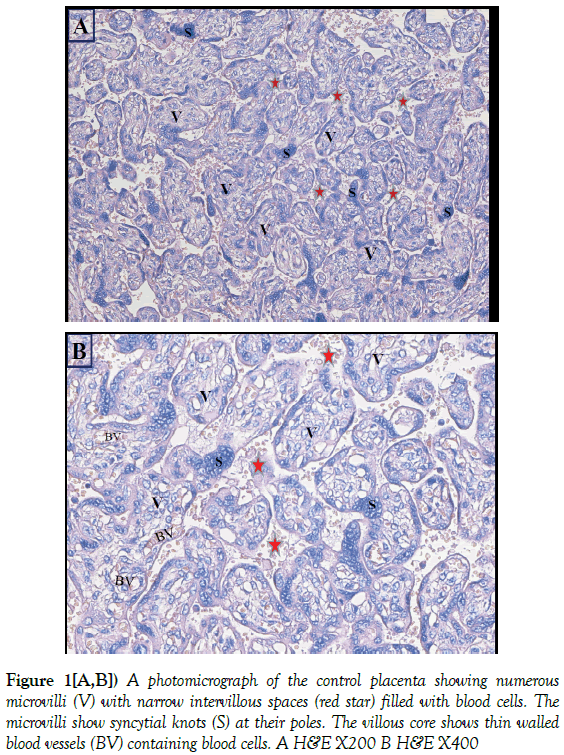
2. Light microscopic study: Light microscopic study of the control placenta showed a large number of densely packed microvilli. The intervillous spaces were narrow and explicit and filled with blood cells. The microvilli showed syncytial knots which are aggregations of the nuclei of the Sy occupying variable areas at the poles of the microvilli. The villous core showed small and thin walled blood vessels showing a number of blood cells (Figure 1).
Figure 1[A,B]) A photomicrograph of the control placenta showing numerous microvilli (V) with narrow intervillous spaces (red star) filled with blood cells. The microvilli show syncytial knots (S) at their poles. The villous core shows thin walled blood vessels (BV) containing blood cells. A H&E X200 B H&E X400
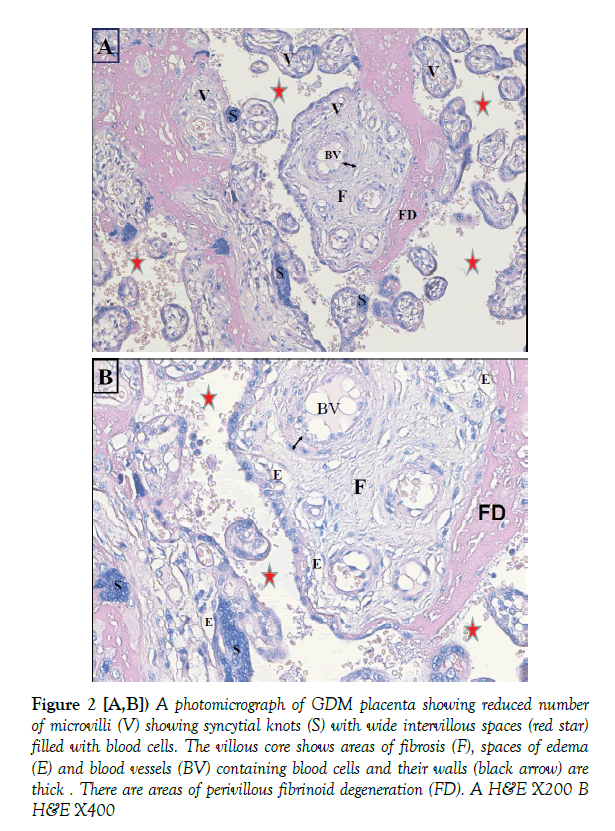
Light microscopic study of the placenta of GDM showed reduced number of microvilli compared with the control with syncytial knots. The intervillous spaces were wide. A number of the microvilli showed areas of fibrosis and scattered spaces of edema in the villous core. The blood vessels in some of the micovilli were dilated and showed thickening of their walls. There were areas of perivillous fibrinoid degeneration shown in bright pink appearance (Figure 2).
Figure 2 [A,B]) A photomicrograph of GDM placenta showing reduced number of microvilli (V) showing syncytial knots (S) with wide intervillous spaces (red star) filled with blood cells. The villous core shows areas of fibrosis (F), spaces of edema (E) and blood vessels (BV) containing blood cells and their walls (black arrow) are thick . There are areas of perivillous fibrinoid degeneration (FD). A H&E X200 B H&E X400
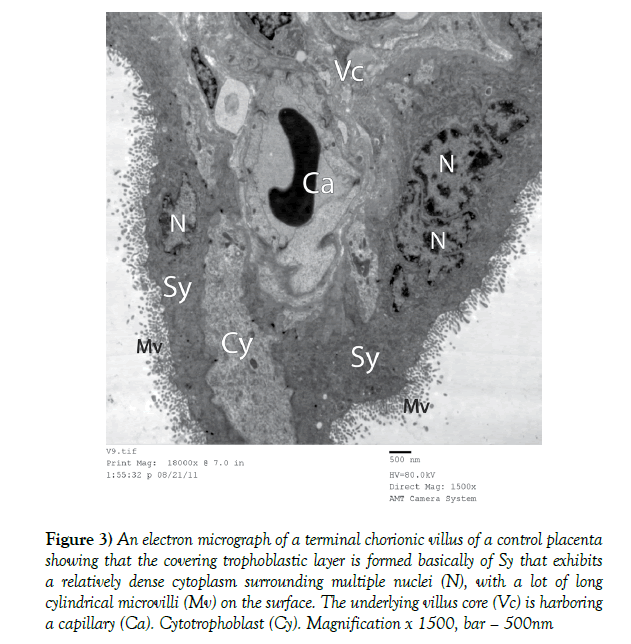
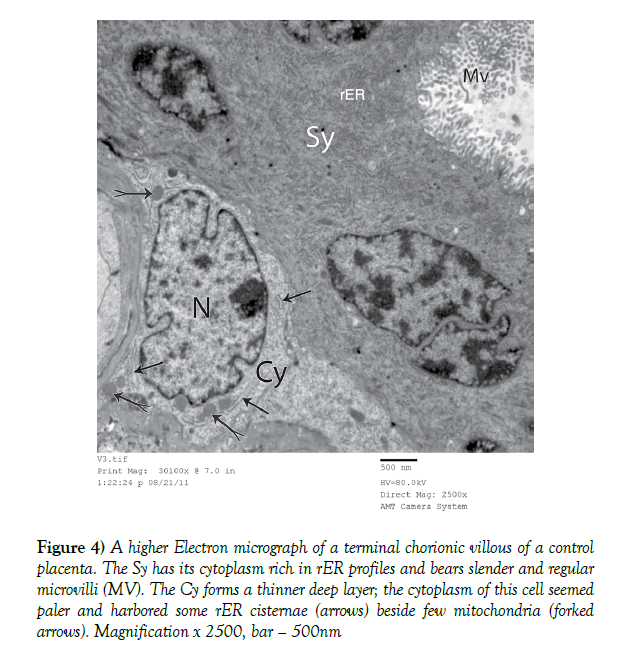
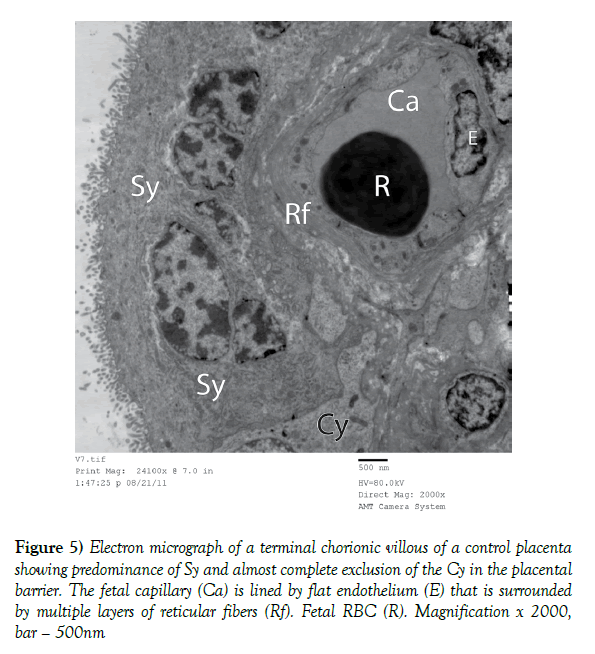
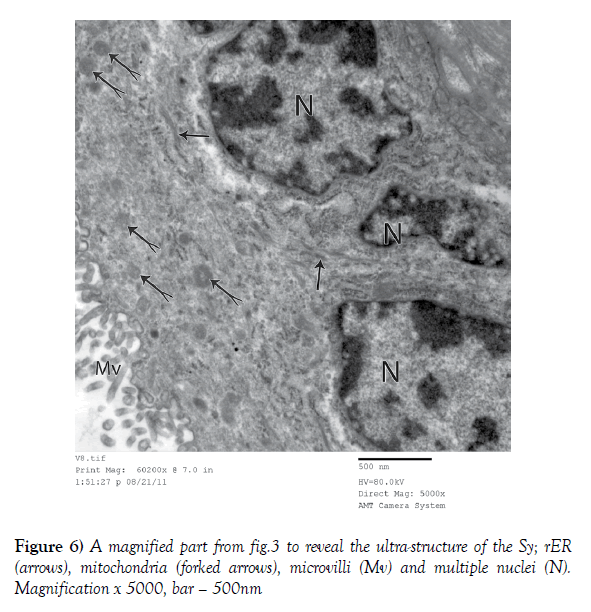
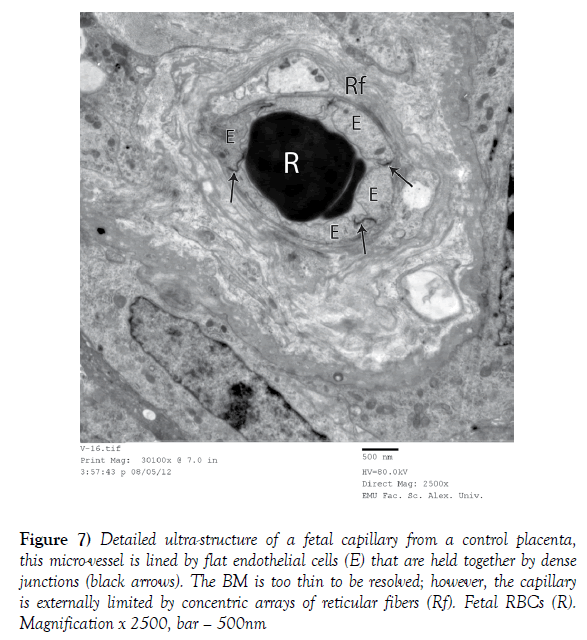
3. Electron microscopic study: Electron microscopic study of the full term control placenta showed that the terminal chorionic villi had a covering of a thick layer of Sy which bears a lot of cylindrical and regular microvilli (Figures 3 and 4). The cytoplasm of Sy was relatively dense surrounding multiple nuclei with rough endoplasmic reticulum (rER) and mitochondria (Figures 3-6). Deeper to SY there was a thin layer of Cy where the cytoplasm of its cells was paler and harbored some rER cisternae beside few mitochondria (Figure 4). In some areas of the villi, there was only Sy with absence of the Cy (Figures 3 and 5). The underling villous core was harboring fetal capillaries that were lined with flat endothelial cells that were held together by dense junctions. (Figures 5 and 7) The BM was too thin to be resolved. The capillaries were externally limited by concentric arrays of reticular fibers (Figures 5 and 7).
Figure 3) An electron micrograph of a terminal chorionic villus of a control placenta showing that the covering trophoblastic layer is formed basically of Sy that exhibits a relatively dense cytoplasm surrounding multiple nuclei (N), with a lot of long cylindrical microvilli (Mv) on the surface. The underlying villus core (Vc) is harboring a capillary (Ca). Cytotrophoblast (Cy). Magnification x 1500, bar – 500nm
Figure 4) A higher Electron micrograph of a terminal chorionic villous of a control placenta. The Sy has its cytoplasm rich in rER profiles and bears slender and regular microvilli (MV). The Cy forms a thinner deep layer; the cytoplasm of this cell seemed paler and harbored some rER cisternae (arrows) beside few mitochondria (forked arrows). Magnification x 2500, bar – 500nm
Figure 5) Electron micrograph of a terminal chorionic villous of a control placenta showing predominance of Sy and almost complete exclusion of the Cy in the placental barrier. The fetal capillary (Ca) is lined by flat endothelium (E) that is surrounded by multiple layers of reticular fibers (Rf). Fetal RBC (R). Magnification x 2000, bar – 500nm
Figure 7) Detailed ultra-structure of a fetal capillary from a control placenta, this micro-vessel is lined by flat endothelial cells (E) that are held together by dense junctions (black arrows). The BM is too thin to be resolved; however, the capillary is externally limited by concentric arrays of reticular fibers (Rf). Fetal RBCs (R). Magnification x 2500, bar – 500nm
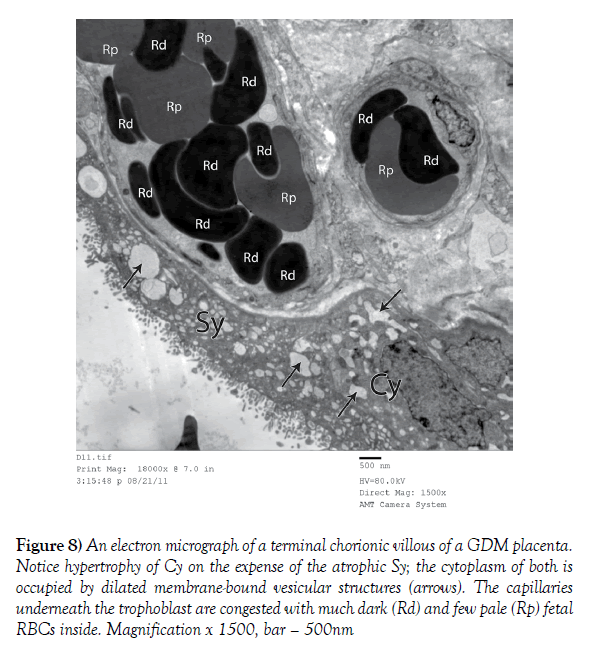
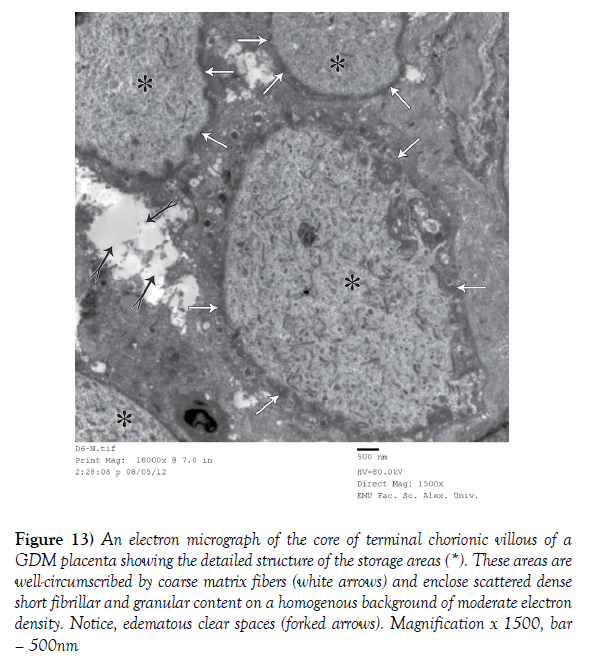
In the GDM placenta, there was hypertrophy of Cy with atrophy of the Sy (Figure 8). The cytoplasm of both types of trophoblast was occupied by a lot of membrane-bounded irregular vesicular structures that contained a low electron-dense material (Figures 8-10). Areas rich in glycogen granules and few mitochondria appeared in the Cy cells (Figure 9). In the Sy, there were inclusion bodies inside the nucleus that assumed a concentric lamellar appearance with a halo around (Figure 10). The microvilli on the surface were scarce and some had terminal club ends (Figure 10). The subtrophoblastic BM was thick and separated from the fetal capillary by a subtrophoblastic space (Figure 9). The fetal capillaries underneath the trophoblast were congested with much dark and few pale RBCs (Figure 8). The endothelial cells lining the capillaries showed aggregates of glycogen granules near the nucleus together with the presence of dilated cisternae of endoplasmic reticulum (Figure 11). There were edematous clear spaces and well defined storage areas in the matrix of the villous core (Figures 12 and 13). These storage areas were well-circumscribed by coarse matrix fibers and enclosed scattered dense short fibrillar and granular content on a homogenous background of moderate electron density (Figure 13). There was fibrosis inside a chorionic villous core where fibroblasts and collagenous bundles were exceptionally prevalent (Figure 14). There were stromal macrophages with their lysosomes containing a material whose density was comparable to that of hemoglobin of the extra-vasated fetal RBCs (Figure 12).
Figure 8) An electron micrograph of a terminal chorionic villous of a GDM placenta. Notice hypertrophy of Cy on the expense of the atrophic Sy; the cytoplasm of both is occupied by dilated membrane-bound vesicular structures (arrows). The capillaries underneath the trophoblast are congested with much dark (Rd) and few pale (Rp) fetal RBCs inside. Magnification x 1500, bar – 500nm
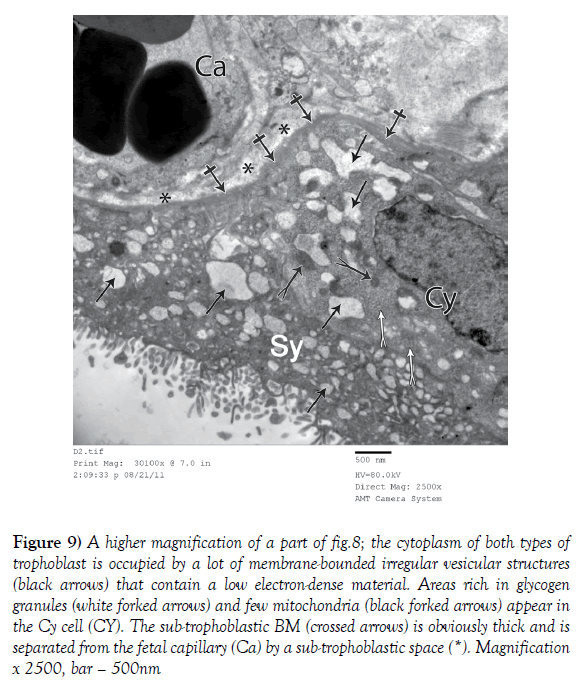
Figure 9) A higher magnification of a part of fig.8; the cytoplasm of both types of trophoblast is occupied by a lot of membrane-bounded irregular vesicular structures (black arrows) that contain a low electron-dense material. Areas rich in glycogen granules (white forked arrows) and few mitochondria (black forked arrows) appear in the Cy cell (CY). The sub-trophoblastic BM (crossed arrows) is obviously thick and is separated from the fetal capillary (Ca) by a sub-trophoblastic space (*). Magnification x 2500, bar – 500nm
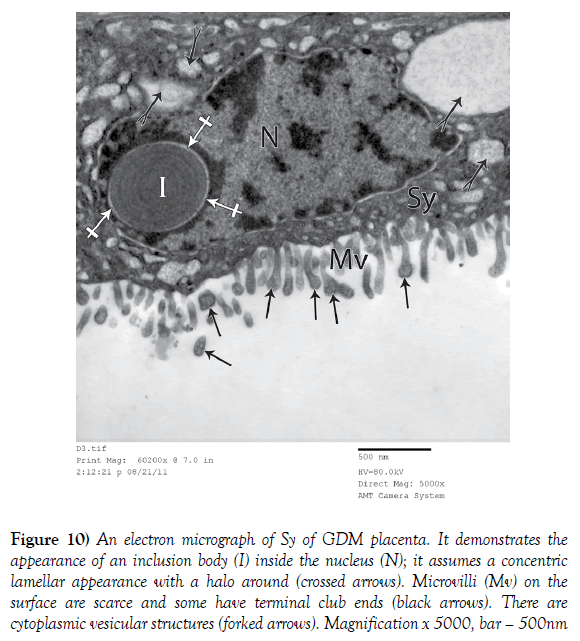
Figure 10) An electron micrograph of Sy of GDM placenta. It demonstrates the appearance of an inclusion body (I) inside the nucleus (N); it assumes a concentric lamellar appearance with a halo around (crossed arrows). Microvilli (Mv) on the surface are scarce and some have terminal club ends (black arrows). There are cytoplasmic vesicular structures (forked arrows). Magnification x 5000, bar – 500nm
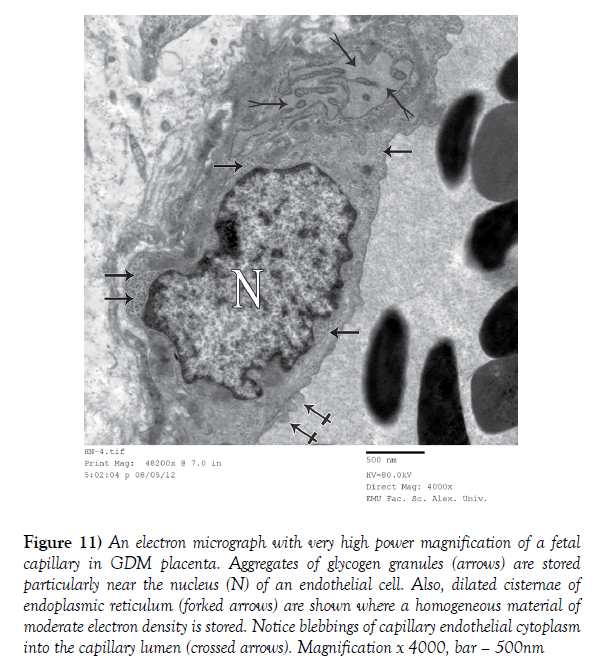
Figure 11) An electron micrograph with very high power magnification of a fetal capillary in GDM placenta. Aggregates of glycogen granules (arrows) are stored particularly near the nucleus (N) of an endothelial cell. Also, dilated cisternae of endoplasmic reticulum (forked arrows) are shown where a homogeneous material of moderate electron density is stored. Notice blebbings of capillary endothelial cytoplasm into the capillary lumen (crossed arrows). Magnification x 4000, bar – 500nm
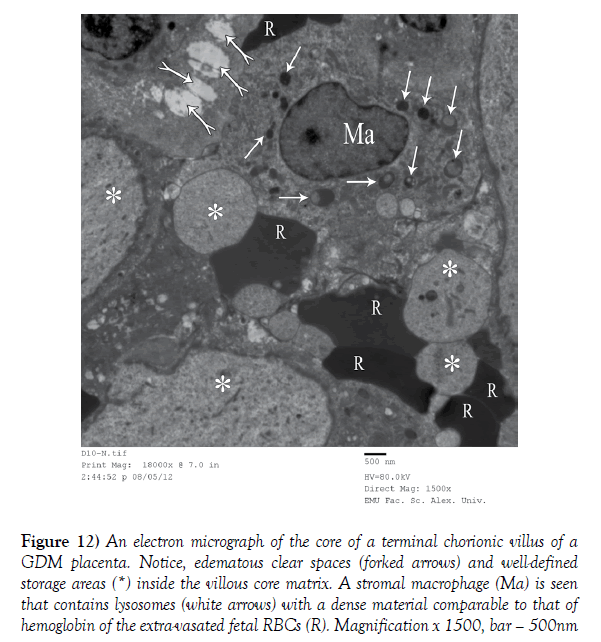
Figure 12) An electron micrograph of the core of a terminal chorionic villus of a GDM placenta. Notice, edematous clear spaces (forked arrows) and well-defined storage areas (*) inside the villous core matrix. A stromal macrophage (Ma) is seen that contains lysosomes (white arrows) with a dense material comparable to that of hemoglobin of the extra-vasated fetal RBCs (R). Magnification x 1500, bar – 500nm
Figure 13) An electron micrograph of the core of terminal chorionic villous of a GDM placenta showing the detailed structure of the storage areas (*). These areas are well-circumscribed by coarse matrix fibers (white arrows) and enclose scattered dense short fibrillar and granular content on a homogenous background of moderate electron density. Notice, edematous clear spaces (forked arrows). Magnification x 1500, bar – 500nm
Discussion
The placenta is a complex organ that has vital roles during fetal growth. Because of its unique position, the placenta is exposed to changes in both maternal and fetal environments. Diabetes mellitus affects both maternal and fetal environments with multiple effects on different body organs including the placenta that shows alterations from non-diabetic placenta [10].
Study of the placenta of the control group showed that the placental terminal villi were formed of a thick layer of Sy with multiple nuclei and a thin layer of Cy with a thin BM. The terminal villi beared regular and cylindrical microvilli. The trophoblast showed some rER and mitochondria. The villous core contained capillaries lined with flat endothelial cells with a thin BM. Same results were obtained by Meng et al. [3] for the structure of the trophoblastic covering and the core of the placental villi.
Placental growth might be considered a reflection of the coordination of trophoblast proliferation and differentiation. Moreover, the degree of trophoblastic maturation and microvillous density are determined by thickness of the trophoblastic layers that in turn affect the fetal-maternal exchange [3].
In the control group, a thin layer of Cy is shown due to proper syncytial fusion. Sy cannot grow by itself but syncytial growth and surface expansion throughout pregnancy together with endometrial invasion depend on continuous proliferation and differentiation of the cells of Cy and finally fusing with Sy, a process known as syncytial fusion which is also important for the syncytial hormonal function [6]. Thin Cy and hence thin placental barrier increases the trans-placental flow, thus ensuring adequate maternal nutrient supply to the fetus [3,6]. Moreover, thin Cy cells ensure proper invasion to the endometrium and its spiral arteries as the Cy at the tips of the villi grows out to penetrate into the decidualised uterus [6]. Placental invasion and establishment of enough maternal blood supply represent the cornerstone in placental development while their dysregulation is associated with pregnancy troubles [3,5].
The placental barrier represents the site of interface between the maternal and fetal circulations. In the control group, it was formed of Sy, a thin layer of Cy and a thin layer of vascular endothelial cells with a thin BM together with a villous core made up of stroma. Gude et al. [13] and Meng et al. [3] mentioned that this structure is suitable to ensure proper oxygenation and nutrition of the embryo which are essential for a successful pregnancy [3,13].
Regarding the rER, it is the intracellular organelle where synthesis of proteins occurs prior to its eventual extrusion into the extracellular matrix to share in the production of placental hormones [6]. Moreover, Mitochondria; by oxidative phosphorylation; adopt a lot of essential functions for placental survival and energy metabolism necessary for this active organ. By oxidative phosphorylation, the cells use enzymes to oxidize nutrients releasing energy. Under normal conditions, increased activity and oxidative phosphorylation of placental mitochondria throughout gestation results in overproduction of reactive oxygen species (ROS) as natural byproducts. Physiological ROS levels play a crucial role in placental development through cellular signaling but increased ROS can inactivate certain enzymes thus exposing the placenta to oxidative stress even in normal pregnancies. This is dealt with by the placental antioxidant defense capacities which induce conversion of ROS to water and molecular oxygen [14,15].
The current study demonstrated certain structural changes in that GDM placentas. The trophoblast had thin Sy, thick Cy, scarse microvilli with thick trophoblastic BM and evident vacuoles. Moreover, The villous core showed obvious edematous spaces, fibroblasts and fibrin deposition and dilatation of endoplasmic reticulum in the cytoplasm of the vascular endothelial cells together with stromal macrophages outside the villous capillaries. There were glycogen storage areas both inside the trophoblast and in the villous core. Also, there were evident areas of perivillous fibrinoid degeneration.
Regarding the thin Sy, thick Cy and scarse microvilli. Similar observations were found by Gul et al. [1], Meng et al. [3], Gheorman et al. [11] and Slukvin et al. [16] who found reduced number of or even absent placental microvilli with immature villous Sy and persistent Cy with GDM.
It was estimated that the maternal diabetic environment induces abnormal uncontrolled cell proliferation in the placenta with increased proliferative activity in the Cy layer with an influence on trophoblastic invasion [6,17]. The cells of the thick Cy at the tips of the villi do not invade properly the endometrial vessels adding to the uteroplacental ischemia and hypoxia. Invasion is a complex process involving a range of invasion inhibiting and invasion promoting factors. The diabetic environment with the accompanying oxidative stress appear to shift the balance towards invasion inhibition [1,18]. This improper invasion reduces the uteroplacental blood flow resulting in hypoxia and increased areas of placental infarction with degeneration of Sy layer leading to shedding and reduction of the number of the villi [3,16]. Moreover, villous Cy proliferation without syncytial fusion, as observed in severe hypoxia, might be accompanied by syncytial degeneration [5].
Oxidative stress and mitochondrial dysfunction are now emerging as key players in diabetic pathogenesis. It was reported that there is twofold increases in trophoblastic mitochondrial ROS production under hyperglycemic conditions compared to normoglycemic states together with the reduction of the placental antioxidant capacity. ROS with the resulting oxidative stress have a deleterious effect on all biological molecular structures leading to inactivation of specific enzymatic reactions together with loss of function and cell death. Thus, the trophoblast is affected by the hyperglycemic environment with reduction of its proliferation [19-21].
Regarding the thickening of the trophoblastic BM seen in GDM placentas. Similar results were obtained by Gheorman et al. [11], Magee et al. [22], Arshad et al. [23] and Meng et al. [3] in placentas of GDM women. The same was found by Gul et al. [1] in the placenta of diabetic rats. BM thickening might be attributed to mucopolysaccharide storage and it could be due to impaired villous trophoblastic activity such as increased production or decreased turnover of BM molecules, especially that the constituents of the BM are mostly produced by the trophoblast [11]. BM thickening was also said to be due to fat droplet accumulation owing to the effect of diabetes on fatty acid oxidation [1,16]. These changes may impair uteroplacental transfer further aggravating the hypoxic state [1].
Moreover, the vacuoles in both trophoblastic layers of GDM placentas detected in the current study were found by Meng et al. [3]. It was hypothesized that slight vacuolation may be present in the normal human placenta as a natural and physiological form of cell degradation which promotes survival [24,25]. On the other hand, GDM with its hyperglycemia and hypoxia may enhance the lysosome/vacuole functions of the trophoblast resulting in a widespread cytoplasmic vacuolation and altered transplacental exchange that may promote cell death [3,25,26].
Regarding the villous core, the present study showed obvious edematous spaces in GDM placentas. Similarly, Gauster et al. [27] and slukvin et al. [16] found edematous spaces in placental villi of GDM women. The same was mentioned by Gul et al. [1] on diabetic rat placentas. Gheorman et al. [11] attributed the villous edema to the mucopolysaccharide deposits with diabetes that consist mainly of hyaluronic acid molecules that can retain water. This edema fluid is interposed as a barrier to the feto-maternal exchange [1].
Also, the present study showed dilatation of endoplasmic reticulum in the cytoplasm of the vascular endothelial cells. The same was mentioned by Meng et al. [3] and in diabetic rats by Gul et al. [1]. The abnormal ultrastructure of rER could have impacts on metabolic functions and synthesis in the placenta as rER is one of the most susceptible organelles to diabetic hyperglycemia and hypoxia [3,15]. Massively dilated rER, however, is an ultrastructural indication of improper processing with a defective extrusion of protein products, and this was attributed to oxidative stress induced cell injury [1,28].
Regarding the glycogen storage areas inside the trophoblast and villous core in GDM placentas. The same results were obtained by Gheorman et al. [11] in human GDM placenta and Gul et al. [1], Yoruk et al. [29] and Padmanabhan and Shafiullah [30] in diabetic rats. This glycogen deposition is said to be related to the level of maternal hyperglycemia that causes elevated insulin levels in the fetal circulation. This fetal hyperinsulinemia will stimulate what is called the buffer action of the placenta by stimulating endothelial glucose uptake and glycogen synthesis [10]. Placenta is glucose dependent and it is the only fetal tissue that can store excess glucose. In addition, glucose itself activates glycogen synthase and deactivates glycogen phosphorylase that destroys glycogen [1,29]. Also, it was said that the high affinity glucose transporter 3 (GLUT3) is expressed in the placental endothelium, where it colocalizes with glycogenin, the protein precursor for glycogen synthesis [1]. These areas filled with glycogen may increase the transfer distance from the maternal blood and affect the placental blood flow and exchange [1,31].
The fibroblasts and fibrin deposition in the villous core observed in the current study were also found by Gheorman et al. [11], Mayhew and Sampson [18] and Gauster et al. [27] in diabetic placentas. The same was described by Meng et al. [3], Shams et al. [32] and Verma et al. [33]. Of course, the interruption of placental blood flow causes placental infarcts and fibrosis. Small placental infarcts with minimal fibrosis are considered to be normal at term while large infarcts and marked fibrosis are associated with vascular abnormalities as in GDM. This fibrin deposits might further affect transport of oxygen and nutrients to the fetus [18,32].
Also, perivillous fibrinoid degeneration was detected in GDM placentas in the present study. This was also mentioned by Arshad et al. [23], Verma et al. [33], Jarmuzek et al. [34] and Gabbay-Benziv and Baschat [35]. An explanation was offered as fibrinoid degeneration is a special form of necrosis that may be due to deposition of molecules derived from the hpoxic degenerative processes or complexes of antigens and antibodies of hypersensitivity reactions together with fibrin that leaked outside the vessels giving the characteristic pink appearance. This fibrinoid material may replace the degenerative Sy at the materno-fetal exchange surfaces, thus acting as a barrier [34,36].
Moreover, the present study showed stromal macrophages outside the villous capillaries of GDM placentas. Guo et al. [37] mentioned that these stromal macrophages represent stromal vascular fraction that was mentioned in many regeneration processes and diabetes-related complications. It was suggested that stromal vascular fraction achieves healing and regeneration of the necrotic tissues by angiogenesis, and extracellular matrix secretion [37].
Conclusion
Finally, this study showed that hyperglycemia and hypoxia are two key factors in the patho-physiological process of GDM complications and it is hyperglycemia that induces hypoxia and oxidative stress in the placenta. Also, the thickened placental barrier, edematous spaces, fibrin deposition and trophoblastic vacuoles, all of which potentially reduce transplacental transport and exchange with aggravation of ischemia and hypoxia.
REFERENCES
- Gul M, Bayat N, Cetin A, et al. Histopathological, Ultrastructural and Apoptotic Changes in Diabetic Rat Placenta. Balkan Med J. 2015;32:296-302.
- Jayabalan N, Nair S, Nuzhat Z, et al. Cross Talk between Adipose Tissue and Placenta in Obese and Gestational Diabetes Mellitus Pregnancies via Exosomes. Front Endocrinol (Lausanne). 2017;8:239.
- Meng Q, ShaoL, Luo L, et al. Ultrastructure of Placenta of Gravidas with Gestational Diabetes Mellitus. Obstet Gynecol Int. 2015;283124.
- Singh V. Extraembryonic membranes and twinning. In Textbook of Clinical Embryology. Elsevier. 2012;57-75.
- Aires MB, Dos Santos AC. Effects of maternal diabetes on trophoblast cells. World J Diabet. 2015;6:338-44.
- Benirschke K, Kaufmann P, Baergen R. Basic Structure of the Villous Trees. In Pathology of the Human Placenta. 2006;50-120.
- Standards of medical care in diabetes—2011. American Diabetes Association. 2011;S11–S61.
- Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med. 2011;28:652-7.
- Ngala RA, Fondjo LA, Gmagna P, et al. Placental peptides metabolism and maternal factors as predictors of risk of gestational diabetes in pregnant women. A case-control study. PLoS One. 2017;12:e0181613.
- Desoye G, Hauguel-de Mouzon S. Human Placenta in Gestational Diabetes Mellitus. The insulin and cytokine network. Diabetes Care. 2007;30:S120-6.
- Gheorman L, Pleşea IE, Gheorman V. Histopathological considerations of placenta in pregnancy with diabetes. Rom J Morphol Embryol. 2012;53:329-36.
- Hayat MA. Principles and techniques of electron microscopy: biological applications. 1989;24-74.
- Gude NM, Roberts CT, Kalionis B, et al. Growth and function of the normal human placenta. Thromb Res. 2004;114:397-407.
- Lemasters JJ, Theruvath TP, Zhong Z, et al. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395-1401.
- Pagliarini DJ, Calvo SE, Chang B, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112-23.
- Slukvin II, Salamat MS, Chandra S. Morphologic studies of the placenta and autopsy findings in neonatal-onset glutaric acidemia type II. Pediatr dev Pathol. 2002;5:315-21.
- Zorn TM, Zuniga M, Madrid E, et al. Maternal diabetes affects cell proliferation in developing rat placenta. Histol Histopathol. 2011;26:1049-56.
- Mayhew TM, Sampson C. Maternal diabetes mellitus is associated with altered deposition of fibrin-type fibrinoid at the villous surface in term placentae. Placenta. 2003; 24:524-31.
- Joshi M, Kotha SR, Malireddy S, et al. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem. 2014;386:233-49.
- Frohlich JD, Huppertz B, Abuja PM, et al. Oxygen Modulates the Response of First-Trimester Trophoblasts to Hyperglycemia. Am J Pathol. 2012;180:153-64.
- Jauniaux E, Burton GJ. The role of oxidative stress in placental-related diseases of pregnancy. J Gynecol Obstet Biol Reprod. 2016;45:775-85.
- Magee TR, Ross MG, Wedekind L, et al. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J Diabetes Complications. 2014;28:448-59.
- Arshad R, Kanpurwala MA, Karim N, et al. Effects of Diet and Metformin on placental morphology in Gestational Diabetes Mellitus. Pak J Med Sci. 2016;32:1522-27.
- Kobayashi S. Choose Delicately, Reuse Adequately: the newly recycled process of Autophagy. Biol Pharm Bull. 2015;38:1098-103.
- Curtis S, Jones CJ, Garrod A, et al. Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction. J Matern Fetal Neonat med. 2013; 26:339-46.
- Holmes VA, Young IS, Patterson CC, et al. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabet care. 2011;34:1683-8.
- Gauster M, Desoye G, Totsch M, et al. The placenta and gestational diabetes mellitus. Curr Diabetes Rep. 2012;12:16-23.
- Shapiro F, Mulhern H, Weis MA, et al. Rough endoplasmic reticulum abnormalities in a patient with spondyloepimetaphyseal dysplasia with scoliosis, joint laxity and finger deformities. Ultrastruct pathol. 2006;30:393-400.
- Yoruk M, Kanter M, Meral I, et al. Localization of glycogen in the placenta and fetal and maternal livers of cadmium-exposed diabetic pregnant rats. Biol Trace Elem Res. 2003;96:217-26.
- Padmanabhan R, Shafiullah M. Intrauterine growth retardation in experimental diabetes: possible role of the placenta. Arch Physiol Biochem. 2001;109:260-71.
- Ruiz-Palacios M, Ruiz-Alcaraz AJ, Sanchez-Campillo M, et al. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Ann Nutr Metab. 2017; 70:16-25.
- Shams F, Rafique M, Samoo NA, et al. Fibrinoid necrosis and hyalinization observed in normal, diabetic and hypertensive placentae. J Coll Physicians Surg PaK. 2012;22:769-72.
- Verma R, Mishra S, Kaul JM. Cellular changes in the placenta in pregnancies complicated with diabetes. Int J Morphol. 2010;28:259-64.
- Jarmuzek P, Wielgos M, Bomba-Opon D. Placental pathologic changes in gestational diabetes mellitus. Neuro Endocrinol Lett. 2015;36:101-5.
- Gabbay-Benziv R, Baschat AA. Gestational diabetes as one of the "great obstetrical syndromes"-the maternal, placental, and fetal dialog. Best Pract Res Clin Obstet Gynaecol. 2015;29:150-5.
- Augustine G, Pulikkathodi M, Renjith S, et al. A study of placental histological changes in gestational diabetes mellitus on account of fetal hypoxia. Int J Med Sci And Pub Health. 2016;5:2457-60.
- Guo J, Nguyen A, Banyard DA, et al. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J Plat Reconstr aesthet Surg. 2016; 69:180-8.